Two-Step Performance Optimization of CsPbBr3 Perovskite Nanocrystals for Wide Color Gamut Displays
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussions
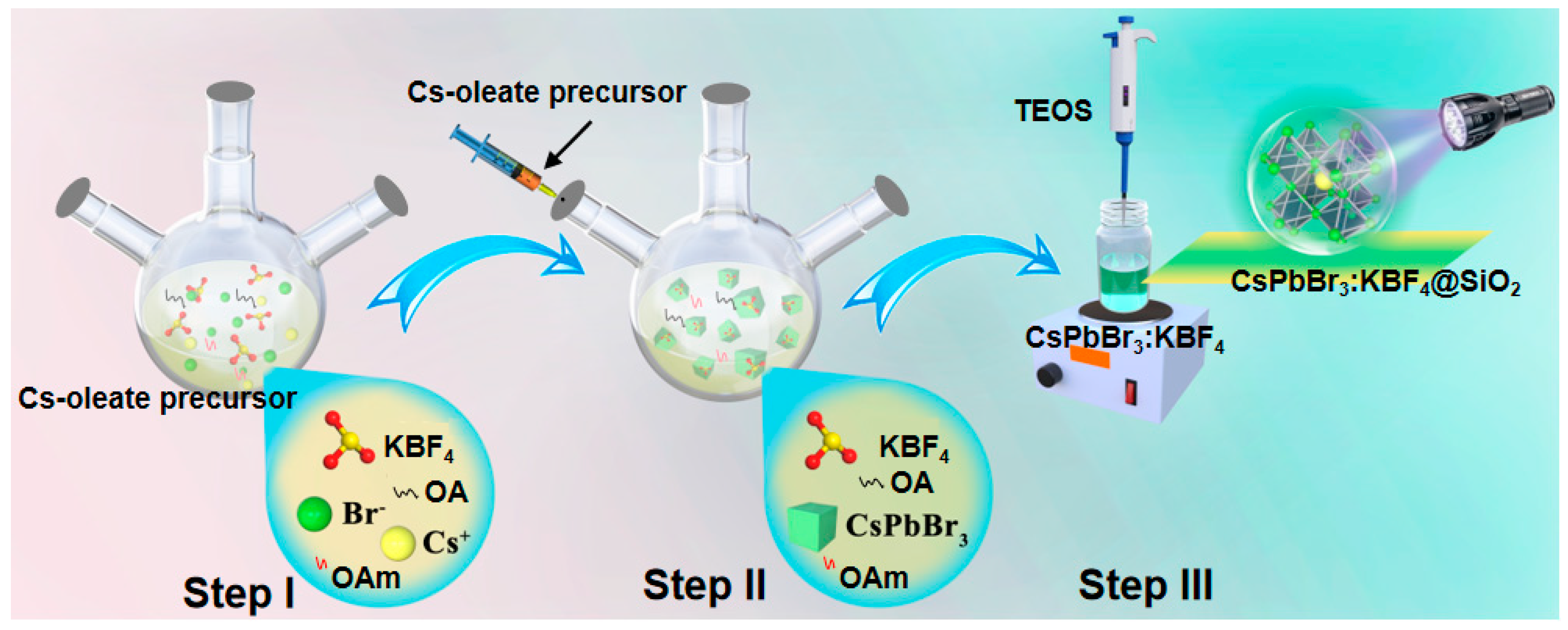
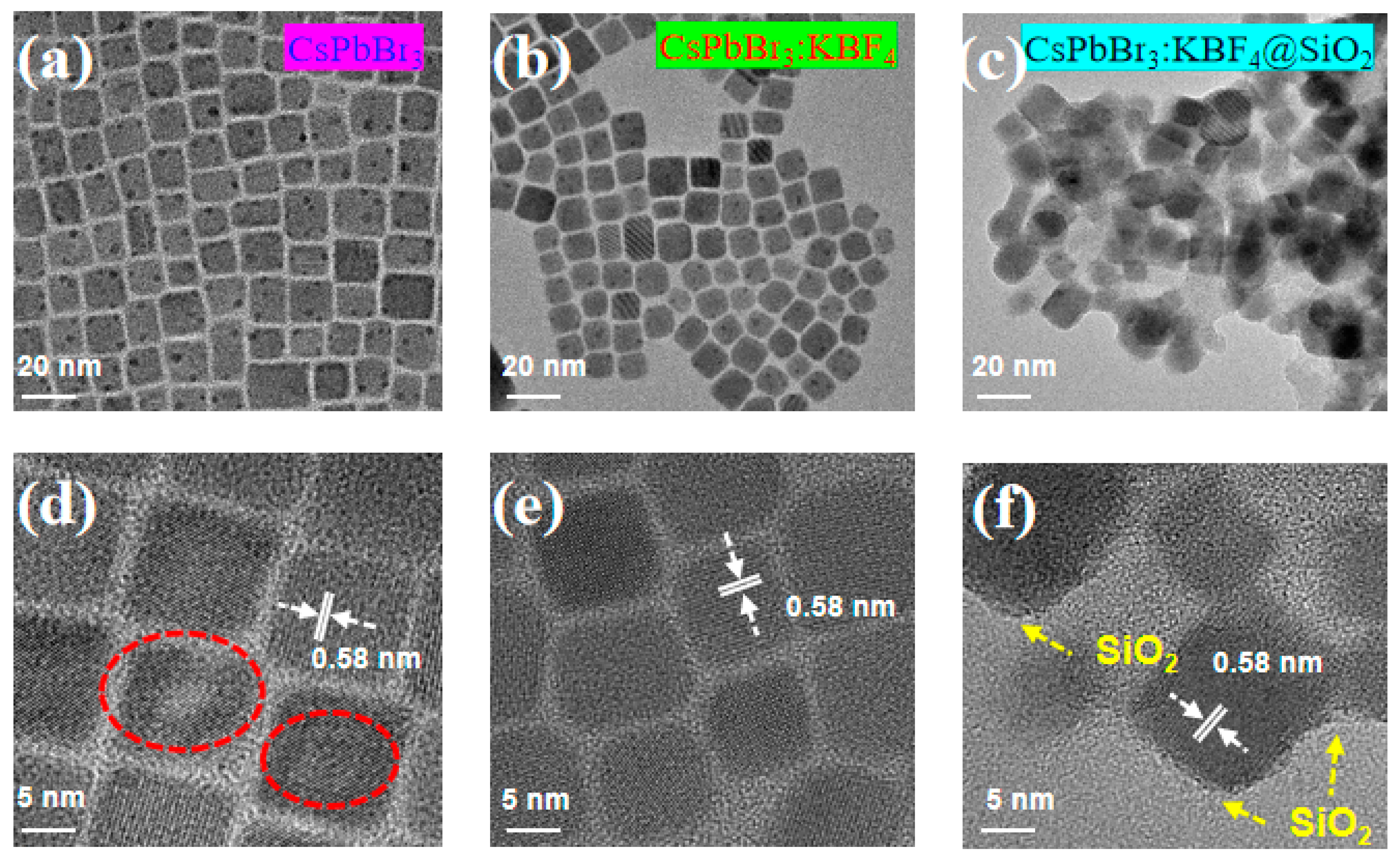
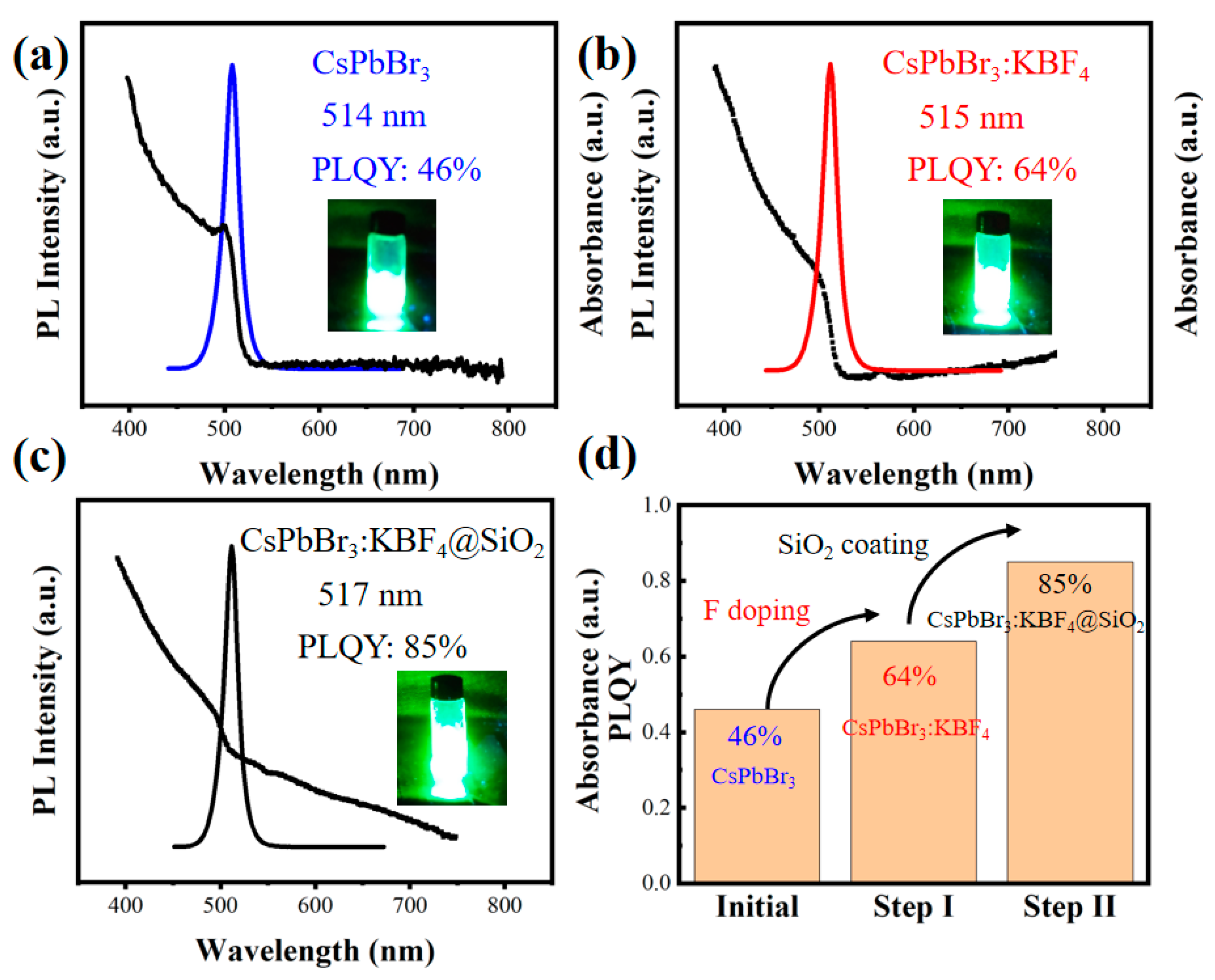
3.1. Preparation and Characterization
3.2. Performance and Stability Analysis
3.3. LED Application
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Kavanagh, S.R.; Scanlon, D.O.; Walsh, A.; Hoye, R.L.Z. Perovskite-inspired materials for photovoltaics and beyond-from design to devices. Nanotechnology 2021, 32, 132004. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Liu, X.; Hu, Y.F.; Lou, Z.D.; Hou, Y.B.; Teng, F. Property modulation of two-dimensional lead-free perovskite thin films by aromatic polymer additives for performance enhancement of field-effect transistors. ACS Appl. Mater. Interfaces 2021, 13, 22424–24272. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, D.; Li, M.; Guo, C.; Jiang, X.; Li, M.L.; Xu, T.T.; Zhu, J.P.; Fan, B.B.; Liu, W.; et al. CsPbBr3 nanocrystals prepared by high energy ball milling in one-step and structural transformation from CsPbBr3 to CsPb2Br5. Appl. Surf. Sci. 2021, 543, 148782. [Google Scholar] [CrossRef]
- Caicedo, D.S.; Gunder, R.; Marquez, J.A.; Levcenko, S.; Schwarzburg, K.; Unold, T.; Abou, R.D. Effects of postdeposition annealing on the luminescence of mixed-phase CsPb2Br5/CsPbBr3 thin films. J. Phys. Chem. C 2020, 124, 19514–19521. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, D.Z.; Qin, C.J. Perovskite light-emitting diodes. CCS Chem. 2020, 4, 859–869. [Google Scholar] [CrossRef]
- Wang, H.Y.; Sun, Y.; Chen, J.; Wang, F.C.; Han, R.Y.; Zhang, C.Y.; Kong, J.F.; Li, L.; Yang, J. A Review of perovskite-based photodetectors and their applications. Nanomaterials 2023, 24, 4390. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.X.; Chen, E.G.; Ye, Y.; Xu, S.; Guo, T.L. Highly stabilized gradient alloy quantum dots and silica hybrid nanospheres by core double shells for photoluminescence devices. J. Phys. Chem. Lett. 2020, 11, 1428–1434. [Google Scholar] [CrossRef]
- Chen, E.G.; Lin, J.Y.; Yang, T.; Chen, Y.; Zhang, X.; Ye, Y.; Sun, J.; Yan, Q.; Guo, T.L. Asymmetric quantum-dot pixelation for color-converted white balance. ACS Photonics 2021, 8, 2158–2165. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z.P. High-quality ruddlesden-popper perovskite film formation for high-performance perovskite solar cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Liu, F.Z.; Tam, H.W.; Wong, M.K.; Ag, A.; Surya, C.; Chen, W.; Zeng, H.B. Perovskite solar cells-an overview of critical issues. Prog. Quant. Electron. 2017, 53, 1–37. [Google Scholar] [CrossRef]
- Li, L.; Ye, S.; Qu, J.L.; Zhou, F.F.; Song, J.; Shen, G.Z. Recent advances in perovskite photodetectors for image sensing. Small 2021, 17, 2005606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Li, G.H.; Cui, Y.X.; Ji, T.; Hao, Y.Y. Research progress in perovskite photodetectors. Laser Optoelectron. Prog. 2019, 1, 010001. [Google Scholar]
- Yin, W.X.; Li, M.K.; Dong, W.; Luo, Z.; Li, Y.X.; Qan, J.Y.; Zhang, J.Q.; Zhang, W.; Zhang, Y.; Kershaw, S.V.; et al. Multidentate ligand polyethylenimine enables bright color-saturated blue light-emitting diodes based on CsPbBr3 nanoplatelet. ACS Energy Lett. 2021, 2, 477. [Google Scholar] [CrossRef]
- Song, L.; Huang, L.X.; Liu, Y.; Hu, Y.S.; Guo, X.Y.; Chang, Y.L.; Geng, C.; Xu, S.; Zhang, Z.H.; Zhang, Y.H.; et al. Efficient and stable blue perovskite light-emitting devices based on inorganic Cs4PbBr6 spaced low-dimensional CsPbBr3 through synergistic control of amino alcohols and polymer additives. ACS Appl. Mater. Interfaces 2021, 13, 33199–33208. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.H.; Wang, C.H.; Hu, X.P.; Ye, Y.Y.; Zhong, L.J.; Chen, E.G.; Ye, Y.; Xu, S.; Sun, J.; Yan, Q.; et al. Water-driven photoluminescence reversibility in CsPbBr3/PDMS-PUa composite. Nano Res. 2022, 15, 6466–6476. [Google Scholar] [CrossRef]
- Giuri, A.; Munir, R.; Listorti, A.; Corcione, C.E.; Gigli, G.; Rizzo, A.; Amassian, A.; Colella, S. Implication of polymeric template agent on the formation process of hybrid halide perovskite films. Nanotechnology 2021, 32, 265707. [Google Scholar] [CrossRef]
- Wang, S.Z.; Amin, A.A.Y.; Wu, L.Z.; Cao, M.H.; Zhang, Q.; Ameri, T. Perovskite nanocrystals: Synthesis, stability, and optoelectronic applications. Small Struct. 2021, 3, 2000124. [Google Scholar] [CrossRef]
- Chang, S.; Bai, Z.L.; Zhong, H.Z. In situ fabricated perovskite nanocrystals: A revolution in optical materials. Adv. Opt. Mater. 2018, 18, 1800380. [Google Scholar] [CrossRef]
- Kim, J.I.; Zeng, Q.S.; Park, S.; Lee, H.; Park, J.; Kim, T.; Lee, T.W. Strategies to extend the lifetime of perovskite downconversion films for display applications. Adv. Mater. 2023, 2209784. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Song, J.Z.; Li, J.H.; Li, X.M.; Xu, L.M.; Dong, Y.H.; Zeng, H.B. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162. [Google Scholar] [CrossRef] [PubMed]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.D.; Alivisatos, A.P. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef] [PubMed]
- Min, X.H.; Xie, Q.F.; Wang, Z.G.; Wang, X.Z.; Chen, M.X. Improving the stability and optical properties of CsPbI3 perovskite nanocrystals by 1-Octadecanethiol through surface modification. Compos. Mater. Chem. Phys. 2021, 276, 125404. [Google Scholar] [CrossRef]
- Murugadoss, V.; Kang, D.Y.; Lee, W.J.; Jang, I.G.; Kim, T.G. Fluorine-induced surface modification to obtain stable and low energy loss zinc oxide/perovskite interface for photovoltaic application. Adv. Compos. Hybrid Mater. 2022, 2, 1385–1395. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Qi, D.P.; Guo, P.Z.; Liu, Y.; Zhu, B.W.; Yang, H.; Liu, Y.Q.; Li, B.; Zhang, C.G.; Yu, J.C.; et al. Thickness-gradient films for high gauge factor stretchable strain sensors. Adv. Mater. 2015, 27, 6230–6237. [Google Scholar] [CrossRef]
- Wang, X.D.; Dong, L.; Zhang, H.L.; Yu, R.M.; Pan, C.F.; Wang, Z.L. Recent process in electronic skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, D.J.; Xu, K.Y.; Hu, J.; Huang, D.C.; Hong, M.C.; Zhu, H.M. Organic polystyrene and inorganic silica double shell protected lead halide perovskite nanocrystals with high emission efficiency and superior stability. Nano Res. 2023, 16, 10507–10514. [Google Scholar] [CrossRef]
- Chen, Q.S.; Wu, J.; Ou, X.Y.; Huang, B.L.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.W.; Han, S.Y.; Liang, L.L.; Yi, Z.G.; et al. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561, 88. [Google Scholar] [CrossRef]
- Lin, X.Y.; You, G.F.; Yao, L.; Wang, L.J.; Cao, J.B.; Li, L.H.; Li, K.; Yang, E.; Zhen, H.Y.; Ling, Q.D. Enhanced photovoltaic performance of donor-acceptor type polymer donors by employing asymmetric pi bridges. Sol. Energy 2021, 224, 938–946. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Shao, Y.B.; Zhang, J.; Chen, C.; Wang, Q. The phototh ermal stability study of silica-coated CsPbBr3 perovskite nanocrystals. J. Solid State Chem. 2022, 311, 123086. [Google Scholar] [CrossRef]
- Baker, R.W.; Forfar, L.; Liang, X.X.; Cameron, P.J. Using design of experiment to obtain a systematic understanding of the effect of synthesis parameters on the properties of perovskite nanocrystals. React Chem. Eng. 2021, 6, 709. [Google Scholar] [CrossRef]
- Luo, C.H.; Huang, S.H.; Zhang, T.; Jiang, C.L.; Qi, R.J.; Liu, M.Q.; Lin, H.C.; Travas, S.J.; Peng, H. Water driven photoluminescence enhancement and recovery of CH3NH3PbBr3/Silicon oil/PDMS-urea composite. J. Alloys Compd. 2020, 834, 155088. [Google Scholar] [CrossRef]
- Voznyy, O.; Levina, L.; Fan, F.J.; Walters, G.; Fan, F.Z.; Kiani, A.; Lp, A.H.; Thon, S.M.; Proppe, A.H.; Liu, M.X.; et al. Origins of Stokes Shift in PbS Nanocrystals. Nano Lett. 2017, 12, 7191–7195. [Google Scholar] [CrossRef] [PubMed]
- Ghobeira, R.; Tabaei, P.S.E.; Morent, R.; De, G.N. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Davies, J.J.; Davies, P.R.; Guan, S.L.; Lee, R.; Morgan, D.J.; Palgrave, R. Advanced XPS characterization: XPS-based multi-technique analyses for comprehensive understanding of functional materials. Mater. Chem. Front. 2021, 5, 7931–7963. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Davies, J.J.; Davies, P.R.; Guan, S.L.; Lee, R.; Morgan, D.J.; Palgrave, R. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J. Cell Sci. 2006, 119, 2935–2944. [Google Scholar]
- Deng, J.; Xun, J.; He, R. Facile and rapid synthesis of high performance perovskite nanocrystals CsPb(X/Br)3 (X=Cl, I) at room temperature. Opt. Mater. 2020, 99, 109528. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, J.H.; Lin, J.Y.; Hu, X.P.; Wang, C.H.; Chen, E.G.; Sun, J.; Yan, Q.; Guo, T.L. Quantum-dot array with a random rough interface encapsulated by atomic layer deposition. Opt. Lett. 2022, 47, 166–169. [Google Scholar] [CrossRef]
- Ren, X.X.; Zhang, X.; Xie, H.X.; Cai, J.H.; Wang, C.H.; Chen, E.G.; Xu, S.; Ye, Y.; Sun, J.; Yan, Q.; et al. Perovskite quantum dots for emerging displays: Recent progress and perspectives. Nanomaterials 2022, 12, 2243. [Google Scholar] [CrossRef]
- Lee, X.H.; Lin, C.C.; Chang, Y.Y.; Chen, H.X.; Sun, C.C. Power management of direct-view LED backlight for liquid crystal display. Opt. Laser. Technol. 2012, 46, 142–144. [Google Scholar] [CrossRef]
- Wang, C.H.; Cai, J.H.; Ye, Y.Y.; Hu, X.P.; Zhong, L.J.; Xie, H.X.; Chen, E.G.; Ye, Y.; Xu, S.; Sun, J.; et al. Full-visible-spectrum perovskite quantum dots by anion exchange resin assisted synthesis. Nanophotonics 2022, 11, 1355–1366. [Google Scholar] [CrossRef]
- Meyns, M.P. Polymer enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Appl. Mater. Interfaces 2016, 30, 19579–19586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, G.S.; Wei, F.; Wu, J. Fluidization and agglomerate structure of SiO2 nanoparticles. Powder Technol. 2002, 124, 152–159. [Google Scholar]
- Polakiewicz, A.; Dodiuk, H.; Kenig, S. Super-hydrophilic coatings based on silica nanoparticles. Powder Technol. 2014, 5, 466–478. [Google Scholar] [CrossRef]






| Fitting Parameter | CsPbBr3 | CsPbBr3:KBF4 | CsPbBr3:KBF4@SiO2 |
|---|---|---|---|
| τ1 (ns) | 9.2 | 8.6 | 7.9 |
| τ2 (ns) | 36.8 | 39.6 | 43.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Chen, X.; Zhang, W.; Yang, L.; Lin, Z.; Zhao, W.; Ye, Y.; Xu, S.; Guo, T.; Chen, E. Two-Step Performance Optimization of CsPbBr3 Perovskite Nanocrystals for Wide Color Gamut Displays. Photonics 2023, 10, 1113. https://doi.org/10.3390/photonics10101113
Cai J, Chen X, Zhang W, Yang L, Lin Z, Zhao W, Ye Y, Xu S, Guo T, Chen E. Two-Step Performance Optimization of CsPbBr3 Perovskite Nanocrystals for Wide Color Gamut Displays. Photonics. 2023; 10(10):1113. https://doi.org/10.3390/photonics10101113
Chicago/Turabian StyleCai, Junhu, Xiaogang Chen, Wenyan Zhang, Longwen Yang, Zexi Lin, Wenxiao Zhao, Yun Ye, Sheng Xu, Tailiang Guo, and Enguo Chen. 2023. "Two-Step Performance Optimization of CsPbBr3 Perovskite Nanocrystals for Wide Color Gamut Displays" Photonics 10, no. 10: 1113. https://doi.org/10.3390/photonics10101113
APA StyleCai, J., Chen, X., Zhang, W., Yang, L., Lin, Z., Zhao, W., Ye, Y., Xu, S., Guo, T., & Chen, E. (2023). Two-Step Performance Optimization of CsPbBr3 Perovskite Nanocrystals for Wide Color Gamut Displays. Photonics, 10(10), 1113. https://doi.org/10.3390/photonics10101113






