Abstract
The availability of detritus is a key factor influencing aquatic biota and can significantly affect decomposition processes. In this study, we investigated how varying quantities of surrounding detritus impact leaf litter decay rates. It was tested in flowing and still-water microcosms to highlight context-dependent effects of surrounding detritus on leaf litter decomposition. To isolate the effect of detritus amount, experiments were conducted in laboratory microcosms simulating lotic and lentic ecosystems, each containing leaf fragments for decomposition assessments. Four detritus quantities were tested, with invertebrates either allowed or restricted from moving among detritus patches. Leaf decomposition rates were influenced by the amount of surrounding detritus, with slower decay observed at higher detritus conditions, regardless of invertebrate mobility. Detritivore distribution responded to both detritus quantity and oxygen availability, showing a preference for high detritus conditions. Additionally, detritus quantity affected microbial activity with a quadratic response, as indicated by leaf respiration rates. Overall, our findings indicate that the amount of surrounding detritus modulates leaf litter decomposition independently of invertebrate density, by influencing oxygen dynamics and, consequently, the activity of biological decomposers.
1. Introduction
Detritus decomposition is the major carbon recycling pathway in most ecosystems and constitutes the energy resource for decomposer micro- and macrofaunal species in many terrestrial and aquatic ecosystems [1,2]; from 70 to 90% of all primary production enters the detrital food web [3]. Leaf litter inputs from riparian vegetation contribute significantly to organic matter pools, supporting diverse microbial and invertebrate communities that mediate decomposition [4].
In aquatic ecosystems, litter input from riparian or littoral vegetation has been shown to affect higher trophic levels [4], although the most apparent effect is exerted at the detritivore level, where the energy and nutrients are made available through the decomposition process.
Among macrophytes, Phragmites australis (Cav. Trin ex Steud., 1841) is one of the most widespread and productive species in wetlands and littoral zones worldwide, often contributing large amounts of detritus to aquatic habitats [5,6].
Although numerous studies are available in the literature on the influence of environmental factors, biotic agents and species diversity in litter breakdown, the role of detritus amount as a regulating factor of litter decomposition remains underexplored. Most existing research has focused on how factors such as temperature [7,8], salinity [9,10] and nutrient availability [11] influence litter decomposition in aquatic environments. However, the role of structural ecosystem characteristics in reed litter decomposition has also been demonstrated [12,13,14]. In addition, several studies have highlighted the role of invertebrates and microbial decomposers [15,16,17] as well as the impact of detritivore diversity and richness [18,19] in regulating leaf litter decomposition in aquatic systems.
Alterations of litter input to ecosystems have been observed to affect benthic invertebrate community and ultimately leaf decomposition showing lower leaf decomposition rates in relation to a reduction or alteration of litter input [20].
The form and size of decomposing material have been the focus of several investigations on litter decomposition [21,22] and consistent relationships between loss rates and amount of leaf litter under decomposition were found both in terrestrial [23] and aquatic ecosystems [24].
In some cases, litter amount was manipulated to investigate the effect on litter decomposition [25,26], and litter bags or packs of different sizes were used to test the potential dependence of leaf decomposition rates from litter accumulation in aquatic ecosystems [27].
Detritus accumulation can alter microhabitat conditions, influencing oxygen availability, microbial activity, and invertebrate behavior [28,29]. High detritus amounts may create anoxic conditions, slowing decomposition, while also providing habitat complexity that affects detritivore distribution and feeding [30]. Most methods used to estimate litter decomposition through mass loss involve the use of unconstrained leaves, stacked leaves loosely tied together to form litter packs, or leaves enclosed in bags of various mesh sizes (litter bags [31]), including modified litter bags [32]. Depending on the specific objectives of this study, either whole leaves [33] or leaf discs [34] may be used within these bags.
Understanding how varying amounts of detritus affect decomposition processes is fundamental to improving our ability to predict the fate and turnover of organic matter in aquatic ecosystems. These systems are increasingly affected by changes in detritus input regimes, driven by both anthropogenic land-use changes—such as agriculture, urbanization, and wetland drainage—and climate-induced alterations in primary productivity patterns [35]. This understanding becomes particularly crucial in wetlands dominated by P. australis, a species known for its high primary productivity and extensive litter production, that contributes substantial amounts of detritus to these ecosystems. The fate of P. australis litter and its decomposition dynamics are still not fully understood, particularly in relation to the detritus amounts regulating litter breakdown.
In this work, we investigate the extent to which the quantity of surrounding detritus influences the decomposition dynamics of P. australis leaf litter within controlled aquatic microcosms.
By manipulating detritus quantity and invertebrate mobility, we aim to investigate the potential effects of litter availability on litter decomposition rates and describe the relationships among detritus amount, oxygen, invertebrate density and litter decomposition.
The use of microcosms provides a controlled environment to test these interactions under replicable conditions, thereby offering insights into how detritus loading may regulate organic matter turnover in P. australis-dominated wetland systems.
This study hypothesizes the following: (i) the presence of surrounding detritus will affect P. australis decomposition in the aquatic microcosms, and (ii) the effects of surrounding detritus on litter decay rates could be related to dissolved oxygen and invertebrate density.
Decomposition rates were assessed using standardized leaf fragments of P. australis subjected to experimental treatments that varied in the quantity of background organic material, hereafter referred to as surrounding detritus. This term denotes the additional detrital matter present in the microcosm environment but not physically attached to the target leaf material, simulating the natural accumulation of organic debris in aquatic habitats.
In addition to varying detritus amounts, the experiment also incorporated the presence of detritivorous invertebrates, with particular attention to their mobility among microsites differing in detritus load. Two detritivore movement scenarios were considered: one in which organisms were allowed to move freely between patches with contrasting detritus quantities, and another in which movement was restricted, thereby limiting the organisms’ exposure to spatial heterogeneity in resource availability. This dual approach enabled us to assess not only the direct effects of detritus quantity on decomposition but also the role of detritivore behavior and spatial dynamics in mediating these effects.
2. Materials and Methods
2.1. Materials
We selected senescent leaves of P. australis as a trophic resource and Gammarus sp. as key detritivore species. All the experimental components were collected from Acquatina lagoon (40°26′53″ N, 18°13′57″ E), a small lagoon ecosystem (0.45 km2) located on the Adriatic coast. The lagoon has a freshwater input in the northern part, the Giammatteo channel, and a connection with the sea at the south edge, being characterized by a latitudinal gradient of salinity and an internal patchiness of habitats. This ensured consistency in water characteristics, resource quality, and consumer requirements. It is a wetland environment characterized by the presence of P. australis reed beds [36].
All experiments were conducted at a constant temperature of 18.0 ± 1.0 °C in a thermostatically controlled environmental chamber. Maintaining a stable thermal regime was essential to minimize both direct and indirect effects of temperature variability on decomposition processes and detritivore activity.
To avoid potential confounding effects arising from the use of mixed detrital sources—an issue previously highlighted in decomposition studies [37,38]—we selected P. australis as the only detrital resource in all experimental treatments. P. australis was chosen not only for its relevance as a model species in decomposition studies but also because it constitutes the dominant emergent macrophyte in freshwater and brackish wetlands throughout the Mediterranean region [39]. Senescent leaves of P. australis were collected from a reed stand at the end of summer, air-dried for one week, then oven-dried at 60 °C for a minimum of three days. Before the experiment, dried leaves were leached in flowing tap water for 72 h, followed by microbial conditioning through the addition of 10 mL of natural water for at least three days [40].
The water used in the experimental microcosms was collected from the same natural wetland site, where P. australis material was sourced, in order to ensure ecological consistency between the detrital resource and the surrounding aquatic environment. To remove larger particulate matter and prevent the introduction of macroinvertebrates or coarse debris that could interfere with experimental conditions, the collected water was carefully filtered through a 20 µm mesh net. Following filtration, the water was stored in a thermostatically controlled room at a constant temperature, identical to that used in the experimental setup.
Detritivores belonging to the genus Gammarus were also collected from Acquatina lagoon, where the genus is known to be resident [41]. Gammarus species are recognized as shredder detritivores that feed on coarse particulate organic matter [42]. Individuals of Gammarus sp. with similar body size (9–10 mm in length) were collected from the same population and acclimated in filtered natural water at 18 °C for 4–6 days before experiments.
Experiments were performed in custom-designed polymethyl methacrylate (PMMA) systems simulating environments with both flowing and still water conditions. The flowing-water system (Figure 1a) consisted of four artificial channels, each 288 cm in length, 30 cm in height, and 38 cm in width. Each channel was subdivided into four compartments (40 cm in length) using frames fitted with a 0.2 mm mesh net to prevent detritus displacement or detritivore movement between compartments. A recirculating pump system, equipped with a refrigerator unit (18.0 ± 0.5 °C), provided a continuous mixing and recirculation of water through the system (approximately 300 L·h−1) and restored the oxygen level in the water. The still-water system was composed by twenty-five circular arenas (hereafter referred to as “patches”) interconnected as shown in Figure 1b. These included twenty-four patches arranged in three concentric rings and a single central patch, all connected both radially and laterally. Each patch measured 14 cm in diameter and 1.5 cm in height, and was connected to adjacent patches by narrow channels 2.4 cm in width. All patches were identified with a number.
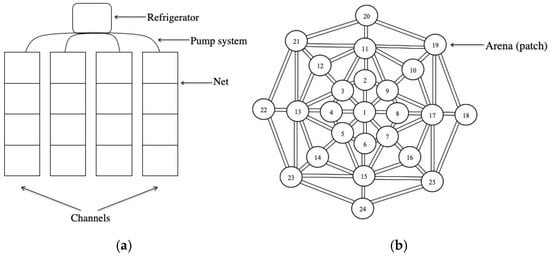
Figure 1.
Experimental aquatic microcosms consisted of channels (flowing-water system (a)) and patches (still-water system (b)).
2.2. Experimental Design
In the flowing-water system, water movement was unidirectional and the migration of detritivores between compartments was effectively inhibited. On the other hand, in the still-water system, water remained largely stationary, allowing detritivores to move freely, exploit different patches, and disperse throughout the entire system.
Recognizable leaf fragments (based on shape and size) were placed in the systems for the estimation of decomposition rates. Some of these fragments were periodically replaced, after collection, with pre-treated backup fragments that had undergone the same initial conditioning and were stored in a separate tank within the same thermostatic room.
To assess the effect of surrounding detritus on decomposition (first working hypothesis), large, preconditioned leaves of P. australis were distributed around the focal leaf fragments in each experimental unit. Four detritus treatments were established: control (no surrounding detritus), low, medium, and high detritus amounts. The amount of surrounding detritus was estimated based on potential input from a standing biomass of P. australis ranging from 1 to 3 kg/m2, according to [43], and assuming an average detritivore ingestion rate of 0.1 gfood·gdetritivore−1·d−1 [44] and an average 4% contribution of decomposer on total leaf dry weight [45] to supply adequate biomass of microbial resource.
Accordingly, the detritus treatments corresponded to the addition of 0.06 g (low), 0.20 g (medium), and 0.40 g (high) of detritus per individual Gammarus sp. introduced at the start of the experiment, plus a ‘control’ consisting of leaf fragments without any surrounding detritus. Treatments were randomly assigned to the four compartments (5 replicates) in the flowing-water system and to the patches (6 replicates) of the still-water system.
Each compartment in the flowing-water channels contained 35 leaf fragments (approximately 1.00 × 2.00 cm), the surrounding detritus, and 50 Gammarus sp. individuals. On days 4, 8, 12, 16, 20, 24, and 28, five leaf fragments were retrieved from each compartment to determine their weight-to-surface area ratio and to estimate decomposition rates. At the end of the 28-day experiment, the remaining Gammarus sp. individuals were counted in each compartment to assess the effect of invertebrate density on leaf litter decomposition.
Both research hypotheses about the effect of surrounding detritus on decomposition, as well as the potential relationships between litter decomposition rates, dissolved oxygen, and invertebrate density, were also tested in the still-water system. Here, each patch received 10 circular leaf fragments of similar mass, 9 Gammarus sp. individuals, and the designed amount of surrounding detritus. Invertebrate counts were performed on day 4 and 7 to monitor survival, and any dead individuals were replaced to maintain constant biomass throughout the experiment. At the end of the experiment, the number of individuals was recorded, and the leaf fragments were collected for analysis.
Water levels in all systems were maintained by regularly adding prefiltered water. Oxygen concentrations were monitored throughout the experimental period using a hand-held multiparameter probe. Colonization of leaf fragments by microbial decomposers was assessed by measuring their respiration rates.
2.3. Methods
After collection, P. australis leaves were cut in fragments, dried for 72 h at 60 °C and weighed on micro-analytical balance (±0.005 mg). Decomposition was estimated with a negative exponential model based on decrease in the biomass or weight-to-surface ratio of leaf fragments over time. In the patch experiment, leaf surface area was kept constant, whereas in the channel experiment, different fragments were sampled randomly. Data from the channel experiment were normalized by surface area to allow for comparison. In both cases, decomposition rates were calculated by plotting weight-loss data over the time.
The leaf fragments placed in the experimental channels were also analyzed using an image analysis system (Leica Camera AG, Wetzlar, Germany), coupled with Leica QWin software (QWin 3.1.0).
Total decomposer activity was assessed by microrespirometry, without attempting taxonomic identification of the decomposers. The respiration rate of three leaf fragments per experimental condition was measured using a flow-through respirometer. For this purpose, 5 mL glass syringes were used as respiration chambers, with a Clarke-type microelectrode (Strathkelvin Instruments LTD Model 781, Glasgow, UK) as an oxygen detector. Oxygen uptake was measured in a flow-through system [46], at the end of the equilibration period, over a 15-min time interval. Oxygen consumption was calculated from the difference in dissolved oxygen concentration before and after the respirometric cell, taking into account for flow-rate and chamber volume, following the method described by Vignes et al. (2012) [47].
Detritivores were analyzed using the Leica image analysis system and QWin software at the beginning of the experiment to select individuals with similar body size. They were visually counted during the experiments and again at its conclusion. Since the detritivores’ mortality rate ranged between 10% and 15% for each count, we replaced the dead specimens to maintain a constant invertebrate biomass and, consequently, a stable predatory pressure on the leaf litter (i.e., the detritivores’ contribution to decomposition).
2.4. Data Analysis
The decay rate was calculated as a negative exponential model , where is the dry mass remaining at time t, is the initial dry mass and k is the rate of decomposition [48]. Differences in leaf decay rate between treatments were assessed using Student’s t-test at 5% significant level. Differences in the number of invertebrates surviving at the end of the experiments under different detritus conditions (control, low, medium, and high detritus) were evaluated using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test.
Temporal changes in invertebrates colonizing leaf patches and dissolved oxygen concentration under the various experimental conditions were assessed using two-way ANOVA, with detritus level (control, low, medium, high) and time as factors, followed by Tukey’s post hoc test.
Ordinary least squares regressions were used to analyze the relationships between decomposition rates and invertebrate abundance, as well as between invertebrate abundance and dissolved oxygen levels. Respiration rates of leaf fragments in relation to different detritus levels were analyzed through a quadratic regression. Results are reported with 95% confidence intervals [49].
All statistical computations were performed using Statistica 6 software unless otherwise specified. Prior the analysis, data were tested for homogeneity of variance using Bartlett’s test to ensure compliance with ANOVA assumptions. The Kolmogorov–Smirnov test was used to assess the normality of the data before applying any parametric tests.
3. Results
P. australis decay rates varied significantly across experimental conditions. In the channels, decay rates ranged from 0.017 ± 0.002 d−1 under medium detritus conditions to 0.023 ± 0.002 d−1 in the control (null detritus), with a significant difference between null and high detritus conditions (Student’s t-test, d.f. 6, t = 2.48, p < 0.05) (Figure 2a). In the patches, decay rates ranged from 0.012 ± 0.006 d−1 under high detritus conditions to 0.027 ± 0.003 d−1 in absence of detritus (Figure 2b), with a significant difference between these two conditions, control and high detritus (Student’s t-test, d.f. = 10, t = 2.32, p < 0.05).
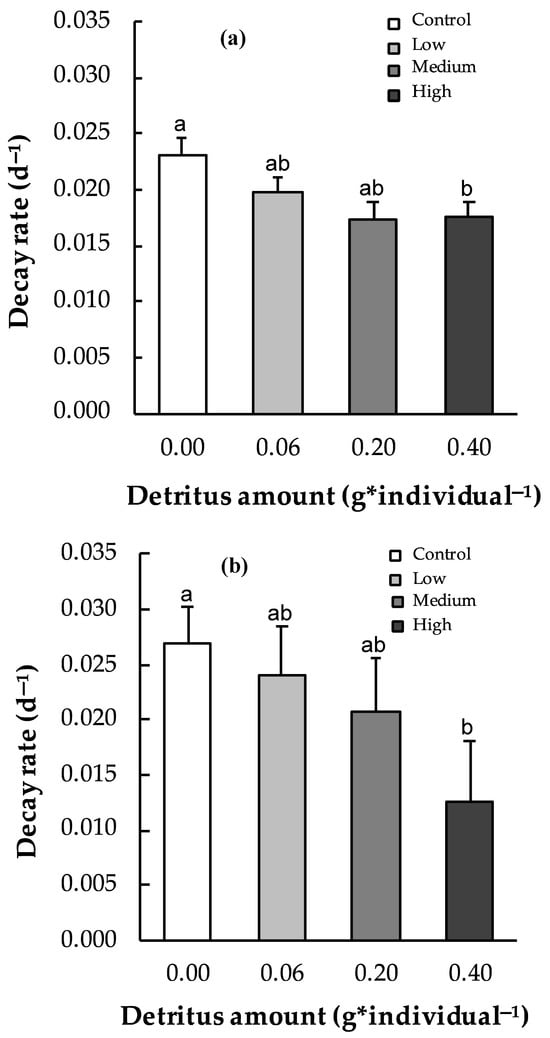
Figure 2.
Decay rates (d−1) of P. australis leaf fragments under each experimental condition (control, low, medium, and high detritus) in the channel (a) and patch (b) microcosms. Error bars represent standard errors; different letters indicate statistically significant differences (p < 0.05).
After 28 days, the number of surviving detritivores in the channels differed among treatments (one-way ANOVA, n = 16; d.f. = 3, 12; F = 6.87, p < 0.01; Figure 3a). The highest abundance was observed under high detritus conditions (20.8 ± 3.8), significantly greater than under medium (9.3 ± 2.0) and null detritus (5.8 ± 1.3) conditions (Tukey’s HSD test, p < 0.05 and p < 0.01, respectively).
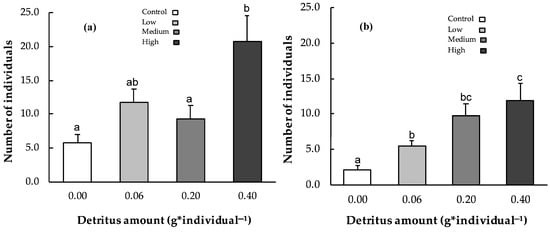
Figure 3.
Invertebrate abundance across all experimental conditions (control, low, medium, and high detritus) in channel (a) and patch (b) microcosms. Error bars indicate standard errors; different letters indicate statistically significant differences (p < 0.05).
Across all sampling times, the number of invertebrates colonizing leaf patches ranged from 2.1 ± 0.6 in the control to 11.8 ± 2.5 under high detritus conditions (Figure 3b). Two-way ANOVA revealed significant effects of detritus amounts, but not sampling times, on invertebrate abundance (p < 0.001; Table 1). Statistically significant differences were observed between the null detritus conditions and all other treatments (low [Tukey’s HSD, p < 0.05], medium [Tukey’s HSD, p < 0.001], and high detritus [Tukey’s HSD, p < 0.001]), as well as between low and high detritus (Tukey’s HSD, p < 0.05). In the patches, dissolved oxygen concentration ranged on average from 2.76 ± 0.07 mg·L−1 under high detritus conditions to 4.41 ± 0.08 mg·L−1 in the control (Figure 4). Two-way ANOVA showed that both detritus amount and time significantly influenced oxygen levels (see Table 1), with all comparisons significant except low detritus vs. medium detritus conditions (Tukey’s test p < 0.001 for all). P. australis decomposition rates exhibited an inverse relationship with detritivore abundance (y = 0.33 − 0.14x; r = −0.94; R2 = 0.99; p < 0.05). The effect of microbial decomposers on leaf decomposition, assessed through respiration rates, exhibited a significant quadratic response to detritus level (y = −2.25 × 10−5 + 0.009x − 0.002 x2, R2 = 0.99, p < 0.05; Figure 5). Minimum respiration rates were recorded under null (0.0070 ± 0.0024 µmmol·h−1·mg−1) and high (0.0069 ± 0.0007 µmmol·h−1·mg−1) detritus conditions. However, no statistically significant differences were found among respiration values.

Table 1.
Two-way ANOVA assessing the effects of detritus amount and experimental time on invertebrate distribution and dissolved oxygen concentration in the still-water system (Tukey’s test, p < 0.001).
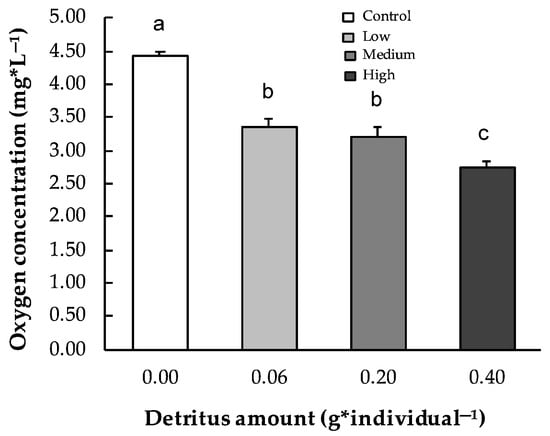
Figure 4.
Water oxygen concentration (mg·L−1) across all experimental conditions (control, low, medium, and high detritus) in patch microcosm. Error bars indicate standard errors; different letters indicate statistically significant differences (p < 0.001).
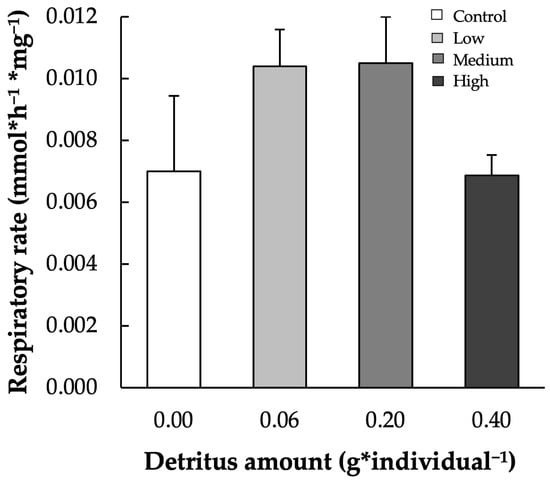
Figure 5.
Respiration rate (µmmol·h−1·mg−1) across all experimental conditions (control, low, medium, and high detritus) in patch microcosm. Error bars indicate standard errors; there are no statistically significant differences.
4. Discussion
The initial hypothesis—that the amount of surrounding detritus can significantly influence the decomposition of leaf litter in controlled aquatic microcosms—has been supported by the results of this study. Our experimental work shows that variations in detritus availability affect not only the decay rates of P. australis leaf litter, but also the associated biological processes, including the abundance and activity of detritivorous invertebrates and microbial communities involved in organic matter breakdown.
Notably, the responses to detritus quantity were not uniform across experimental conditions: distinct patterns were observed between microcosms simulating flowing-systems and those representing still-water systems.
Our results highlight the multifaceted nature of decomposition dynamics in aquatic ecosystems and underline the complex interplay between ecosystem physical characteristics, detritus quantity, and biotic agents of decomposition.
Previous investigations on the effects of litter alteration on decomposition dynamics have revealed equivocal responses and often contradictory results, underscoring the complexity of the interactions between drivers of decomposition process. Results from experimental works in aquatic environments showed strong interactions between litter quality and decomposition [50], while not significant influences were found on litter decomposition processes by the detritus amount in a certain condition [51].
A progressive reduction in decomposition rates was observed in the patches as the amount of detritus increased. This trend stands in clear contrast to the pattern observed in the channels, where decomposition rates remained relatively stable or showed a slight increase, even with greater detritus accumulation. These suggest that the physical structure and flow conditions may enhance the influence of detritus amount on microbial and macroinvertebrate decomposer communities. Indeed, recent studies have already highlighted the role of litter quality and ecosystem physicochemical properties as drivers of litter decomposition in streams [52].
In the patches, high detritus levels likely led to oxygen depletion (average 2.76 mg·L−1), inhibiting aerobic microbial activity and slowing decomposition. This pattern is consistent with previous studies, where anoxia suppresses microbial respiration and alters detrital breakdown pathways [53].
Detritivore abundance showed a positive response to detritus enrichment, particularly under high detritus conditions in the channels, which hosted the highest number of individuals. The significantly lower detritivore abundance under both medium and null detritus conditions could indicate that a threshold level of detrital input may be necessary to sustain detritivore populations in the channels. Interestingly, the lowest abundance was observed under null detritus, as expected, but the medium detritus conditions also resulted in a significantly reduced number of survivors compared to the high-detritus conditions.
This non-linear response of invertebrate abundance may be related to intraspecific competition under low and medium conditions, or to altered refuge availability.
Detritivores preferred conditions with surrounding litter material to those without detritus also in the patches, highlighting a positive effect of surrounding detritus on invertebrate density; however, this did not translate into faster decomposition, where oxygen limitations likely outweighed the increased biological activity.
The inverse correlation between detritivore abundance and decomposition rate (R2 = 0.99, p < 0.05) in the patches may reflect competition or behavioral shifts (e.g., reduced feeding) under deteriorating oxygen conditions or overcrowding such as those that may occur with warming [54]. Additionally, detritivores may exploit the surrounding detritus within patches contained more palatable or easily digestible detritus in relation to the focal leaf fragments used in the decomposition assays, so they may have selectively fed elsewhere, reducing their apparent effect on the measured litter. It is also well known that, while the role of invertebrates in litter decomposition is well established, their abundance in aquatic ecosystems can vary considerably, and their contribution to the decomposition process can be secondary to that of microbial communities [55].
These observations were confirmed by the respiration rates of leaf fragments in the different experimental conditions. The quadratic response of microbial respiration to detritus availability suggests that microbial decomposer activity is optimized at intermediate detritus levels, with reduced functioning under both extremes. In absence of surrounding detritus, water oxygen was likely not limiting, and the detritivores probably consumed most of the focal leaf fragments, exerting a predatory effect on the fungi and resulting in low respiration rates. On the other hand, in presence of high amounts of surrounding detritus, the detritivores could be attracted from the resources and the limited conditions of water oxygen determined low growth of fungi, resulting in low decomposition and respiration rates of leaf fragments. So, this non-linear pattern may result from oxygen availability constraints at high detritus conditions and limited resource availability at low levels. These results align with the concept of an intermediate disturbance hypothesis applied to microbial processes [56], where moderate inputs promote optimal conditions for microbial metabolism, while extreme conditions inhibit activity.
5. Conclusions
All the results obtained in our study highlight that, while the two experimental microcosms differed in several aspects beyond hydrodynamics, including experimental duration, invertebrate density, and management, the main result on the influence of surrounding detritus on leaf litter decomposition was confirmed. In light of what these factors could have contributed, individually or interactively, to the observed differences in leaf litter decomposition, we emphasize the need for future experiments that systematically manipulate single variables in a fully factorial design to clarify the causal mechanisms underlying leaf litter decomposition in aquatic systems.
Ultimately, our findings underscore the context-dependent nature of detritus effects on decomposition, highlighting important ecological implications for this study and management of aquatic ecosystems. The influence of detritus availability on decomposition is not uniform across environments but is modulated by the physical and hydrological characteristics of the system. In more dynamic environments such as the channel microcosms used in our study, increased detritus inputs appear to enhance decomposition rates by promoting the colonization and activity of detritivorous invertebrates. This effect is likely facilitated by improved oxygen diffusion and continuous water flow, which together create favorable conditions for aerobic microbial processes and efficient nutrient cycling.
Conversely, in more quiescent environments—such as the patch microcosms—high detritus accumulation can have the opposite effect. In these settings, excessive organic matter may lead to the formation of anaerobic microsites, resulting in reduced oxygen availability that inhibits microbial activity and suppresses the abundance or functional capacity of benthic detritivores.
The divergent responses observed in our controlled experiments suggest that further research is needed—particularly studies that incorporate large-scale and long-term experimental frameworks capable of capturing spatial and temporal variability across ecosystem types. Such research could not only enhance theoretical models of organic matter processing but also provide valuable guidance for ecosystem management. This is especially relevant in restored or constructed wetlands, where detritus accumulation is often a common feature and may influence restoration success by altering decomposition rates, nutrient cycling, and overall ecosystem functioning.
Author Contributions
Conceptualization, A.B. and F.S.; methodology, F.S., F.V., D.S. and A.B.; software, F.S. and F.V.; validation, F.S., D.S. and A.B.; formal analysis, F.S. and F.V.; investigation, D.S.; resources, A.B.; data curation, D.S. and F.S.; writing—original draft preparation, F.S. and F.V.; writing—review and editing, F.S.; visualization, M.P.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by PRIN project (2022HRL2HL)—Role of habitat degradation and food web structure in the performance of wetlands as carbon sink.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wetzel, R.G. Death, detritus, and energy flow in aquatic ecosystems. Freshw. Biol. 1995, 33, 83–89. [Google Scholar] [CrossRef]
- Benbow, M.E.; Receveur, J.P.; Lamberti, G.A. Death and Decomposition in Aquatic Ecosystems. Front. Ecol. Evol. 2020, 8, 17. [Google Scholar] [CrossRef]
- O’Neill, R.V.; Reichle, D.A. Dimensions of ecosystem theory. In Forests: Fresh Perspectives from Ecosystem Analysis; Waring, R.H., Ed.; Oregon Press University Press: Corvallis, OR, USA, 1991; pp. 11–26. [Google Scholar]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 1997, 277, 102–104. [Google Scholar] [CrossRef]
- Windham, L.; Ehrenfeld, J.G. Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecol. Appl. 2003, 13, 883–897. [Google Scholar] [CrossRef]
- Duke, S.T.; Francoeur, S.N.; Judd, K.E. Effects of Phragmites australis Invasion on Carbon Dynamics in a Freshwater Marsh. Wetlands 2015, 35, 311–321. [Google Scholar] [CrossRef]
- Sangiorgio, F.; Basset, A.; Pinna, M.; Sabetta, L.; Abbiati, M.; Ponti, M.; Minocci, M.; Orfanidis, S.; Nicolaidou, A.; Moncheva, S.; et al. Environmental factors affecting Phragmites australis litter decomposition in Mediterranean and Black Sea transitional waters. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, S16–S26. [Google Scholar] [CrossRef]
- Ferreira, V.; Chauvet, E. Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Glob. Change Biol. 2011, 17, 551–564. [Google Scholar] [CrossRef]
- Abelho, M.; Ribeiro, R.; Moreira-Santos, M. Salinity Affects Freshwater Invertebrate Traits and Litter Decomposition. Diversity 2021, 13, 599. [Google Scholar] [CrossRef]
- Quintino, V.; Sangiorgio, F.; Ricardo, F.; Mamede, R.; Pires, P.; Freitas, R.; Rodrigues, A.M.; Basset, A. In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuar. Coast. Shelf Sci. 2009, 85, 497–506. [Google Scholar] [CrossRef]
- Gessner, M.O. Breakdown and nutrient dynamics of submerged Phragmites shoots in the littoral zone of a temperate hardwater lake. Aquat. Bot. 2000, 66, 9–20. [Google Scholar] [CrossRef]
- Pinna, M.; Sangiorgio, F.; Fonnesu, A.; Basset, A. Spatial analysis of plant detritus processing in a Mediterranean River type: The case of the River Tirso Basin, Sardinia Italy. J. Environ. Sci. 2003, 15, 227–240. [Google Scholar] [CrossRef]
- Sangiorgio, F.; Pinna, M.; Basset, A. Inter- and intra-habitat variability of plant detritus decomposition in a transitional environment (Lake Alimni, Adriatic Sea). Chem. Ecol. 2004, 20, 353–366. [Google Scholar] [CrossRef]
- Sangiorgio, F.; Fonnesu, A.; Pinna, M.; Sabetta, L.; Basset, A. Influence of Drought and Abiotic Factors on Phragmites australis Leaf Decomposition in the River Pula, Sardinia Italy. J. Freshw. Ecol. 2006, 21, 411–420. [Google Scholar] [CrossRef]
- Gessner, M.O.; Chauvet, E. Importance of Stream Microfungi in Controlling Breakdown Rates of Leaf Litter. Ecology 1994, 75, 1807–1817. [Google Scholar] [CrossRef]
- Graça, M.A.S. The Role of Invertebrates on Leaf Litter Decomposition in Streams—A Review. Internat. Rev. Hydrobiol. 2011, 86, 383–393. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Ferreira, R.C.F.; Coimbra, C.N. Litter processing along a stream gradient: The role of invertebrates and decomposers. J. N. Am. Benthol. Soc. 2001, 20, 408–420. [Google Scholar] [CrossRef]
- Boyero, L.; López-Rojo, N.; Tonin, A.M.; Pérez, J.; Correa-Araneda, F.; Pearson, R.G.; Bosch, J.; Albariño, R.J.; Anbalagan, S.; Barmuta, L.A.; et al. Impacts of detritivore diversity loss on instream decomposition are greatest in the tropics. Nat. Commun. 2021, 12, 3700. [Google Scholar] [CrossRef]
- McKie, B.G.; Woodward, G.; Hladyz, S.; Nistorescu, M.; Preda, E.; Popescu, C.; Giller, P.S.; Malmqvist, B. Ecosystem functioning in stream assemblages from different regions: Contrasting responses to variation in detritivore richness, evenness and density. J. Anim. Ecol. 2008, 77, 495–504. [Google Scholar] [CrossRef]
- Encalada, A.C.; Calles, J.; Ferreira, V.; Canhoto, C.; Graça, M.A.S. Riparian land use and the relationship between the benthos and litter decomposition in tropical montane streams. Freshw. Biol. 2010, 55, 1719–1733. [Google Scholar] [CrossRef]
- Reice, S.R. Environmental patchiness and the breakdown of leaf litter in a woodland stream. Ecology 1974, 54, 1271–1282. [Google Scholar] [CrossRef]
- Benfield, E.F.; Paul, R.W., Jr.; Webster, J.R. Influence of exposure technique on leaf breakdown rates in streams. Oikos 1979, 33, 386–391. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, L.; Zhou, G.; Huang, W.; Liu, J. Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests. Plant Soil 2015, 392, 139–153. [Google Scholar] [CrossRef]
- Pozo, J.; Oterrnin, A.; Basaguren, A. Is loss rate dependent on leaf-litter amount? Verh. Int. Ver. Theor. Angew. Limnol. 2002, 28, 556–559. [Google Scholar] [CrossRef]
- Tiegs, S.D.; Peter, F.D.; Robinson, C.T.; Uehlinger, U.; Gessner, M.O. Leaf decomposition and invertebrate colonization responses to manipulated litter quantity in streams. J. N. Am. Benthol. Soc. 2008, 27, 321–331. [Google Scholar] [CrossRef]
- González, J.M.; Mora, N.; Molina, R. Initial mass of leaf litter influences mass loss and invertebrate assemblages in two mountain streams. Hydrobiologia 2025, 852, 1531–1544. [Google Scholar] [CrossRef]
- Campbell, I.C.; Enierga, G.M.; Fuchshuber, L. The influence of pack size and position, leaf type, and shredder access on the processing rate of Atherosperma moschatum leaves in an Australian cool temperate rainforest stream. Internat. Rev. Hydrobiol. 1994, 79, 557–568. [Google Scholar] [CrossRef]
- Bruder, A.; Schindler, M.H.; Moretti, M.S.; Gessner, M.O. Litter decomposition in a temperate and a tropical stream: The effects of species mixing, litter quality and shredders. Freshw. Biol. 2014, 59, 438–449. [Google Scholar] [CrossRef]
- Ferreira, V.; Castela, J.; Rosa, P.; Tonin, A.M.; Boyero, L.; Graça, M.A.S. Aquatic hyphomycetes, benthic macroinvertebrates and leaf litter decomposition in streams naturally differing in riparian vegetation. Aquat. Ecol. 2016, 50, 711–725. [Google Scholar] [CrossRef]
- Lecerf, A.; Dobson, M.; Dang, C.K.; Chauvet, E. Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 2005, 146, 432–442. [Google Scholar] [CrossRef]
- Barlöcher, F. Pitfalls of traditional techniques when studying decomposition of vascular plant remains in aquatic habitats. Limnetica 1997, 13, 1–11. [Google Scholar] [CrossRef]
- Bedford, A.P. A modified litter bag design for use in lentic habitats. Hydrobiologia 2004, 529, 187–193. [Google Scholar] [CrossRef]
- Benfield, E.F.; Jones, D.S.; Patterson, M.F. Leaf pack processing in a pastureland stream. Oikos 1977, 29, 99–103. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Hynes, H.B.N. Experimental study on the role of autumn-shed leaves in aquatic environments. J. Ecol. 1968, 56, 229–243. [Google Scholar] [CrossRef]
- Wall, C.B.; Spiegel, C.J.; Diaz, E.M.; Tran, C.H.; Fabiani, A.; Broe, T.Y.; Perez-Coronel, E.P.; Jackrel, S.L.; Mladenov, N.; Symons, C.C.; et al. Fire transforms effects of terrestrial subsidies on aquatic ecosystem structure and function. Glob. Change Biol. 2023, 30, e17058. [Google Scholar] [CrossRef] [PubMed]
- Basset, A.; Maci, S.; Mazzola, A.; Rosati, I.; Roselli, L.; Tramati, C.; Vizzini, S.; Pinna, M. Acquatina Lagoon: A model ecosystem to study community patterns. In Proceedings of the VI EUROLAG & VII LAGUNET Conference 2013, Lecce, Italy, 16–19 December 2013. [Google Scholar]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Abelho, M. Leaf-litter mixtures affect breakdown and macroinvertebrate colonization rates in a stream ecosystem. Internat. Rev. Hydrobiol. 2009, 94, 436–451. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The utilisation of reed (Phragmites australis): A review. Mires Peat 2013, 13, 1. Available online: http://www.mires-and-peat.net/pages/volumes/map13/map1301.php (accessed on 2 May 2025).
- Mancinelli, G.; Sabetta, L.; Sangiorgio, F. On the influence of temporal resolution in mesh bag decomposition studies. Internat. Rev. Hydrobiol. 2009, 92, 135–145. [Google Scholar] [CrossRef]
- Longo, E.; Mancinelli, G. Size at the onset of maturity (SOM) revealed in length weight relationships of brackish amphipods and isopods: An information theory approach. Estuar. Coast. Shelf Sci. 2014, 136, 119–128. [Google Scholar] [CrossRef]
- Tachet, H.; Bournaud, M.; Richoux, P. Introduction à l’Étude des Macroinvertébrés des Eaux Douces (Systématique Élémentaire et Aperçu Écologique); Université Lyon: Lyon, France, 1997; Available online: https://www.persee.fr/doc/linly_0366-1326_1981_num_50_5_14287_t2_0011_0000_3 (accessed on 2 May 2025).
- Windham, L.; Meyerson, L. Effects of Common Reed (Phragmites australis) Expansions on Nitrogen Dynamics of Tidal Marshes of the Northeastern U.S. Estuaries 2003, 26, 452–464. [Google Scholar] [CrossRef]
- Maltby, L.; Clayton, S.; Wood, R.; McLoughlin, N. Evaluation of the Gammarus pulex in situ feeding assay as a biomonitor of water quality: Robustness, responsiveness, and relevance. Environ. Toxicol. Chem. 2002, 21, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, C.; Cássio, F. Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Appl. Environ. Microbiol. 2004, 70, 5266–5273. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, J. Respirometria: Tecniche respirometriche per la determinazione del consumo di ossigeno in animali acquatici. In Argomenti di Idrobiologia e Acquacoltura; Carpenè, E., Isani, G., Serra, R., Eds.; CLUEB: Bologna, Italy, 1995. [Google Scholar]
- Vignes, F.; Fedele, M.; Pinna, M.; Mancinelli, G.; Basset, A. Variability of Lekanesphaera monodi metabolic rates with habitat trophic status. Acta Oecol. 2012, 41, 58–64. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Freeman, W.H., Ed.; JSTOR: New York, NY, USA, 1995. [Google Scholar]
- Pérez, J.; Ferreira, V.; Graça, M.A.S.; Boyero, L. Litter Quality Is a Stronger Driver than Temperature of Early Microbial Decomposition in Oligotrophic Streams: A Microcosm Study. Environ. Microbiol. 2021, 82, 897–908. [Google Scholar] [CrossRef]
- Xu, C.; Yu, X.; Duan, H.; Li, J.; Xia, S.; Zhang, Q.; Li, C. The decomposition processes and return of carbon, nitrogen, and phosphorus of Phragmites australis litter with different detritus amount. Hydrobiologia 2023, 850, 3893–3906. [Google Scholar] [CrossRef]
- Yue, K.; De Frenne, P.; Van Meerbeek, K.; Ferreira, V.; Fornara, D.A.; Wu, Q.; Ni, X.; Peng, Y.; Wang, D.; Hedě, P.; et al. Litter quality and stream physicochemical properties drive global invertebrate effects on instream litter decomposition. Biol. Rev. 2022, 97, 2023–2038. [Google Scholar] [CrossRef]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity meets decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef]
- Benavides-Gordillo, S.; Moretti, M.S.; González, A.L.; Moi, D.A.; Aidar, M.P.M.; Kersch-Becker, M.F.; Romero, G.O. Warming and shifts in litter quality drive multiple responses in freshwater detritivore communities. Sci. Rep. 2024, 14, 11137. [Google Scholar] [CrossRef]
- Wissinger, S.A.; Klemmer, A.J.; Braccia, A.; Bush, M.B.; Batzer, D.P. Relationships between macroinvertebrates and detritus in freshwater wetlands. Freshw. Sci. 2021, 40, 681–698. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.; Song, K.; Kwon, M.J.; Lee, J. Intermediate Disturbances Enhance Microbial Enzyme Activities in Soil Ecosystems. Microorganisms 2024, 12, 1401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).