Influence of Geographical Location of Spirulina (Arthrospira platensis) on the Recovery of Bioactive Compounds Assisted by Pulsed Electric Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Extraction Procedure
2.4. Chemical Analysis
2.4.1. Total Phenolic Content (TPC)
2.4.2. Trolox Equivalent Antioxidant Capacity (TEAC)
2.4.3. Oxygen Radical Absorbance Capacity (ORAC)
2.4.4. Chlorophyll a, Chlorophyll b and Carotenoids
2.5. Statistical Analysis
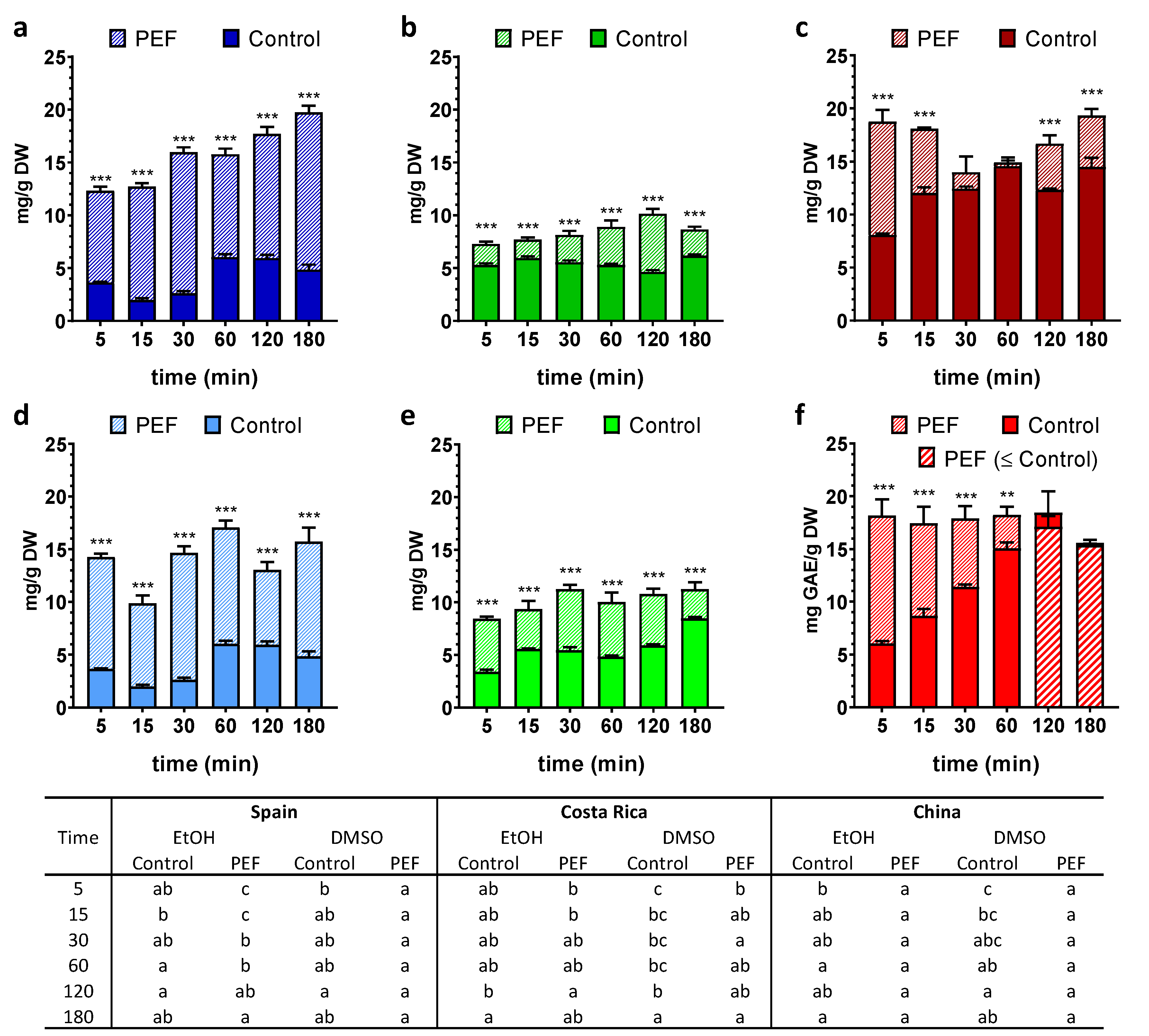
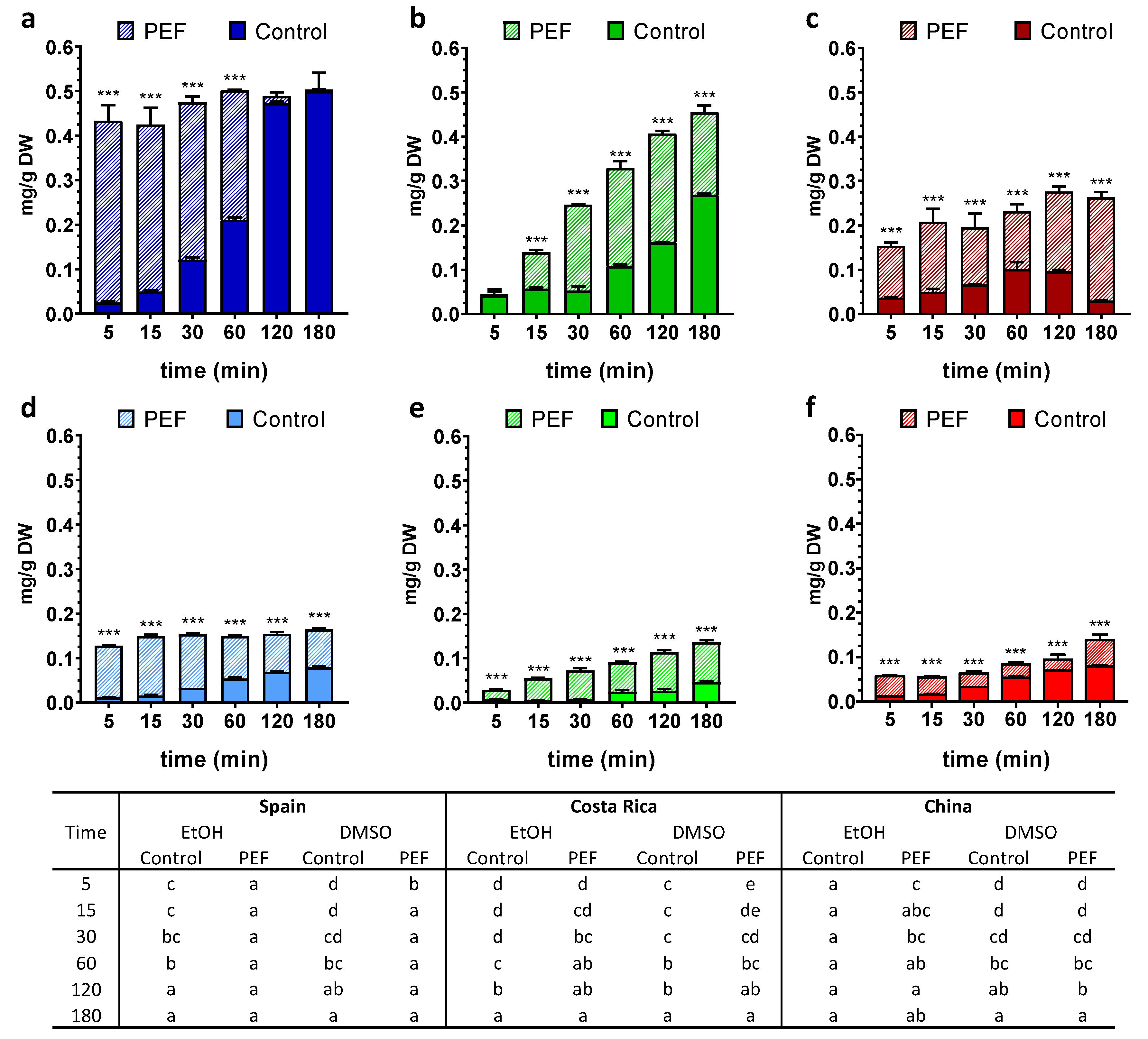
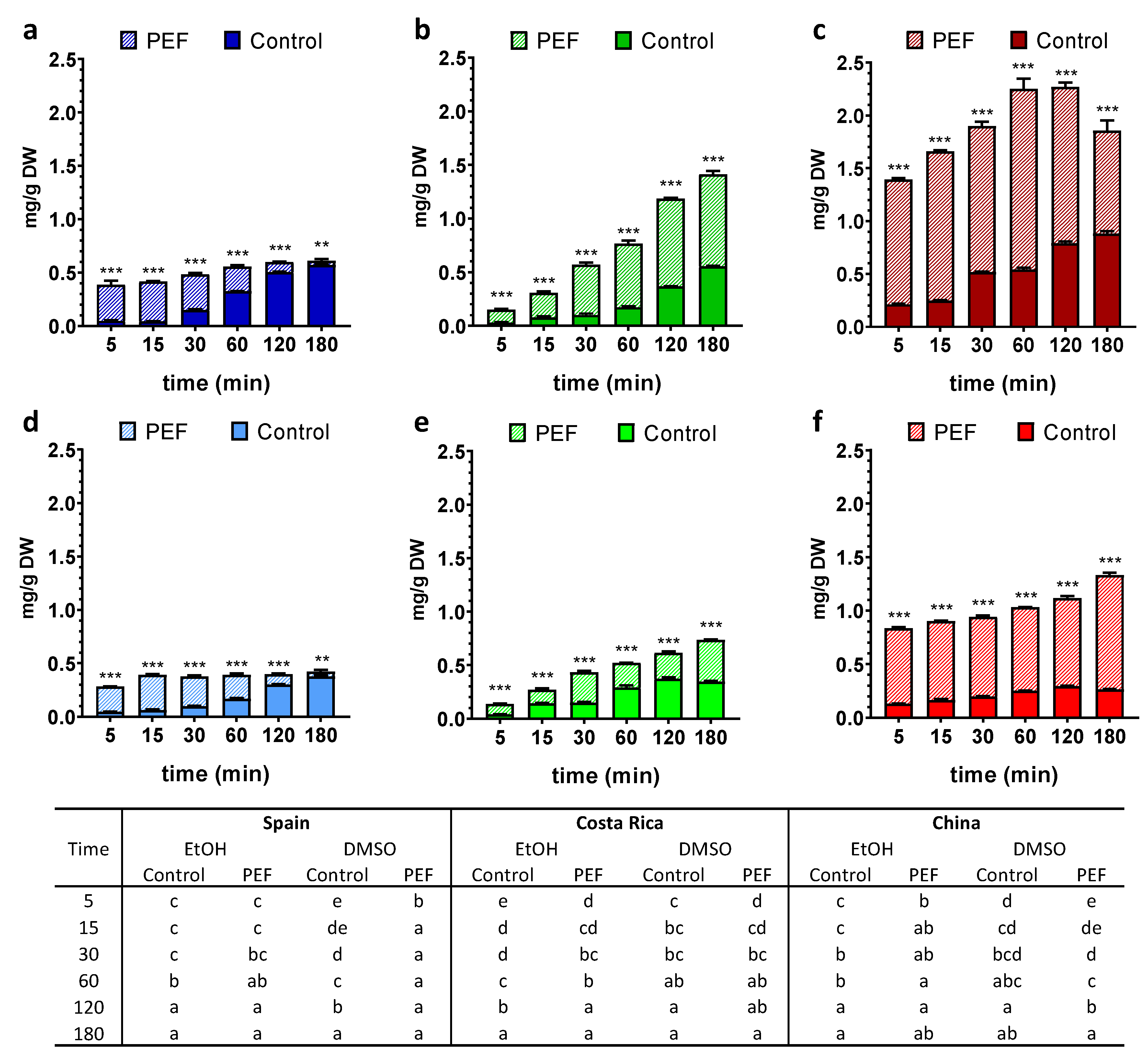
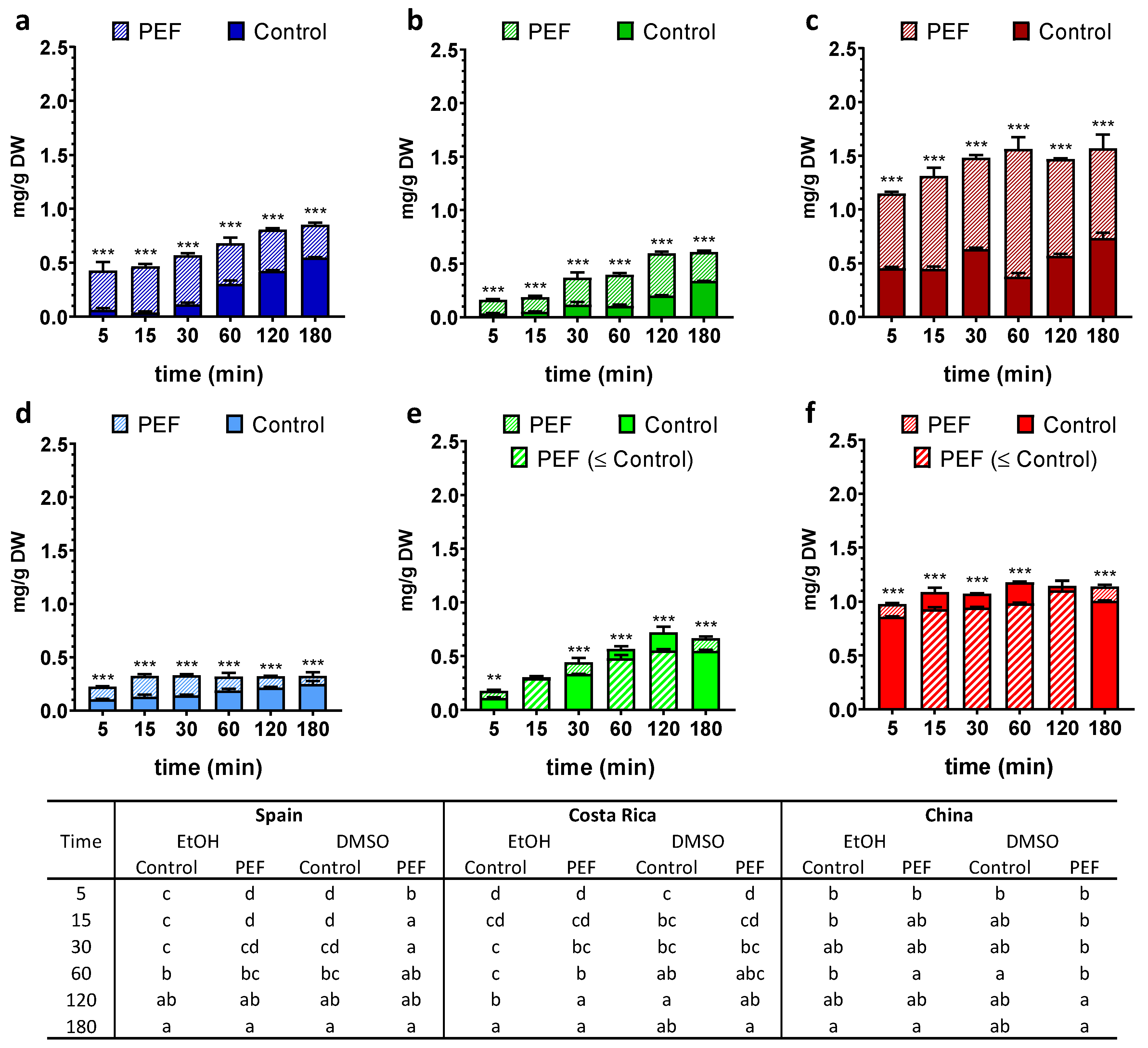
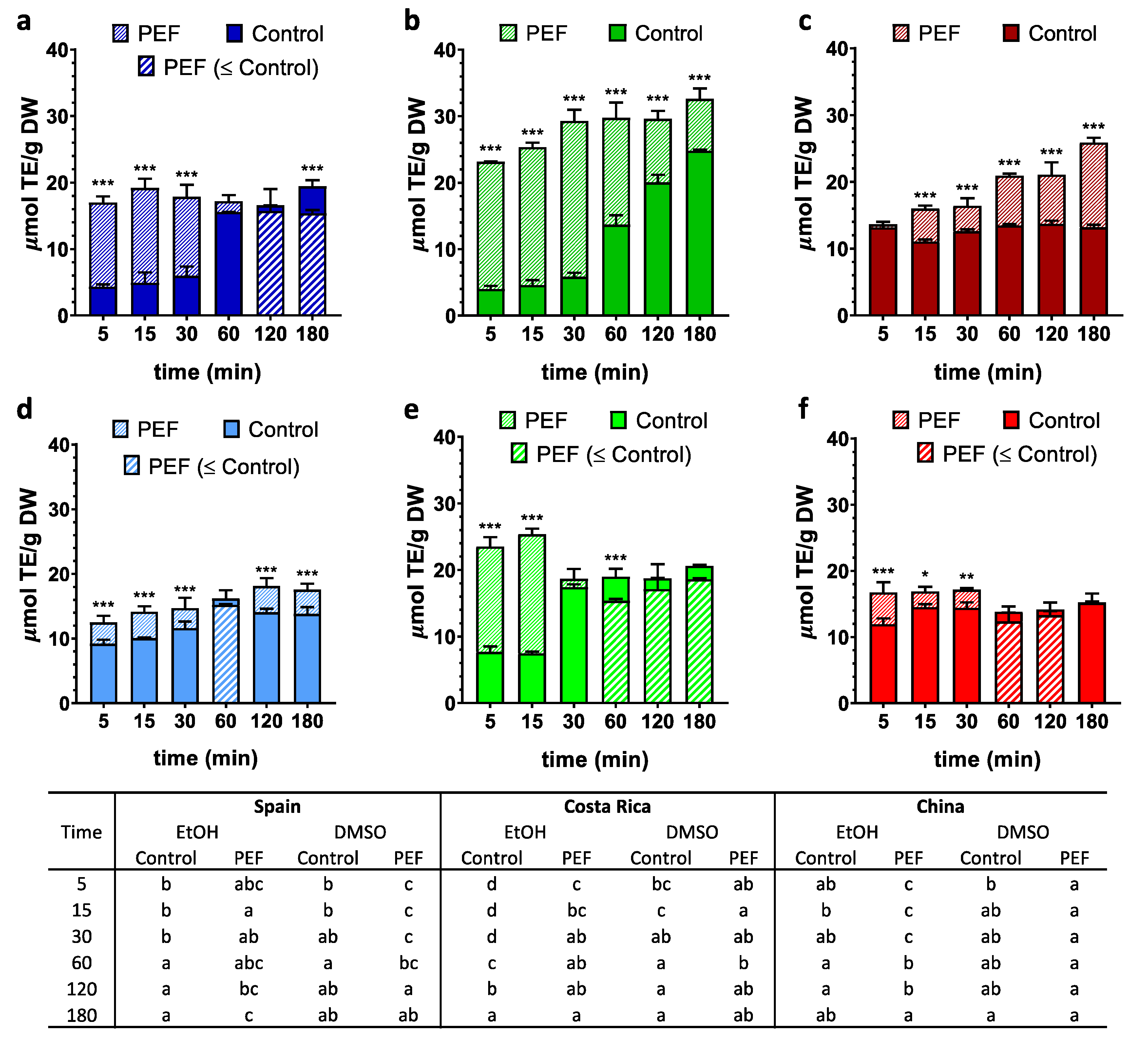
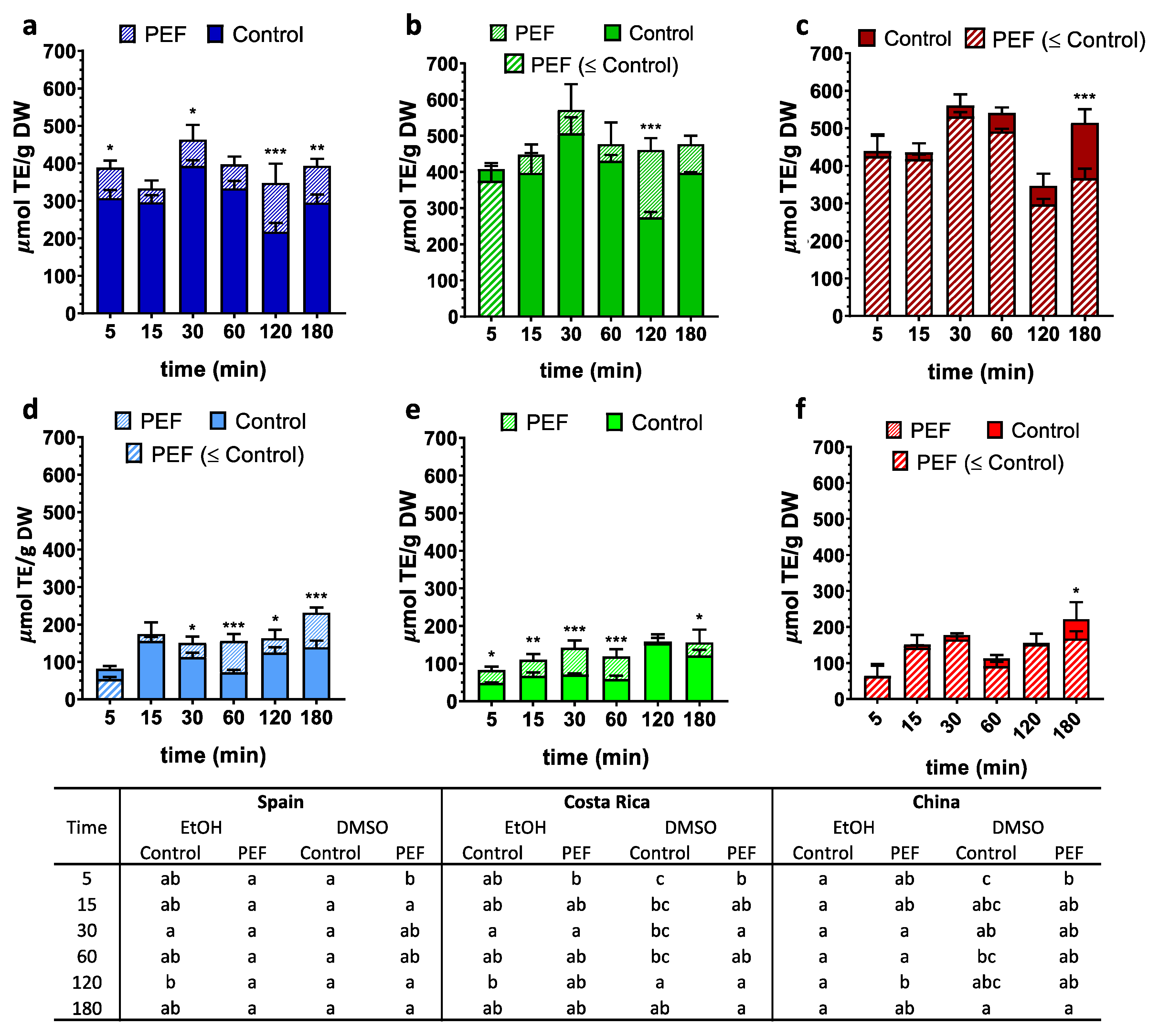
3. Results
3.1. Total Phenolic Content (TPC)
3.2. Chlorophyll a, Chlorophyll b and Carotenoids Contents
3.3. Antioxidant Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Viner-Mozzini, Y.; Jia, Y.; Song, L.; Sukenik, A. Alkyltrimethylammonium (ATMA) Surfactants as Cyanocides—Effects on Photosynthesis and Growth of Cyanobacteria. Chemosphere 2021, 274, 129778. [Google Scholar] [CrossRef] [PubMed]
- Luan, G.; Zhang, S.; Lu, X. Engineering Cyanobacteria Chassis Cells toward More Efficient Photosynthesis. Curr. Opin. Biotechnol. 2020, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Serdaroǧlu, M.; Jiménez-Colmenero, F. Design of Healthier Foods and Beverages Containing Whole Algae. Funct. Ingred. Algae Foods Nutraceuticals 2013, 609–633. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Santos, T.D.; de Freitas, B.C.B.; Moreira, J.B.; Zanfonato, K.; Costa, J.A.V. Development of Powdered Food with the Addition of Spirulina for Food Supplementation of the Elderly Population. Innov. Food Sci. Emerg. Technol. 2016, 37, 216–220. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive Peptides and Carbohydrates from Seaweed for Food Applications: Natural Occurrence, Isolation, Purification, and Identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; Rangel-Yagui, C.D.O.; De Carvalho, J.C.M.; Sato, S. An Investigation of Effect of Replacing Nitrate by Urea in the Growth and Production of Chlorophyll by Spirulina Platensis. Biomass Bioenergy 2002, 23, 261–269. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.S.; Ryu, B.M.; Kim, S.K. Purification of Novel Anti-Inflammatory Peptides from Enzymatic Hydrolysate of the Edible Microalgal Spirulina Maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Anekthanakul, K.; Senachak, J.; Hongsthong, A.; Charoonratana, T.; Ruengjitchatchawalya, M. Natural ACE Inhibitory Peptides Discovery from Spirulina (Arthrospira Platensis) Strain C1. Peptides 2019, 118, 170107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X. Isolation and Identification of Anti-Proliferative Peptides from Spirulina platensis Using Three-Step Hydrolysis. J. Sci. Food Agric. 2017, 97, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Faieta, M.; Neri, L.; Di Michele, A.; Di Mattia, C.D.; Pittia, P. High Hydrostatic Pressure Treatment of Arthrospira (Spirulina) Platensis Extracts and the Baroprotective Effect of Sugars on Phycobiliproteins. Innov. Food Sci. Emerg. Technol. 2021, 70, 102693. [Google Scholar] [CrossRef]
- Santos Assunção, L.; Quênia Muniz Bezerra, P.; Stahl Hermes Poletto, V.; de Oliveira Rios, A.; Graça Ramos, I.; Duarte Ferreira Ribeiro, C.; Aparecida Souza Machado, B.; Izabel Druzian, J.; Alberto Vieira Costa, J.; Larroza Nunes, I. Combination of Carotenoids from Spirulina and PLA/PLGA or PHB: New Options to Obtain Bioactive Nanoparticles. Food Chem. 2021, 346, 128742. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Fraix, A.; Sortino, S.; Agostiano, A.; Cosma, P. Development of Spirulina Sea-Weed Raw Extract/Polyamidoamine Hydrogel System as Novel Platform in Photodynamic Therapy: Photostability and Photoactivity of Chlorophyll A. Mater. Sci. Eng. C 2021, 119, 111593. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of Microalgal Biomass Incorporation into Foods: Nutritional and Sensorial Attributes of the End Products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-Conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of Ultrasound for Green Extraction of Proteins from Spirulina. Mechanism, Optimization, Modeling, and Industrial Prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.-J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of Antioxidant Bioactive Compounds from Tetraselmis Chuii and Phaedoactylum Tricornutum Using Pulsed Electric Fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Marine Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable Extraction of Valuable Components from Spirulina Assisted by Pulsed Electric Fields Technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of Phycocyanin—A Natural Blue Colorant from Dried Spirulina Biomass: Influence of Processing Parameters and Extraction Techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed Electric Field and PH Assisted Selective Extraction of Intracellular Components from Microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P.; et al. Study on the Microalgal Pigments Extraction Process: Performance of Microwave Assisted Extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Banerjee, S.; Ramaswamy, S. Dynamic Process Model and Economic Analysis of Microalgae Cultivation in Open Raceway Ponds. Algal Res. 2017, 26, 330–340. [Google Scholar] [CrossRef]
- Hossain, N.; Hasan, M.H.; Mahlia, T.M.I.; Shamsuddin, A.H.; Silitonga, A.S. Feasibility of Microalgae as Feedstock for Alternative Fuel in Malaysia: A Review. Energy Strategy Rev. 2020, 32, 100536. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- de la Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ramakritinan, C.M.; Kumaraguru, A.K. Solvent Extraction and Spectrophotometric Determination of Pigments of Some Algal Species from the Shore of Puthumadam, Southeast Coast of India. Int. J. Ocean. Oceanogr. 2010, 4, 29–34. [Google Scholar]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira Platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Xu, G.; Fu, X. Improving the Lipid Extraction Yield from Chlorella Based on the Controllable Electroporation of Cell Membrane by Pulsed Electric Field. Bioresour. Technol. 2021, 330, 124933. [Google Scholar] [CrossRef] [PubMed]
- ’t Lam, G.P.; Postma, P.R.; Fernandes, D.A.; Timmermans, R.A.H.; Vermuë, M.H.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Olivieri, G. Pulsed Electric Field for Protein Release of the Microalgae Chlorella Vulgaris and Neochloris Oleoabundans. Algal Res. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current Applications and New Opportunities for the Use of Pulsed Electric Fields in Food Science and Industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed Electric Field Assisted Extraction of Nutritionally Valuable Compounds from Microalgae Nannochloropsis Spp. Using the Binary Mixture of Organic Solvents and Water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Minas Da Piedade, M.E. Solubility of Nicotinic Acid in Water, Ethanol, Acetone, Diethyl Ether, Acetonitrile, and Dimethyl Sulfoxide. J. Chem. Thermodyn. 2012, 47, 362–371. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-Assisted Green Solvent Extraction of High-Added Value Compounds from Microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant Properties and Lipid Composition of Selected Microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramon-Mascarell, F.; Martí-Quijal, F.J.; Castagnini, J.M.; Phimolsiripol, Y.; Ruksiriwanich, W.; Rajoka, M.S.R.; Mehwish, H.M.; Barba, F.J. Influence of Geographical Location of Spirulina (Arthrospira platensis) on the Recovery of Bioactive Compounds Assisted by Pulsed Electric Fields. Separations 2022, 9, 257. https://doi.org/10.3390/separations9090257
Ramon-Mascarell F, Martí-Quijal FJ, Castagnini JM, Phimolsiripol Y, Ruksiriwanich W, Rajoka MSR, Mehwish HM, Barba FJ. Influence of Geographical Location of Spirulina (Arthrospira platensis) on the Recovery of Bioactive Compounds Assisted by Pulsed Electric Fields. Separations. 2022; 9(9):257. https://doi.org/10.3390/separations9090257
Chicago/Turabian StyleRamon-Mascarell, Francesc, Francisco J. Martí-Quijal, Juan Manuel Castagnini, Yuthana Phimolsiripol, Warintorn Ruksiriwanich, Muhammad Shahid Riaz Rajoka, Hafiza Mahreen Mehwish, and Francisco J. Barba. 2022. "Influence of Geographical Location of Spirulina (Arthrospira platensis) on the Recovery of Bioactive Compounds Assisted by Pulsed Electric Fields" Separations 9, no. 9: 257. https://doi.org/10.3390/separations9090257
APA StyleRamon-Mascarell, F., Martí-Quijal, F. J., Castagnini, J. M., Phimolsiripol, Y., Ruksiriwanich, W., Rajoka, M. S. R., Mehwish, H. M., & Barba, F. J. (2022). Influence of Geographical Location of Spirulina (Arthrospira platensis) on the Recovery of Bioactive Compounds Assisted by Pulsed Electric Fields. Separations, 9(9), 257. https://doi.org/10.3390/separations9090257










