Paleolimnological Approaches to Track Anthropogenic Eutrophication in Lacustrine Systems Across the American Continent: A Review
Abstract
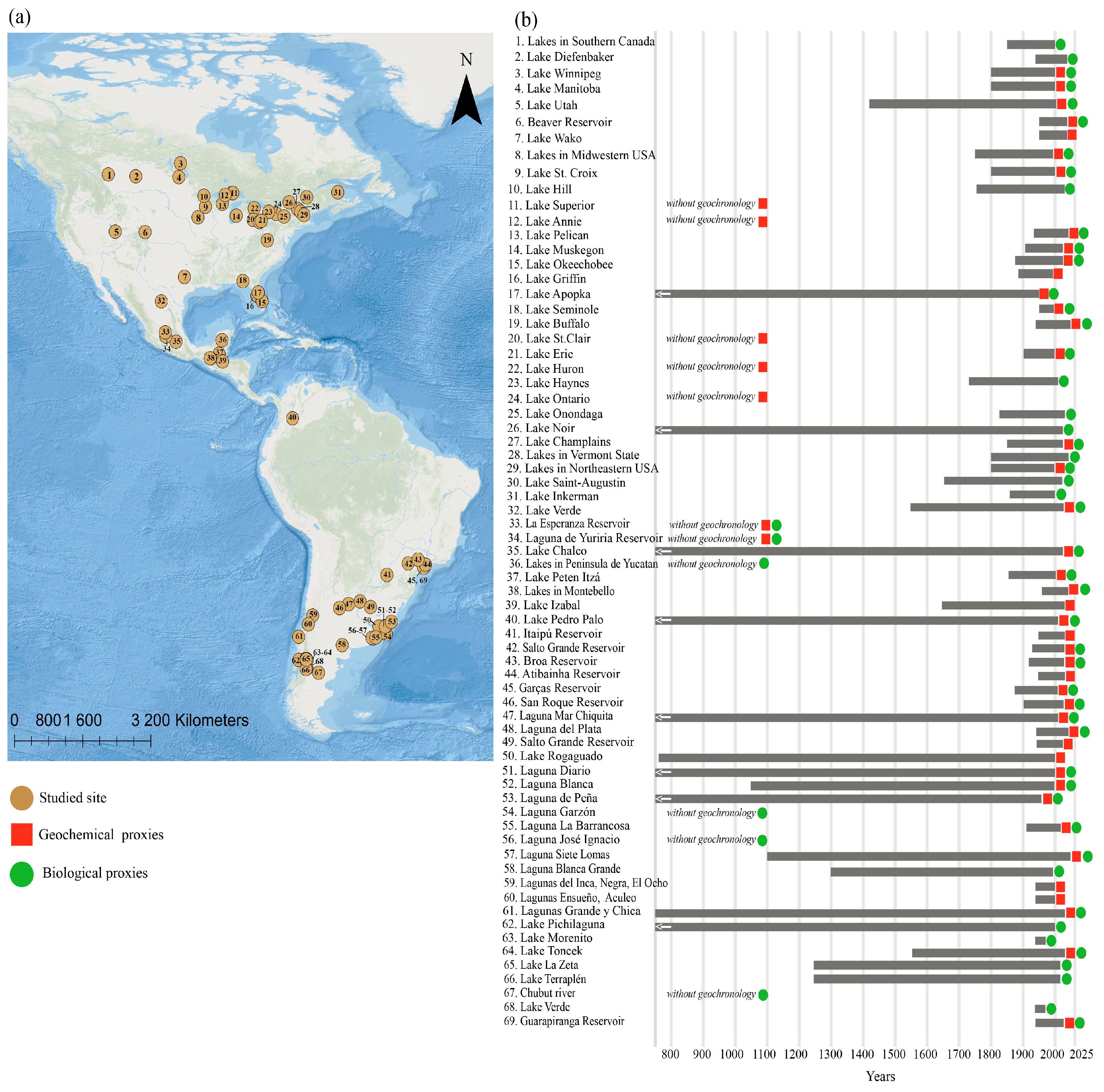
1. Eutrophication in the Context of Global Change
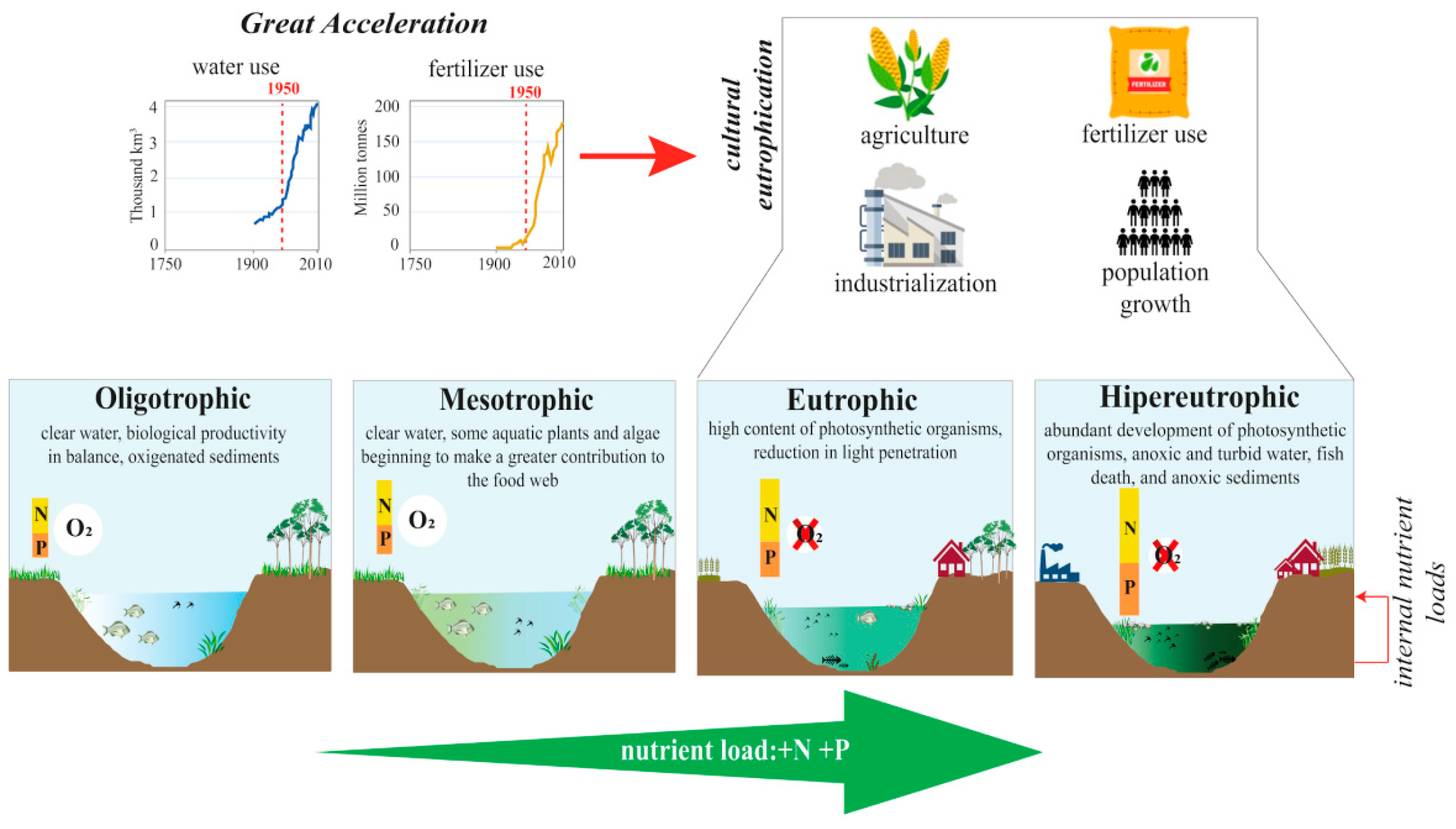
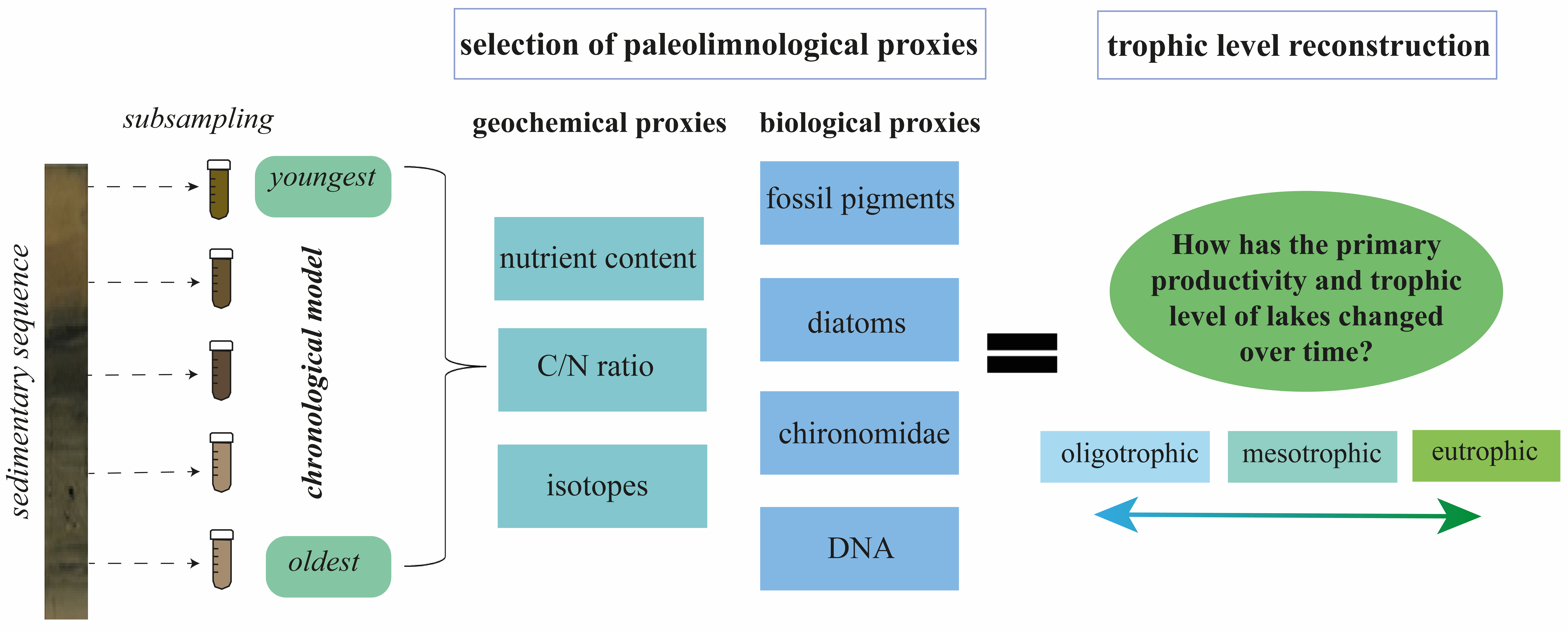
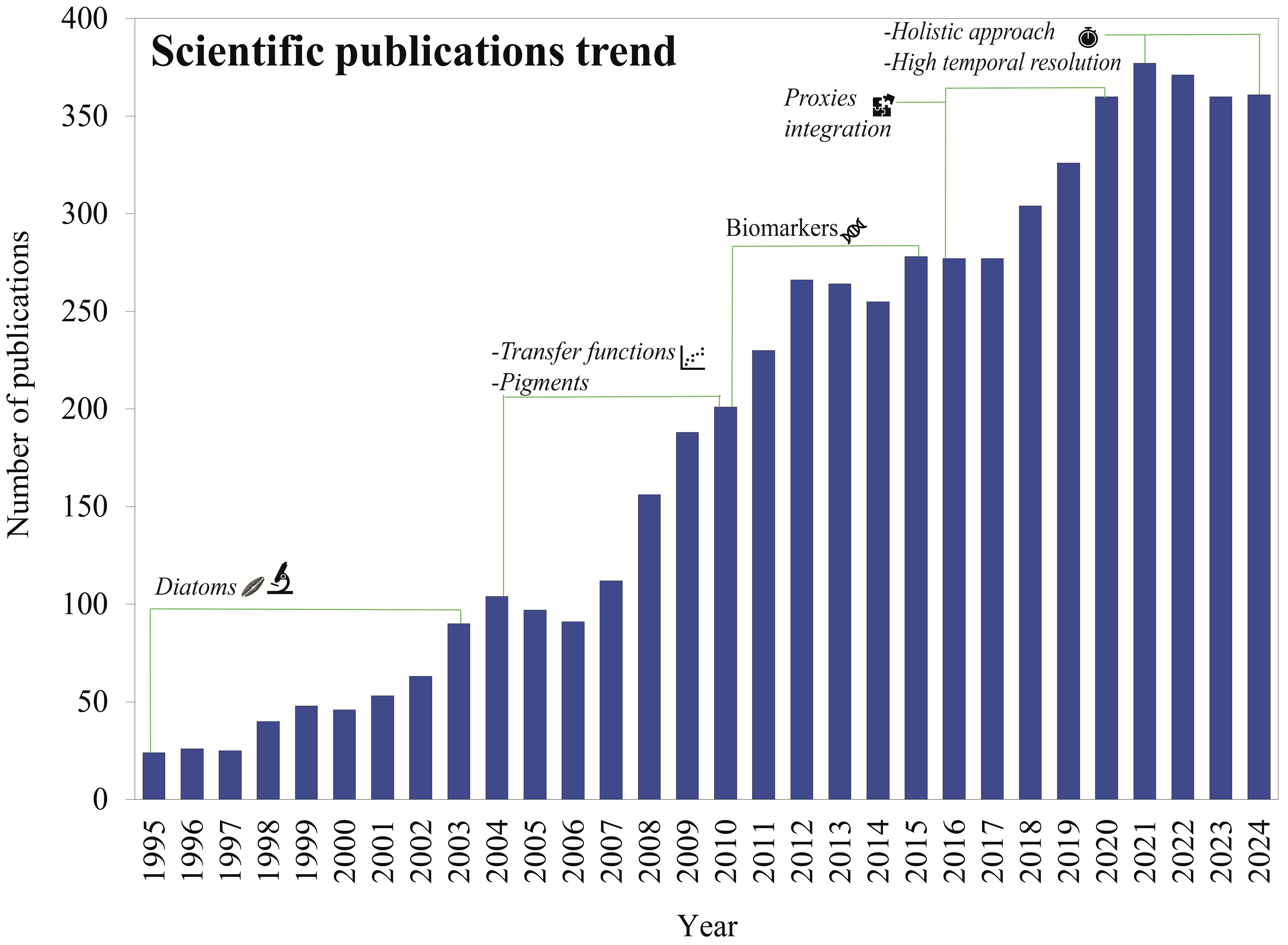
2. Use of Paleolimnology to Reconstruct the Trophic State of Lacustrine Systems
3. Geochemical Proxies
3.1. Nitrogen and Phosphorus Content
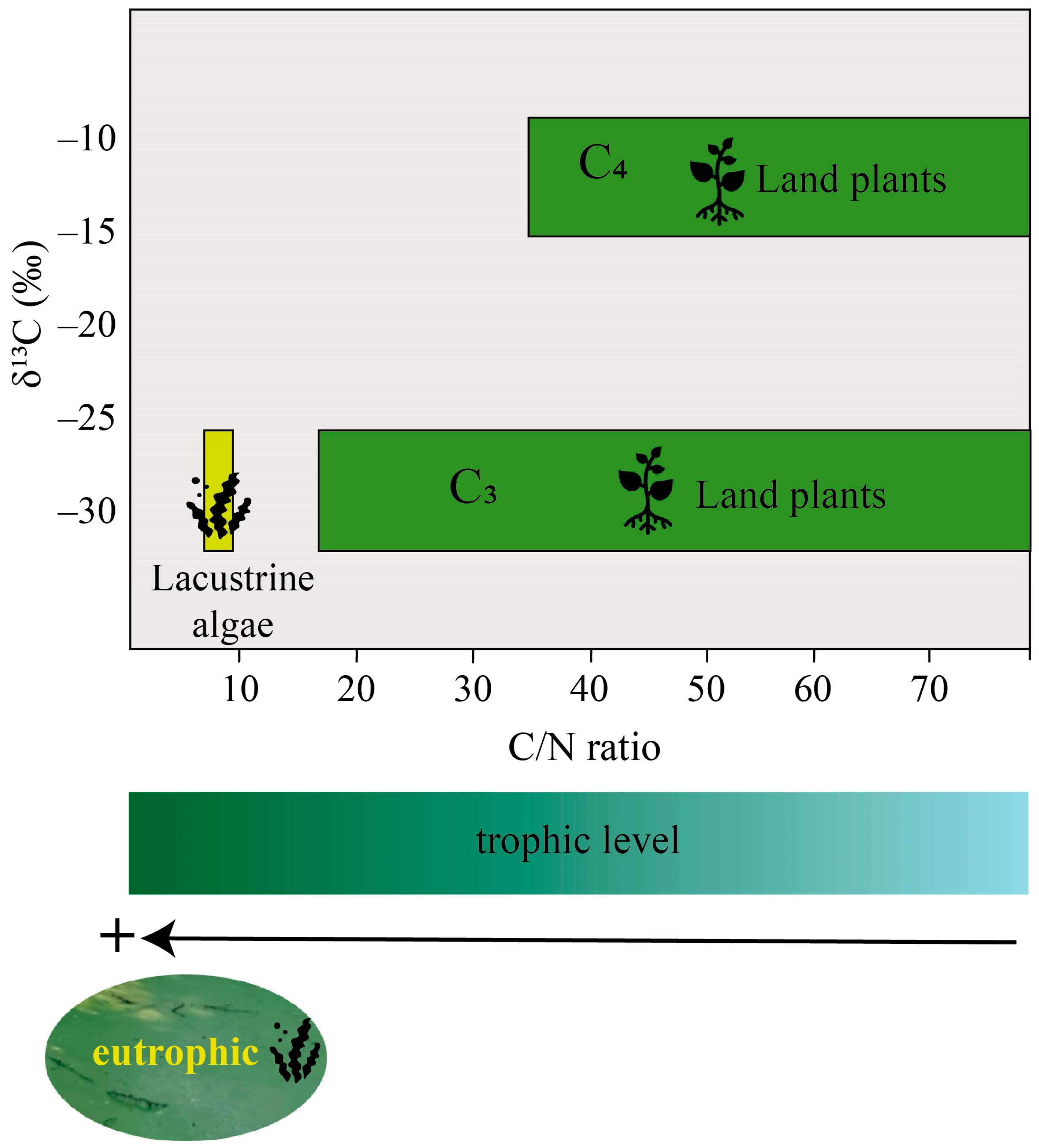
3.2. C/N Ratio
3.3. Stable Isotopes
4. Biological Proxies
4.1. Diatoms
4.2. Fossil Pigments
4.3. Chironomids
4.4. Biological Analyses Based on Sedimentary DNA
| Biological Proxy | Basis | Location | Reference |
|---|---|---|---|
| Diatoms | changes in species composition and abundance | Laguna Blanca, Uruguay | [81] |
|
transfer function
DI-TP | Lake St. Croix, USA | [107] | |
| changes in species composition and abundance | Garças Reservoir, Brazil | [54] | |
| changes in species composition and abundance | Laguna Lonkoy, Argentina | [117] | |
|
transfer functions DI-TP
DI-pH | Lakes Haynes, Canada | [118] | |
| changes in species composition and abundance | Lake Verde, Mexico | [111] | |
| changes in species composition and abundance | Guarapiranga Reservoir, Brazil | [55] | |
|
transfer function
DI-TP | Lakes in midwestern, USA | [121] | |
|
transfer functions DI-TP
DI-COND | Lake Onondaga, USA | [116] | |
| changes in species composition and abundance | Laguna Peña, Uruguay | [124] | |
|
transfer function
DI-TP | Lake Muskegon, USA | [122] | |
| changes in species composition and abundance | Lake Balamtetik, Mexico | [108] | |
|
DI-COND
changes in species composition and abundance | Chubut River, Argentina | [126] | |
| changes in species composition and abundance | Laguna Blanca Grande, Argentina | [112] | |
| species composition and abundance | Laguna Garzón and Laguna José Ignacio, Uruguay | [125] | |
| changes in species composition and abundance |
Lake Pedro Palo,
Colombia | [88] | |
| changes in species composition and abundance | Lake Pichilaguna, Chile | [127] | |
|
transfer functions DI-TP
DI-pH | Lakes in Vermont, USA | [120] | |
| changes in species composition and abundance | Lake Peñasquito | [118] | |
| changes in species composition and abundance | Laguna Siete Lomas, Argentina | [53] | |
| changes in species composition and abundance | Lake Montebello, Mexico | [68] | |
| Fossil pigments | changes in total and marker pigments concentration | Lake Okeechobee, USA | [73] |
| changes in total and cyanopigments concentration | Lake St. Croix, USA | [107] | |
| changes in total and marker pigments concentration | Lake Saint-Augustin, Canada | [137] | |
| changes in total and marker pigments concentration | Laguna Mar Chiquita, Argentina | [133] | |
| changes in total and cyanopigments concentration | Lake Diefenbaker, Canada | [144] | |
| changes in total and marker pigments concentration |
Lake
Seminole, USA | [138] | |
| changes in total and marker pigments concentration | Lake Inkerman, Canada | [139] | |
| changes in total and marker pigments concentration | Lake Winnipeg, Canada | [145] | |
| changes in total and cyanopigments concentration | Lakes in southern Canada | [140] | |
| changes in total and marker pigments concentration | Laguna La Barrancosa, Argentina | [57] | |
| changes in total and marker pigments concentration | San Roque Reservoir, Argentina | [62] | |
| changes in total and marker pigments concentration | Lakes in northeastern USA | [142] | |
| changes in total and marker pigments concentration | Broa Reservoir, Brazil | [136] | |
| changes in total and marker pigments concentration | Atibainha Reservoir, Brazil | [147] | |
| changes in total pigments concentration | Laguna del Plata Mar Chiquita system, Argentina | [58] | |
| changes in total and marker pigment-s concentration | Lake Manitoba, Canada | [51] | |
| changes in total pigments concentration | Laguna de Yuriria and La Esperanza Reservoirs | [67] | |
| changes in total and marker pigments concentration | San Roque Reservoir, Argentina | [52] | |
| changes in total and marker pigments concentration | Laguna Siete Lomas | [53] | |
| changes in total and marker pigments concentration | Lake Montebello, Mexico | [68] | |
| Chironomids | changes in species composition and abundance | Lake Morenito, Argentina | [156] |
| species composition and abundance | Lakes in the Peninsula de Yucatán, Mexico, Guatemala, Belize | [110] | |
| transfer function Chir-DO | Lake Onondaga, USA | [116] | |
| changes in species composition and abundance | Lake Toncek and Lake Verde, Argentina | [155] | |
| changes in species composition and abundance | Lake La Zeta and Lake Terraplén, Argentina | [157] | |
| transfer function Chir- DO | Lake Erie, Canada and USA | [158] | |
| DNAsed | specific genes sxtU--Cylindrospermopsis raciborskii | Laguna Blanca, Uruguay | [164] |
|
microsatellite markers
Daphnia pulicaria | Lake Hill, USA | [162] | |
| metagenomic Bacteria–Archaea–Eukarya | Lake Chalco, Mexico | [165] | |
|
metagenomic
phytoplankton and higher plants | Lake Utah, USA | [163] |
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The Trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Stoermer, E.F. The Anthropocene (2000); Benner, S., Lax, G., Crutzen, P.J., Pöschl, U., Lelieveld, J., Brauch, H.G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 1. [Google Scholar] [CrossRef]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond Six of Nine Planetary Boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y. The Concept of the Anthropocene. Annu. Rev. Environ. Resour. 2017, 42, 77–104. [Google Scholar] [CrossRef]
- Zalasiewicz, J.; Waters, C.N.; Head, M.J.; Poirier, C.; Summerhayes, C.P.; Leinfelder, R.; Grinevald, J.; Steffen, W.; Syvitski, J.; Haff, P.; et al. A Formal Anthropocene Is Compatible with but Distinct from Its Diachronous Anthropogenic Counterparts: A Response to W.F. Ruddiman’s ‘Three Flaws in Defining a Formal Anthropocene’. Prog. Phys. Geogr. 2019, 43, 319–333. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread Global Increase in Intense Lake Phytoplankton Blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R.; Bolgrien, D.; Lathrop, R.C.; Stow, C.A.; Reed, T.; Wilson, M.A. Ecological and Economic Analysis of Lake Eutrophication by Nonpoint Pollution. Aust. Ecol. 1998, 23, 68–79. [Google Scholar] [CrossRef]
- Smith, V.H.; Joye, S.B.; Howarth, R.W. Eutrophication of Freshwater and Marine Ecosystems. Limnol. Oceanogr. 2006, 51 Pt 2, 351–355. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Kapsalis, V.C.; Kalavrouziotis, I.K. Eutrophication—A Worldwide Water Quality Issue. In Chemical Lake Restoration: Technologies, Innovations and Economic Perspectives; Kalavrouziotis, I.K., Kapsalis, V.C., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Lecomte, K.L.; Pasquini, A.I.; Manjarrez-Rangel, C.S.; Puy-Alquiza, M.J.; Segoviano-Garfias, J.d.J.N.; Zanor, G.A. Surface Hydrochemical Dynamic in an Artificial Lake with Anthropic Impact: La Purísima Reservoir, Central Mexico. Environ. Monit. Assess. 2022, 194, 128. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Chalar, G.; Ibarguren, E.; Baeza, S.; De Giacomi, S.; Alvareda, E.; Brum, E.; Paradiso, M.; Mejía, P.; Crossa, M. Nutrient Levels, Trophic Status, and Land-Use Influences on Streams, Rivers, and Lakes in a Protected Floodplain of Uruguay. Limnol. Oceanogr. 2022, 94, 125966. [Google Scholar] [CrossRef]
- Zanor, G.A.; Lecomte, K.L.; Alquiza, M.J.P.Y.; Saldaña-Robles, A.; Manjarrez-Rangel, C.S.; Rubio-Jiménez, C.A.; Pussetto, N. A 16th Century Artificial Reservoir Under Human Pressure: Water Quality Variability Assessment in Laguna de Yuriria, Central Mexico. Environ. Monit. Assess. 2023, 195, 182. [Google Scholar] [CrossRef] [PubMed]
- García-Miranda, F.G.; Muro, C.; Alvarado, Y.; Expósito-Castillo, J.L.; Cabadas-Báez, H.V. Eutrophication Conditions in Two High Mountain Lakes: The Influence of Climate Conditions and Environmental Pollution. Hydrology 2025, 12, 32. [Google Scholar] [CrossRef]
- Cervantes-Astorga, E.; Aguilar-Juárez, O.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. A GIS Methodology to Determine the Critical Regions for Mitigating Eutrophication in Large Territories: The Case of Jalisco, Mexico. Sustainability 2021, 13, 8029. [Google Scholar] [CrossRef]
- Pompêo, M.; Moschini-Carlos, V.; Bitencourt, M.D.; Sòria-Perpinyà, X.; Vicente, E.; Delegido, J. Water Quality Assessment Using Sentinel-2 Imagery with Estimates of Chlorophyll a, Secchi Disk Depth, and Cyanobacteria Cell Number: The Cantareira System Reservoirs (São Paulo, Brazil). Environ. Sci. Pollut. Res. 2021, 28, 34990–35011. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, B.; Aubriot, L.; Olano, H.; Achkar, M. Satellite Assessment of Eutrophication Hot Spots and Algal Blooms in Small and Medium-Sized Productive Reservoirs in Uruguay’s Main Drinking Water Basin. Environ. Sci. Pollut. Res. 2023, 30, 43604–43618. [Google Scholar] [CrossRef] [PubMed]
- Saravani, M.J.; Saadatpour, M.; Shahvaran, A.R. A Web GIS-Based Integrated Water Resources Assessment Tool for Javeh Reservoir. Expert Syst. Appl. 2024, 252, 124198. [Google Scholar] [CrossRef]
- Díaz, I.; Levrini, P.; Achkar, M.; Crisci, C.; Nion, C.F.; Goyenola, G.; Mazzeo, N. Empirical Modeling of Stream Nutrients for Countries without Robust Water Quality Monitoring Systems. Environments 2021, 8, 129. [Google Scholar] [CrossRef]
- Van Heyst, A.; Sinclair, A.; Jamieson, R. Application of Phosphorus Loading Models to Understand Drivers of Eutrophication in a Complex Rural Lake-Watershed System. J. Environ. Manag. 2022, 302, 114010. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Sadabadi, S.A.; Rousseau, A.N.; Laurion, I.; Behmel, S.; Sadeghian, A.; Foulon, E.; Wauthy, M.; Cantin, A.M. Spatiotemporal Insights of Phytoplankton Dynamics in a Northern, Rural-Urban Lake Using a 3D Water Quality Model. J. Environ. Manag. 2024, 370, 122687. [Google Scholar] [CrossRef] [PubMed]
- EPA-822-B00-001; Nutrient Criteria Technical Guidance Manual: Lakes and Reservoirs. U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 2000.
- EPA 841-R-16-113; National Lakes Assessment 2012: A Collaborative Survey of Lakes in the United States. U.S. Environmental Protection Agency, Office of Water and Office of Research and Development: Washington, DC, USA, 2016.
- Fernandez-Figueroa, E.G.; Rogers, S.R.; Waters, M.N.; Wilson, A.E. The Green Convergence: United States Lakes Are Collectively Moving Toward a Eutrophic State. Harmful Algae 2024, 139, 102721. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, I.; Bordet, F.; Chaparro, G. Bloom Forming Cyanobacterial Complexes Co-Occurring in a Subtropical Large Reservoir: Validation of Dominant Eco-Strategies. In Phytoplankton Responses to Human Impacts at Different Scales; Rengefors, K., Gustafsson, S., Hajdu, S., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 175–190. [Google Scholar] [CrossRef]
- Rodriguez, M.I.; Ruiz, M. Limnology of the San Roque Reservoir. In The Suquía River Basin (Córdoba, Argentina): An Integrated Study on Its Hydrology, Pollution, Effects on Native Biota and Models to Evaluate Changes in Water Quality; Báez, M.E., Ed.; Springer: Cham, Switzerland, 2016; pp. 37–59. [Google Scholar] [CrossRef]
- Bonansea, M.; Bazán, R.; Germán, A.; Ferral, A.; Beltramone, G.; Cossavella, A.; Pinotti, L. Assessing Land Use and Land Cover Change in Los Molinos Reservoir Watershed and the Effect on the Reservoir Water Quality. J. S. Am. Earth Sci. 2021, 108, 103243. [Google Scholar] [CrossRef]
- Comisión Nacional del Agua (CONAGUA). Estadísticas del Agua en México 2018; CONAGUA: Ciudad de México, Mexico, 2018; Available online: https://files.conagua.gob.mx/conagua/publicaciones/publicaciones/eam2018.pdf (accessed on 14 July 2025).
- González, M.A.L.; Jarero, E.G.R.; Peña, M.P.; Carrillo, E.J.; Padilla, I.E.; Uriarte, E.L. Variación espacio-temporal del fitoplancton del lago de Chapala, México, durante 2012: Spatio-temporal variation of phytoplankton in Lake Chapala, Mexico, during 2012. E-CUCBA 2024, 11, 68–85. [Google Scholar] [CrossRef]
- Pompêo, M.; Cardoso-Silva, S.; Figueira, R.C.L.; Moschini-Carlos, V. Limnologia de Reservatórios: Do Clássico às Novas Abordagens; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2024. [Google Scholar]
- Goyenola, G.; Kruk, C.; Mazzeo, N.; Nario, A.; Perdomo, C.; Piccini, C.; Meerhoff, M. Producción, Nutrientes, Eutrofización y Cianobacterias en Uruguay: Armando el Rompecabezas. Rev. Lab. Tecnol. Urug. 2021, 22, e558. [Google Scholar] [CrossRef]
- Mazzeo, N.; Ciganda, A.L.; Fernández Nion, C.; Peñas, F.J.; González-Ferreras, A.M.; Crisci, C.; Zurbriggen, C.; Pérez, D.; Barquin, J.; Díaz, I. Inter and Transdisciplinarity Strategies for Evaluating and Improving Water Quality Monitoring Systems: Uruguay as a Study Case. Environ. Sci. Policy 2024, 154, 103699. [Google Scholar] [CrossRef]
- Monastersky, R. Anthropocene: The Human Age. Nature 2015, 519, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Smol, J.P. Paleolimnology: An Important Tool for Effective Ecosystem Management. J. Aquat. Ecosyst. Health 1992, 1, 49–58. [Google Scholar] [CrossRef]
- Appleby, P.G.; Oldfield, F. The Calculation of Lead-210 Dates Assuming a Constant Rate of Supply of Unsupported 210Pb to the Sediment. Catena 1978, 5, 1–8. [Google Scholar] [CrossRef]
- Fritz, S.C.; Juggins, S.; Battarbee, R.W.; Engstrom, D.R. Reconstruction of Past Changes in Salinity and Climate Using a Diatom-Based Transfer Function. Nature 1991, 352, 706–708. [Google Scholar] [CrossRef]
- Appleby, P.G. Chronostratigraphic Techniques in Recent Sediments. In Tracking Environmental Change Using Lake Sediments; Last, W.M., Smol, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 171–203. [Google Scholar] [CrossRef]
- Gregersen, R.; Pearman, J.K.; Atalah, J.; Waters, S.; Vandergoes, M.J.; Howarth, J.D.; Thomson-Laing, G.; Thompson, L.; Wood, S.A. A Taxonomy-Free Diatom eDNA-Based Technique for Assessing Lake Trophic Level Using Lake Sediments. J. Environ. Manag. 2023, 345, 118885. [Google Scholar] [CrossRef] [PubMed]
- Gregory-Eaves, I.; Smol, J.P. Paleolimnology: Approaches and Applications. In Wetzel’s Limnology: Lake and River Ecosystems, 4th ed.; Wetzel, R.G., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 1015–1043. [Google Scholar] [CrossRef]
- Kenney, W.F.; Waters, M.N.; Schelske, C.L.; Brenner, M. Sediment Records of Phosphorus-Driven Shifts to Phytoplankton Dominance in Shallow Florida Lakes. J. Paleolimnol. 2002, 27, 367–377. [Google Scholar] [CrossRef]
- Winston, B.; Hausmann, S.; Escobar, J.; Kenney, W.F. A Sediment Record of Trophic State Change in an Arkansas (USA) Reservoir. J. Paleolimnol. 2014, 51, 393–403. [Google Scholar] [CrossRef]
- Wengrat, S.; Bennion, H.; Ferreira, P.A.d.L.; Figueira, R.C.L.; Bicudo, D.C. Assessing the Degree of Ecological Change and Baselines for Reservoirs: Challenges and Implications for Management. J. Paleolimnol. 2019, 62, 337–357. [Google Scholar] [CrossRef]
- Torres, I.C.; Inglett, P.W.; Brenner, M.; Kenney, W.F.; Reddy, K.R. Stable Isotope (δ13C and δ15N) Values of Sediment Organic Matter in Subtropical Lakes of Different Trophic Status. J. Paleolimnol. 2012, 47, 693–706. [Google Scholar] [CrossRef]
- Bennion, H.; Fluin, J.; Simpson, G.L. Assessing Eutrophication and Reference Conditions for Scottish Freshwater Lochs Using Subfossil Diatoms. J. Appl. Ecol. 2004, 41, 124–138. [Google Scholar] [CrossRef]
- Lami, A.; Turner, S.; Musazzi, S.; Gerli, S.; Guilizzoni, P.; Rose, N.L.; Yang, H.; Wu, G.; Yang, R. Sedimentary Evidence for Recent Increases in Production in Tibetan Plateau Lakes. Hydrobiologia 2010, 648, 175–187. [Google Scholar] [CrossRef]
- Makri, S.; Lami, A.; Lods-Crozet, B.; Marchetto, A.; Guilizzoni, P.; Loizeau, J.-L. Reconstruction of Trophic State Shifts over the Past 90 Years in a Eutrophicated Lake in Western Switzerland, Inferred from the Sedimentary Record of Photosynthetic Pigments. J. Paleolimnol. 2019, 61, 129–145. [Google Scholar] [CrossRef]
- Langdon, P.G.; Ruiz, Z.O.E.; Brodersen, K.P.; Foster, I.D. Assessing Lake Eutrophication Using Chironomids: Understanding the Nature of Community Response in Different Lake Types. Freshw. Biol. 2006, 51, 562–577. [Google Scholar] [CrossRef]
- Ni, Z.; Zhang, E.; Meng, X.; Sun, W.; Ning, D. Chironomid-Based Reconstruction of 500-Year Water-Level Changes in Daihai Lake, Northern China. Catena 2023, 227, 107122. [Google Scholar] [CrossRef]
- Ibrahim, A.; Capo, E.; Wessels, M.; Martin, I.; Meyer, A.; Schleheck, D.; Epp, L.S. Anthropogenic Impact on the Historical Phytoplankton Community of Lake Constance Reconstructed by Multimarker Analysis of Sediment-Core Environmental DNA. Mol. Ecol. 2021, 30, 3040–3056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huo, S.; Yeager, K.M.; Wu, F. Sedimentary DNA Record of Eukaryotic Algal and Cyanobacterial Communities in a Shallow Lake Driven by Human Activities and Climate Change. Sci. Total Environ. 2021, 753, 141985. [Google Scholar] [CrossRef] [PubMed]
- Gushulak, C.A.C.; Chegoonian, A.M.; Wolfe, J.; Gray, K.; Mezzini, S.; Wissel, B.; Hann, B.; Baulch, H.M.; Finlay, K.; Leavitt, P.R. Impacts of Hydrologic Management on the Eutrophication of Shallow Lakes in an Intensive Agricultural Landscape (Saskatchewan, Canada). Freshw. Biol. 2024, 69, 984–1000. [Google Scholar] [CrossRef]
- Mengo, L.; Deon, J.; Halac, S.; Foray, G.; Loizeau, J.-L.; Ariztegui, D.; Chiavassa, S.; Adatte, T.; Pasquini, A.; Spangenberg, J.; et al. Deciphering the Intricate Link between Watershed-Level Land Use Changes and Reservoir Eutrophication in Central Argentina over the 20th-21st Century. Anthropocene 2024, 46, 100437. [Google Scholar] [CrossRef]
- Sánchez Vuichard, G.; Mengo, L.; Halac, S.; Foray, G.; Hassan, G.; Vásquez, C.; Stutz, S. Environmental Changes in the Southeastern Pampa Plain (Southeastern South America) during the Last Millennium Based on Multiple Lacustrine Indicators and Historical Records. J. Paleolimnol. 2024, 72, 179–204. [Google Scholar] [CrossRef]
- Costa-Böddeker, S.; Bennion, H.; de Jesus, T.A.; Albuquerque, A.L.S.; Figueira, R.C.L.; de C. Bicudo, D. Paleolimnologically Inferred Eutrophication of a Shallow, Tropical, Urban Reservoir in Southeast Brazil. J. Paleolimnol. 2012, 48, 751–766. [Google Scholar] [CrossRef]
- Fontana, L.; Albuquerque, A.L.S.; Brenner, M.; Bonotto, D.M.; Sabaris, T.P.P.; Pires, M.A.F.; Cotrim, M.E.B.; Bicudo, D.C. The Eutrophication History of a Tropical Water Supply Reservoir in Brazil. J. Paleolimnol. 2014, 51, 29–43. [Google Scholar] [CrossRef]
- Cardoso-Silva, S.; Mizael, J.O.S.S.; Frascareli, D.; de Lima Ferreira, P.A.; Rosa, A.H.; Vicente, E.; Moschini-Carlos, V. Paleolimnological Evidence of Environmental Changes in Seven Subtropical Reservoirs Based on Metals, Nutrients, and Sedimentation Rates. Catena 2021, 206, 105432. [Google Scholar] [CrossRef]
- Plastani, M.S.; Laprida, C.; Montes de Oca, F.; Massaferro, J.; Panarello, H.O.; Ramón Mercau, J.; Lami, A. Recent Environmental Changes Inferred from Sediments in a Shallow Lake of the Argentinian Pampas. J. Paleolimnol. 2019, 61, 37–52. [Google Scholar] [CrossRef]
- Costamagna, I.; Halac, S.R.; Mengo, L.; Pisani, N.; Ruiz, M.; Piovano, E.L. Deciphering the Environmental Drivers throughout the 20th and 21st Centuries in the Paleolimnological Record of Laguna del Plata, Laguna Mar Chiquita System, Northern Pampean Plain, Argentina. J. S. Am. Earth Sci. 2022, 119, 103979. [Google Scholar] [CrossRef]
- Sánchez Vuichard, G.; Tonello, M.S.; Stutz, S.; Navarro, D.; Vásquez, C. Pampa Plain (Argentina) Wetland History through a Lake Case Study: Kakel Huincul Environmental History during the Last 600 Years. Wetlands 2023, 43, 15. [Google Scholar] [CrossRef]
- Menone, M.L.; Pérez, D.J.; Iturburu, F.G. Anthropic Stressors and Impacts on Pampean Lakes. In Pampean Lakes; Piovano, E., Stutz, S., Morales, J.A., Ariztegui, D., Eds.; Springer: Cham, Switzerland, 2025; pp. 123–145. [Google Scholar] [CrossRef]
- Gangi, D.; Plastani, M.S.; Laprida, C.; Lami, A.; Dubois, N.; Bordet, F.; Gogorza, C.; Frau, D.; de Tezanos Pinto, P. Recent Cyanobacteria Abundance in a Large Sub-Tropical Reservoir Inferred from Analysis of Sediment Cores. J. Paleolimnol. 2020, 63, 195–209. [Google Scholar] [CrossRef]
- Halac, S.; Mengo, L.; Guerra, L.; Lami, A.; Musazzi, S.; Loizeau, J.L.; Ariztegui, D.; Piovano, E.L. Paleolimnological Reconstruction of the Centennial Eutrophication Processes in a Sub-Tropical South American Reservoir. J. S. Am. Earth Sci. 2020, 103, 102707. [Google Scholar] [CrossRef]
- Von Gunten, L.; Grosjean, M.; Beer, J.; Grob, P.; Morales, A.; Urrutia, R. Age Modeling of Young Non-Varved Lake Sediments: Methods and Limits. Examples from Two Lakes in Central Chile. J. Paleolimnol. 2009, 42, 401–412. [Google Scholar] [CrossRef]
- Montes, I.-Y.; Banegas-Medina, A.; Fagel, N.; El Ouahabi, M.; Verleyen, E.; Alvarez, D.; Torrejón, F.; Schmidt, S.; Lepoint, G.; Diaz, G.; et al. Late Holocene Paleonvironmental Evolution of Two Coastal Lakes in Mediterranean Chile and Its Implications for Conservation Planning. Appl. Sci. 2021, 11, 3478. [Google Scholar] [CrossRef]
- Metcalfe, S.E.; Davies, S.J.; Braisby, J.D.; Leng, M.J.; Newton, A.J.; Terrett, N.L.; O’Hara, S.L. Long and Short-Term Change in the Pátzcuaro Basin, Central Mexico. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 247, 272–295. [Google Scholar] [CrossRef]
- Israde-Alcántara, I.; Vázquez, C.G.; Davies, S.; Aston, B.; Miranda, M.C. A 12,000 Year Diatom-Based Paleoenvironmental Record from Lago De Zirahuén, Mexico. In Limnogeology: Progress, Challenges and Opportunities: A Tribute to Elizabeth Gierlowski-Kordesch; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 367–391. [Google Scholar] [CrossRef]
- Manjarrez-Rangel, S.; Halac, S.R.; Piovano, E.L.; Castro, M.C.D.R.; Avilés, R.M.; Zanor, G.A. Análisis de Indicadores Ambientales en Registros Sedimentarios Recientes de Reservorios en México Central. Boletín Soc. Geol. Mex. 2024, 76, 1–32. [Google Scholar] [CrossRef]
- Caballero, M.; Waters, M.N.; Amezcua, M.; Ruiz-Fernández, A.C.; Sigala, I.; Alcocer, J. Recent Human-Induced Ecological Changes in a Neotropical Karst Lake of Southern Mexico. Catena 2025, 256, 109119. [Google Scholar] [CrossRef]
- Metcalfe, S.E.; O’Hara, S.L. Sensibilidad de lagos mexicanos a alteraciones en el medio ambiente: Ejemplos del Eje Neovolcánico. Tecnol. Cienc. Agua 1992, 107–121. Available online: https://www.revistatyca.org.mx/index.php/tyca/article/view/683 (accessed on 14 July 2025).
- Wu, X.; Ma, T.; Du, Y.; Jiang, Q.; Shen, S.; Liu, W. Phosphorus Cycling in Freshwater Lake Sediments: Influence of Seasonal Water Level Fluctuations. Sci. Total Environ. 2021, 792, 148383. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Ji, Z.; Pei, Y. Characteristics and Distribution of Phosphorus in Surface Sediments of a Shallow Lake. J. Environ. Sci. 2023, 124, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.L.W.; Thomas, R.L.; Wong, H.K.T.; Johnston, L.M. Nitrogen and C/N Ratios in the Sediments of Lakes Superior, Huron, St. Clair, Erie, and Ontario. Can. J. Earth Sci. 1977, 14, 2402–2413. [Google Scholar] [CrossRef]
- Engstrom, D.R.; Schottler, S.P.; Leavitt, P.R.; Havens, K.E. A Reevaluation of the Cultural Eutrophication of Lake Okeechobee Using Multiproxy Sediment Records. Ecol. Appl. 2006, 16, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.N.; Lini, A.; Ostrofsky, M.L.; Bunting, L.; Burgess, H.; Leavitt, P.R.; Reuter, D.; Lami, A.; Guilizzoni, P.; Gilles, E. The Eutrophication of Lake Champlain’s Northeastern Arm: Insights from Paleolimnological Analyses. J. Great Lakes Res. 2012, 38 (Suppl. 1), 35–48. [Google Scholar] [CrossRef]
- Cerda, M.; Scali, C.; Valdés, J.; Macario, K.D.; dos Anjos, R.M.; Vogel, V.; Lamego, F.; Nepomuceno, A. Coupling Fallout 210Pb and Stable Isotopes (δ13C, δ15N) for Catchment Urbanization Reconstruction in Southeastern Coastal Zone of Brazil. J. Radioanal. Nucl. Chem. 2016, 310, 1021–1032. [Google Scholar] [CrossRef]
- Inda, H.; García-Rodríguez, F.; del Puerto, L.; Stutz, S.; Figueira, R.C.L.; de Lima Ferreira, P.A.; Mazzeo, N. Discriminating between Natural and Human-Induced Shifts in a Shallow Coastal Lagoon: A Multidisciplinary Approach. Anthropocene 2016, 16, 1–15. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, Eutrophication and Harmful Algal Blooms along the Freshwater to Marine Continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, Phosphorus, and Eutrophication in Streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S. Eutrophication of Lakes Cannot Be Controlled by Reducing Nitrogen Input: Results of a 37-Year Whole-Ecosystem Experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Filstrup, C.T.; Scott, J.T.; White, J.D.; Lind, O.T. Use of Sediment Elemental and Isotopic Compositions to Record the Eutrophication of a Polymictic Reservoir in Central Texas, USA. Lakes Reserv. Res. Manag. 2010, 15, 25–39. [Google Scholar] [CrossRef]
- García-Rodríguez, F.; Mazzeo, N.; Sprechmann, P.; Metzeltin, D.; Sosa, F.; Treutler, H.C.; Gaucher, C. Paleolimnological Assessment of Human Impacts in Lake Blanca, SE Uruguay. J. Paleolimnol. 2002, 28, 457–468. [Google Scholar] [CrossRef]
- Giesche, A.; Lombardo, U.; Finsinger, W.; Veit, H. Reconstructing Holocene Landscape and Environmental Changes at Lago Rogaguado, Bolivian Amazon. J. Paleolimnol. 2021, 65, 235–253. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Lacustrine Organic Geochemistry—An Overview of Indicators of Organic Matter Sources and Diagenesis in Lake Sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Meyers, P.A. Preservation of Elemental and Isotopic Source Identification of Sedimentary Organic Matter. Chem. Geol. 1994, 114, 289–302. [Google Scholar] [CrossRef]
- Hyodo, A.; Longstaffe, F.J. The Palaeoproductivity of Ancient Lake Superior. Quat. Sci. Rev. 2011, 30, 2988–3000. [Google Scholar] [CrossRef]
- Robertson, P.G.; Hamilton, S.K.; Del Grosso, S.J.; Parton, W.J. The Biogeochemistry of Bioenergy Landscapes: Carbon, Nitrogen, and Water Considerations. Ecol. Appl. 2011, 21, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Hennemann, M.C.; Simonassi, J.C.; Petrucio, M.M. Paleolimnological Record as an Indication of Incipient Eutrophication in an Oligotrophic Subtropical Coastal Lake in Southern Brazil. Environ. Monit. Assess. 2015, 187, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.I.; MacKenzie, K.; Boom, A.; Bremond, L.; Gonzalez, N.; Carr, A.S.; Berrio, J.C. Lacustrine responses to middle and late Holocene anthropogenic activities in the northern tropical Andes. J. Paleolimnol. 2021, 65, 123–136. [Google Scholar] [CrossRef]
- Obrist-Farner, J.; Brenner, M.; Curtis, J.H.; Kenney, W.F.; Salvinelli, C. Recent Onset of Eutrophication in Lake Izabal, the Largest Water Body in Guatemala. J. Paleolimnol. 2019, 62, 359–372. [Google Scholar] [CrossRef]
- Holtvoeth, J.; Whiteside, J.H.; Engels, S.; Freitas, F.S.; Grice, K.; Greenwood, P.; Sepúlveda, J. The paleolimnologist’s guide to compound-specific stable isotope analysis–An introduction to principles and applications of CSIA for Quaternary lake sediments. Quat. Sci. Rev. 2019, 207, 101–133. [Google Scholar] [CrossRef]
- Meyers, P.A.; Teranes, J.L. Sediment Organic Matter. In Tracking Environmental Change Using Lake Sediments; Last, W.M., Smol, J.P., Eds.; Developments in Paleoenvironmental Research; Springer: Dordrecht, The Netherlands, 2002; Volume 2. [Google Scholar] [CrossRef]
- Doyle, R.M.; Longstaffe, F.J.; Moser, K.A. An Isotope, Elemental, and n-Alkane Baseline for Organic Matter Sources in Sediments of High-Altitude Lakes in the Uinta Mountains, Utah, USA. J. Paleolimnol. 2023, 69, 123–139. [Google Scholar] [CrossRef]
- Schelske, C.L.; Hodell, D.A. Using Carbon Isotopes of Bulk Sedimentary Organic Matter to Reconstruct the History of Nutrient Loading and Eutrophication in Lake Erie. Limnol. Oceanogr. 1995, 40, 918–929. [Google Scholar] [CrossRef]
- Rosenmeier, M.F.; Brenner, M.; Kenney, W.F.; Whitmore, T.J.; Taylor, C.M. Recent Eutrophication in the Southern Basin of Lake Petén Itzá, Guatemala: Human Impact on a Large Tropical Lake. Hydrobiologia 2004, 511, 161–172. [Google Scholar] [CrossRef]
- Saulnier-Talbot, É. Paleolimnology as a Tool to Achieve Environmental Sustainability in the Anthropocene: An Overview. Geosciences 2016, 6, 26. [Google Scholar] [CrossRef]
- Thevenon, F.; Adatte, T.; Spangenberg, J.E.; Anselmetti, F.S. Elemental (C/N ratios) and isotopic (δ15Norg, δ13Corg) compositions of sedimentary organic matter from a high-altitude mountain lake (Meidsee, 2661 m a.s.l., Switzerland): Implications for Lateglacial and Holocene Alpine landscape evolution. Holocene 2012, 22, 1135–1142. [Google Scholar] [CrossRef]
- Meyers, P.A. Applications of Organic Geochemistry to Paleolimnological Reconstructions: A Summary of Examples from the Laurentian Great Lakes. Org. Geochem. 2003, 34, 261–289. [Google Scholar] [CrossRef]
- Brenner, M.; Whitmore, T.J.; Curtis, J.H.; Hodell, D.A.; Schelske, C.L. Stable Isotope (δ13C and δ15N) Signatures of Sedimented Organic Matter as Indicators of Historic Lake Trophic State. J. Paleolimnol. 1999, 22, 205–221. [Google Scholar] [CrossRef]
- Lu, Y.; Meyers, P.A.; Johengen, T.H.; Eadie, B.J.; Robbins, J.A.; Han, H. δ15N Values in Lake Erie Sediments as Indicators of Nitrogen Biogeochemical Dynamics During Cultural Eutrophication. Chem. Geol. 2010, 273, 1–7. [Google Scholar] [CrossRef]
- Hennemann, M.C.; Petrucio, M.M. High Chlorophyll a Concentration in a Low Nutrient Context: Discussions in a Subtropical Lake Dominated by Cyanobacteria. J. Limnol. 2016, 75, 520–530. [Google Scholar] [CrossRef]
- Glibert, P.M.; Middelburg, J.J.; McClelland, J.W.; Vander Zanden, M.J. Stable Isotope Tracers: Enriching Our Perspectives and Questions on Sources, Fates, Rates, and Pathways of Major Elements in Aquatic Systems. Limnol. Oceanogr. 2019, 64, 950–981. [Google Scholar] [CrossRef]
- Cole, M.L.; Kroeger, K.D.; McClelland, J.W.; Valiela, I. Effects of watershed land use on nitrogen concentrations and δ15N in groundwater. Biogeochemistry 2006, 77, 199–215. [Google Scholar] [CrossRef]
- Botrel, M.; Gregory-Eaves, I.; Maranger, R. Defining drivers of nitrogen stable isotopes (δ15N) of surface sediments in temperate lakes. J. Paleolimnol. 2014, 52, 419–433. [Google Scholar] [CrossRef]
- Priestley, S.C.; Tyler, J.; Liebelt, S.R.; Mosley, L.M.; Wong, W.W.; Shao, Y.; Woolston, Z.; Farrell, M.; Welsh, D.T.; Brookes, J.D.; et al. N and C Isotope Variations Along an Extreme Eutrophication and Salinity Gradient in the Coorong Lagoon, South Australia. Front. Earth Sci. 2022, 9, 727971. [Google Scholar] [CrossRef]
- Gu, B.; Schelske, C.L.; Hodell, D.A. Extreme 13C enrichments in a shallow hypereutrophic lake: Implications for carbon cycling. Limnol. Oceanogr. 2004, 49, 1152–1159. [Google Scholar] [CrossRef]
- Heink, U.; Kowarik, I. What are indicators? On the definition of indicators in ecology and environmental planning. Ecol. Indic. 2010, 10, 584–593. [Google Scholar] [CrossRef]
- Edlund, M.B.; Engstrom, D.R.; Triplett, L.D.; Lafrancois, B.M.; Leavitt, P.R. Twentieth century eutrophication of the St. Croix River (Minnesota-Wisconsin, USA) reconstructed from the sediments of its natural impoundment. J. Paleolimnol. 2009, 41, 641–657. [Google Scholar] [CrossRef]
- Caballero, M.; Mora, L.; Muñoz, E.; Escolero, O.; Bonifaz, R.; Ruiz, C.; Prado, B. Anthropogenic influence on the sediment chemistry and diatom assemblages of Balamtetik Lake, Chiapas, Mexico. Environ. Sci. Pollut. Res. 2020, 27, 15935–15943. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Leavitt, P.; Smol, J.; Zirnhelt, N. Comparison of diatoms, fossil pigments and historical records as measures of lake eutrophication. Freshw. Biol. 1997, 38, 401–417. [Google Scholar] [CrossRef]
- Pérez, L.; Lorenschat, J.; Massaferro, J.; Pailles, C.; Sylvestre, F.; Hollwedel, W.; Brandorff, G.-O.; Brenner, M.; Islebe, G.; Del, M.; et al. Bioindicators of climate and trophic state in lowland and highland aquatic ecosystems of the Northern Neotropics. Rev. Biol. Trop. 2013, 61, 603–644. [Google Scholar] [CrossRef] [PubMed]
- Correa-Metrio, A.; Dechnik, Y.; Lozano-García, S.; Caballero, M. Detrended correspondence analysis: A useful tool to quantify ecological changes from fossil data sets. Bol. Soc. Geol. Mex. 2014, 66, 135–143. [Google Scholar] [CrossRef]
- López-Blanco, C.; Rodríguez-Abaunza, G.A.; Seitz, C.; Perez, L.; Cuña-Rodriguez, C.; Fontana, S.L. A 700-year multiproxy reconstruction on the Argentinian Pampas inferred from the sediments of Laguna Blanca Grande. J. S. Am. Earth Sci. 2021, 105, 103000. [Google Scholar] [CrossRef]
- Falasco, E.; Bona, F.; Badino, G.; Hoffmann, L.; Ector, L. Diatom teratological forms and environmental alterations: A review. Hydrobiologia 2009, 623, 1–35. [Google Scholar] [CrossRef]
- Battarbee, R.W. Palaeolimnological approaches to climate change, with special regard to the biological record. Quat. Sci. Rev. 2000, 19, 107–124. [Google Scholar] [CrossRef]
- Grudzinska, I.; Courtney-Mustaphi, C.; Rey, F.; Gobet, E.; Tinner, W.; Marchetto, A.; Heiri, O. Neolithic human activity caused eutrophication in small central European lakes. Catena 2024, 236, 107738. [Google Scholar] [CrossRef]
- Rowell, H.C.; Enache, M.D.; Quinlan, R.; Smith, A.J.; Bloomfield, J.A.; Charles, D.F.; Effler, S.W. Quantitative paleolimnological inference models applied to a high-resolution biostratigraphic study of lake degradation and recovery, Onondaga Lake, New York (USA). J. Paleolimnol. 2016, 55, 241–258. [Google Scholar] [CrossRef]
- Hassan, G.S. Diatom-based reconstruction of middle to late Holocene paleoenvironments in Lake Lonkoy, southern Pampas, Argentina. Diatom Res. 2013, 28, 473–486. [Google Scholar] [CrossRef]
- Caballero, M.; Vazquez, G.; Alcocer, J.; Mora Palomino, L.N. Diatom diversity and distribution in neotropical karst lakes under anthropogenic stress. EGUsphere 2024, 2024, 1–23. [Google Scholar] [CrossRef]
- Watchorn, M.A.; Hamilton, P.B.; Patterson, R.T. The paleolimnology of Haynes Lake, Oak Ridges Moraine, Ontario, Canada: Documenting anthropogenic and climatic disturbances. Environ. Earth Sci. 2013, 68, 1823–1834. [Google Scholar] [CrossRef]
- Biberovic, I.; Diamond, S.E.; Heathcote, A.J.; Lini, A.; Morales-Williams, A.M. Diatom-based transfer functions for pH and total phosphorus in Vermont, USA lakes. J. Paleolimnol. 2024, 73, 23–34. [Google Scholar] [CrossRef]
- Heathcote, A.J.; Ramstack Hobbs, J.M.; Anderson, N.J.; Frings, P.; Engstrom, D.R.; Downing, J.A. Diatom floristic change and lake paleoproduction as evidence of recent eutrophication in shallow lakes of the midwestern USA. J. Paleolimnol. 2015, 53, 17–34. [Google Scholar] [CrossRef]
- Liu, B.; McLean, C.E.; Long, D.T.; Steinman, A.D.; Stevenson, R.J. Eutrophication and recovery of a lake inferred from sedimentary diatoms originating from different habitats. Sci. Total Environ. 2018, 628–629, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Caballero, M. The structure and species composition of the diatom communities in tropical volcanic lakes of eastern Mexico. Diatom Res. 2013, 28, 77–91. [Google Scholar] [CrossRef]
- Cuña-Rodríguez, C.; Piovano, E.; Del Puerto, L.; Inda, H.; García-Rodríguez, F. On the Relationship between Holocene Environmental Variability and the Diatom Composition in the Peña Lagoon, SE Uruguay. Ameghiniana 2018, 55, 423–436. [Google Scholar] [CrossRef]
- Tudurí, A.; Perez Becoña, L.; Venturini, N.; Rodríguez-Gallego, L.; García-Rodríguez, F.; González, L.; Lescano, C.; Costa, S.; del Puerto, L.; Bergamino, L. Trophic assessment in South American Atlantic coastal lagoons: Linking water, sediment and diatom indicators. Mar. Pollut. Bull. 2021, 165, 112119. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.A.; Vélez-Agudelo, C.; Isla, F.I. Diatom responses to natural and anthropogenic environmental changes in a Patagonian river, Argentina. J. S. Am. Earth Sci. 2020, 102, 102677. [Google Scholar] [CrossRef]
- Sepúlveda-Zúñiga, E.; Maidana, N.I.; Villacís, L.A.; Sagredo, E.A.; Moreno, P.I. The last millennium viewed from a fine-resolution freshwater diatom record from northwestern Patagonia. Quat. Sci. Rev. 2022, 296, 107806. [Google Scholar] [CrossRef]
- Ryves, D.B.; Juggins, S.; Fritz, S.C.; Battarbee, R.W. Experimental diatom dissolution and the quantification of microfossil preservation in sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 172, 99–113. [Google Scholar] [CrossRef]
- Neil, K.; Gajewski, K. An 11,000-yr record of diatom assemblage responses to climate and terrestrial vegetation changes, southwestern Québec. Ecosphere 2018, 9, e02505. [Google Scholar] [CrossRef]
- Fritz, S.C.; Benito, X.; Steinitz-Kannan, M. Long-term and regional perspectives on recent change in lacustrine diatom communities in the tropical Andes. J. Paleolimnol. 2019, 61, 251–262. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Hodgson, D.A. Sedimentary Pigments. In Tracking Environmental Change Using Lake Sediments; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Springer: Dordrecht, The Netherlands, 2002; Volume 3, pp. 295–325. [Google Scholar] [CrossRef]
- Guilizzoni, P.; Marchetto, A.; Lami, A.; Gerli, S.; Musazzi, S. Use of sedimentary pigments to infer past phosphorus concentration in lakes. J. Paleolimnol. 2011, 45, 433–445. [Google Scholar] [CrossRef]
- Coianiz, L.; Ariztegui, D.; Piovano, E.L.; Lami, A.; Guilizzoni, P.; Gerli, S.; Waldmann, N. Environmental change in subtropical South America for the last two millennia as shown by lacustrine pigments. J. Paleolimnol. 2015, 53, 233–250. [Google Scholar] [CrossRef]
- Romero-Viana, L.; Keely, B.J.; Camacho, A.; Vicente, E.; Miracle, M.R. Photoautotrophic community changes in Lagunillo del Tejo (Spain) in response to lake level fluctuation: Two centuries of sedimentary pigment records. Org. Geochem. 2009, 40, 376–386. [Google Scholar] [CrossRef]
- Swain, E.B. Measurement and interpretation of sedimentary pigments. Freshw. Biol. 1985, 15, 53–75. [Google Scholar] [CrossRef]
- de Oliveira Soares Silva Mizael, J.; Cardoso-Silva, S.; Frascareli, D.; Pompêo, M.L.M.; Moschini-Carlos, V. Ecosystem history of a tropical reservoir revealed by metals, nutrients and photosynthetic pigments preserved in sediments. Catena 2020, 184, 104242. [Google Scholar] [CrossRef]
- Deshpande, B.N.; Tremblay, R.; Pienitz, R.; Vincent, W.F. Sedimentary pigments as indicators of cyanobacterial dynamics in a hypereutrophic lake. J. Paleolimnol. 2014, 52, 171–184. [Google Scholar] [CrossRef]
- Waters, M.N.; Schelske, C.L.; Brenner, M. Cyanobacterial dynamics in shallow Lake Apopka (Florida, USA) before and after the shift from a macrophyte-dominated to a phytoplankton-dominated state. Freshw. Biol. 2015, 60, 1571–1580. [Google Scholar] [CrossRef]
- Ady, F.D.; Patoine, A. Impacts of land use and climate variability on algal communities since ~1850 CE in an oligotrophic estuary in northeastern New Brunswick, Canada. J. Paleolimnol. 2016, 55, 151–165. [Google Scholar] [CrossRef]
- Maheaux, H.; Leavitt, P.R.; Jackson, L.J. Asynchronous Onset of Eutrophication among Shallow Prairie Lakes of the Northern Great Plains, Alberta, Canada. Glob. Change Biol. 2016, 22, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Buchaca, T.; Kosten, S.; Lacerot, G.; Mazzeo, N.; Kruk, C.; Huszar, V.L.; Lotter, A.F.; Jeppesen, E. Pigments in surface sediments of South American shallow lakes as an integrative proxy for primary producers and their drivers. Freshw. Biol. 2019, 64, 1437–1452. [Google Scholar] [CrossRef]
- Ewing, H.A.; Weathers, K.C.; Cottingham, K.L.; Leavitt, P.R.; Greer, M.L.; Carey, C.C.; Steele, B.C.; Fiorillo, A.U.; Sowles, J.P. “New” Cyanobacterial Blooms Are Not New: Two Centuries of Lake Production Are Related to Ice Cover and Land Use. Ecosphere 2020, 11, e03170. [Google Scholar] [CrossRef]
- Buchaca, T.; Catalan, J. On the Contribution of Phytoplankton and Benthic Biofilms to the Sediment Record of Marker Pigments in High Mountain Lakes. J. Paleolimnol. 2008, 40, 369–383. [Google Scholar] [CrossRef]
- Tse, T.J.; Doig, L.E.; Leavitt, P.R.; Quiñones-Rivera, Z.J.; Codling, G.; Lucas, B.T.; Liber, K.; Giesy, J.P.; Wheater, H.; Jones, P.D. Long-Term Spatial Trends in Sedimentary Algal Pigments in a Narrow River-Valley Reservoir, Lake Diefenbaker, Canada. J. Great Lakes Res. 2015, 41 (Suppl. 2), 56–66. [Google Scholar] [CrossRef]
- Bunting, L.; Leavitt, P.R.; Simpson, G.L.; Wissel, B.; Laird, K.R.; Cumming, B.F.; St. Amand, A.; Engstrom, D.R. Increased Variability and Sudden Ecosystem State Change in Lake Winnipeg, Canada, Caused by 20th Century Agriculture. Limnol. Oceanogr. 2016, 61, 2090–2107. [Google Scholar] [CrossRef]
- Gushulak, C.A.; Mezzini, S.; Moir, K.E.; Simpson, G.L.; Bunting, L.; Wissel, B.; Leavitt, P.R. Impacts of a century of land-use change on the eutrophication of large, shallow, prairie Lake Manitoba in relation to adjacent Lake Winnipeg (Manitoba, Canada). Freshw. Biol. 2024, 69, 47–63. [Google Scholar] [CrossRef]
- Cardoso-Silva, S.; Mizael, J.O.S.S.; Frascareli, D.; Figueira, R.C.L.; Pompêo, M.; Vicente, E.; Moschini-Carlos, V. Geochemistry and sedimentary photopigments as proxies to reconstruct past environmental changes in a subtropical reservoir. Environ. Sci. Pollut. Res. 2022, 29, 28495–28509. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, P.R. A Review of Factors That Regulate Carotenoid and Chlorophyll Deposition and Fossil Pigment Abundance. J. Paleolimnol. 1993, 9, 109–127. [Google Scholar] [CrossRef]
- Moss, B. Studies on the Degradation of Chlorophyll a and Carotenoids in Freshwaters. New Phytol. 1968, 67, 49–59. [Google Scholar] [CrossRef]
- Heiri, O.; Brooks, S.J.; Birks, H.J.B.; Lotter, A.F. A 274-lake calibration data-set and inference model for chironomid-based summer air temperature reconstruction in Europe. Quat. Sci. Rev. 2011, 30, 3445–3456. [Google Scholar] [CrossRef]
- Verbruggen, F.; Heiri, O.; Meriläinen, J.J.; Lotter, A.F. Subfossil chironomid assemblages in deep, stratified European lakes: Relationships with temperature, trophic state and oxygen. Freshw. Biol. 2011, 56, 407–423. [Google Scholar] [CrossRef]
- Lindegaard, C. Classification of water-bodies and pollution. In The Chironomidae; Epler, J.H., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 385–404. [Google Scholar] [CrossRef]
- Laprida, C.; Massaferro, J.; Ramón Mercau, M.J.; Cusminsky, G. Paleobioindicadores del fin del mundo: Ostrácodos y quironómidos del extremo sur de Sudamérica en ambientes lacustres Cuaternarios. Lat. Am. J. Sedimentol. Basin Anal. 2014, 21, 97–117. Available online: https://lajsba.sedimentologia.org.ar/lajsba/article/view/134 (accessed on 14 July 2025).
- Massaferro, J. Paleoecología: El uso de los quironómidos fósiles (Diptera: Chironomidae) en reconstrucciones paleoambientales durante el Cuaternario en la Patagonia. Rev. Soc. Entomol. Argent. 2009, 68, 209–217. [Google Scholar]
- Montes de Oca, F.; Motta, L.; Plastani, M.S.; Laprida, C.; Lami, A.; Massaferro, J. Reconstructing recent environmental changes using non-biting midges (Diptera: Chironomidae) in two high mountain lakes from northern Patagonia, Argentina. J. Paleolimnol. 2018, 59, 175–187. [Google Scholar] [CrossRef]
- Massaferro, J.; Guevara, S.R.; Rizzo, A.; Arribére, M. Short-term environmental changes in Lake Morenito (41° S, 71° W, Patagonia, Argentina) from the analysis of sub-fossil chironomids. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 23–30. [Google Scholar] [CrossRef]
- Serra, M.N.; Massaferro, J.; Villarosa, G. Volcanic and environmental impacts on subfossil chironomids from Northern Patagonia (Argentina) over the last 700 years. Limnology 2021, 22, 337–346. [Google Scholar] [CrossRef]
- Perlov, D.; Reavie, E.D.; Quinlan, R. Anthropogenic stressor impacts on hypolimnetic dissolved oxygen in Lake Erie: A chironomid-based paleolimnological assessment. J. Great Lakes Res. 2023, 49, 953–968. [Google Scholar] [CrossRef]
- Apothéloz-Perret-Gentil, L.; Cordonier, A.; Straub, F.; Iseli, J.; Esling, P.; Pawlowski, J. Taxonomy-Free Molecular Diatom Index for High-Throughput eDNA Biomonitoring. Mol. Ecol. Resour. 2017, 17, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Armbrecht, L.; Weber, M.E.; Raymo, M.E.; Peck, V.L.; Williams, T.; Warnock, J.; Kato, Y.; Hernández-Almeida, I.; Hoem, F.; Reilly, B.; et al. Ancient Marine Sediment DNA Reveals Diatom Transition in Antarctica. Nat. Commun. 2022, 13, 5787. [Google Scholar] [CrossRef] [PubMed]
- Parducci, L.; Bennett, K.D.; Ficetola, G.F.; Alsos, I.G.; Suyama, Y.; Wood, J.R.; Pedersen, M.W. Ancient Plant DNA in Lake Sediments. New Phytol. 2017, 214, 924–942. [Google Scholar] [CrossRef] [PubMed]
- Frisch, D.; Morton, P.K.; Culver, B.W.; Edlund, M.B.; Jeyasingh, P.D.; Weider, L.J. Paleogenetic Records of Daphnia pulicaria in Two North American Lakes Reveal the Impact of Cultural Eutrophication. Glob. Change Biol. 2017, 23, 708–718. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Devey, M.; Leavitt, P.R.; Power, M.J.; Brothers, S.; Brahney, J. Anthropogenic Forcing Leads to an Abrupt Shift to Phytoplankton Dominance in a Shallow Eutrophic Lake. Freshw. Biol. 2024, 69, 335–350. [Google Scholar] [CrossRef]
- Martínez de la Escalera, G.; Antoniades, D.; Bonilla, S.; Piccini, C. Application of Ancient DNA to the Reconstruction of Past Microbial Assemblages and for the Detection of Toxic Cyanobacteria in Subtropical Freshwater Ecosystems. Mol. Ecol. 2014, 23, 5791–5802. [Google Scholar] [CrossRef] [PubMed]
- Moguel, B.; Pérez, L.; Alcaraz, L.D.; Blaz, J.; Caballero, M.; Muñoz-Velasco, I.; Becerra, A.; Laclette, J.P.; Ortega-Guerrero, B.; Romero-Oliva, C.S.; et al. Holocene Life and Microbiome Profiling in Ancient Tropical Lake Chalco, Mexico. Sci. Rep. 2021, 11, 13848. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.W.; Overballe-Petersen, S.; Ermini, L.; Der Sarkissian, C.; Haile, J.; Hellstrom, M.; Spens, J.; Thomsen, P.F.; Bohmann, K.; Cappellini, E.; et al. Ancient and Modern Environmental DNA. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130383. [Google Scholar] [CrossRef] [PubMed]
- Zinger, L.; Bonin, A.; Alsos, I.G.; Bálint, M.; Bik, H.; Boyer, F.; Chariton, A.A.; Creer, S.; Coissac, E.; Deagle, B.E.; et al. DNA Metabarcoding—Need for Robust Experimental Designs to Draw Sound Ecological Conclusions. Mol. Ecol. 2019, 28, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjarrez-Rangel, C.S.; Halac, S.R.; Mengo, L.D.V.; Piovano, E.L.; Zanor, G.A. Paleolimnological Approaches to Track Anthropogenic Eutrophication in Lacustrine Systems Across the American Continent: A Review. Limnol. Rev. 2025, 25, 33. https://doi.org/10.3390/limnolrev25030033
Manjarrez-Rangel CS, Halac SR, Mengo LDV, Piovano EL, Zanor GA. Paleolimnological Approaches to Track Anthropogenic Eutrophication in Lacustrine Systems Across the American Continent: A Review. Limnological Review. 2025; 25(3):33. https://doi.org/10.3390/limnolrev25030033
Chicago/Turabian StyleManjarrez-Rangel, Cinthya Soledad, Silvana Raquel Halac, Luciana Del Valle Mengo, Eduardo Luis Piovano, and Gabriela Ana Zanor. 2025. "Paleolimnological Approaches to Track Anthropogenic Eutrophication in Lacustrine Systems Across the American Continent: A Review" Limnological Review 25, no. 3: 33. https://doi.org/10.3390/limnolrev25030033
APA StyleManjarrez-Rangel, C. S., Halac, S. R., Mengo, L. D. V., Piovano, E. L., & Zanor, G. A. (2025). Paleolimnological Approaches to Track Anthropogenic Eutrophication in Lacustrine Systems Across the American Continent: A Review. Limnological Review, 25(3), 33. https://doi.org/10.3390/limnolrev25030033







