Impact of Phenol on Membranes during Bipolar Membrane Electrodialysis for High Salinity Pesticide Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Experimental Methods
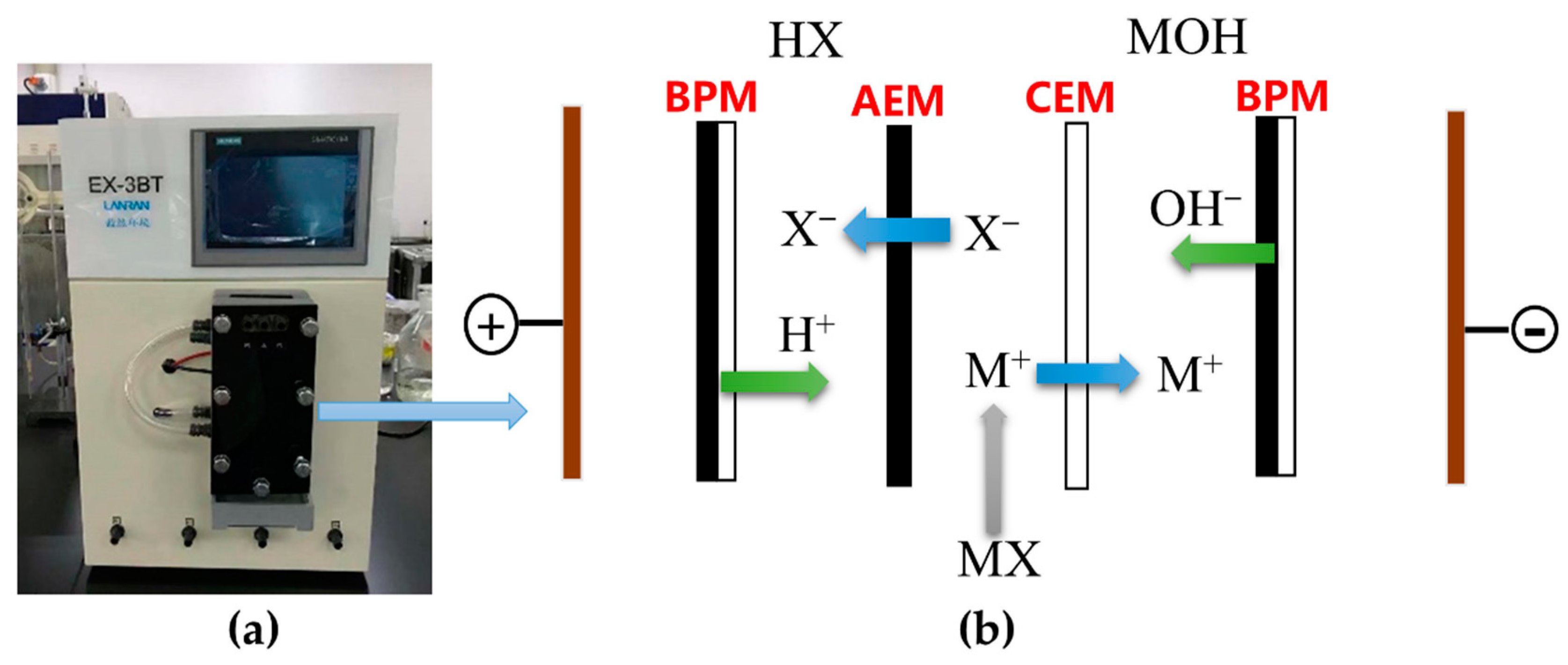
2.1. Chemicals and BMED Membrane Setup
2.2. BMED Experimental Procedure and Membrane Soaking Experiments
2.2.1. BMED Experimental Procedure
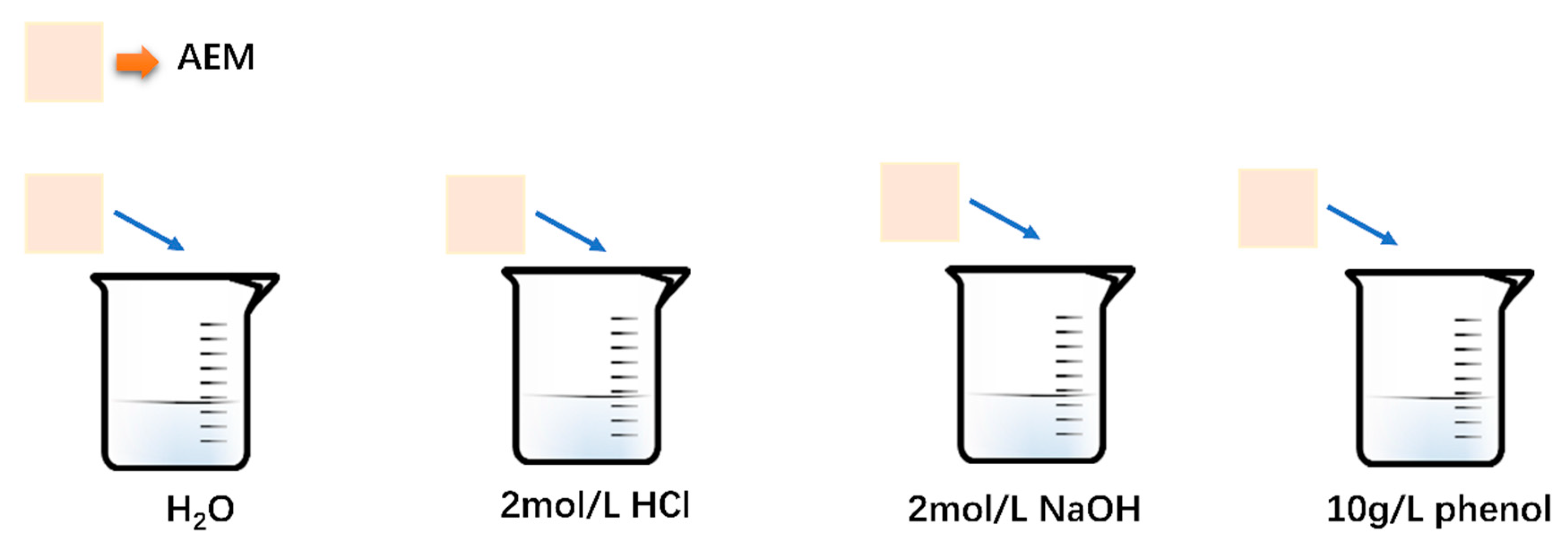
2.2.2. Membrane Soaking Tests
2.3. Analytical Methods and IEMs Characterization
2.3.1. Analytical Methods of the BMED Processes
2.3.2. Characterization of the Ion Exchange Membranes
3. Results and Discussion
3.1. The Fouling Effect of Phenol Concentration on the BMED Stack for a Short-Running Process
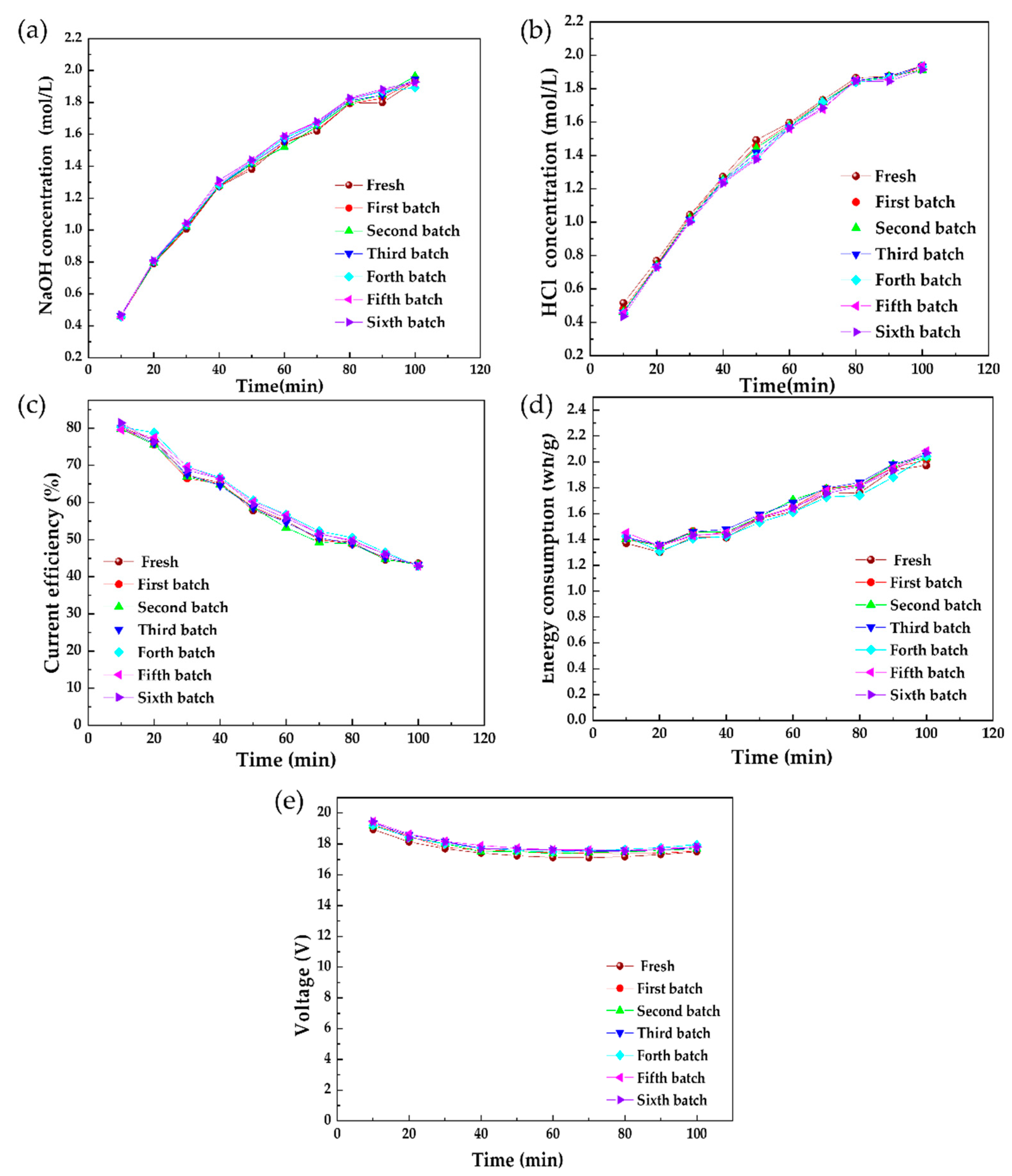
3.1.1. The Effect of Phenol Concentration on the BMED Performance
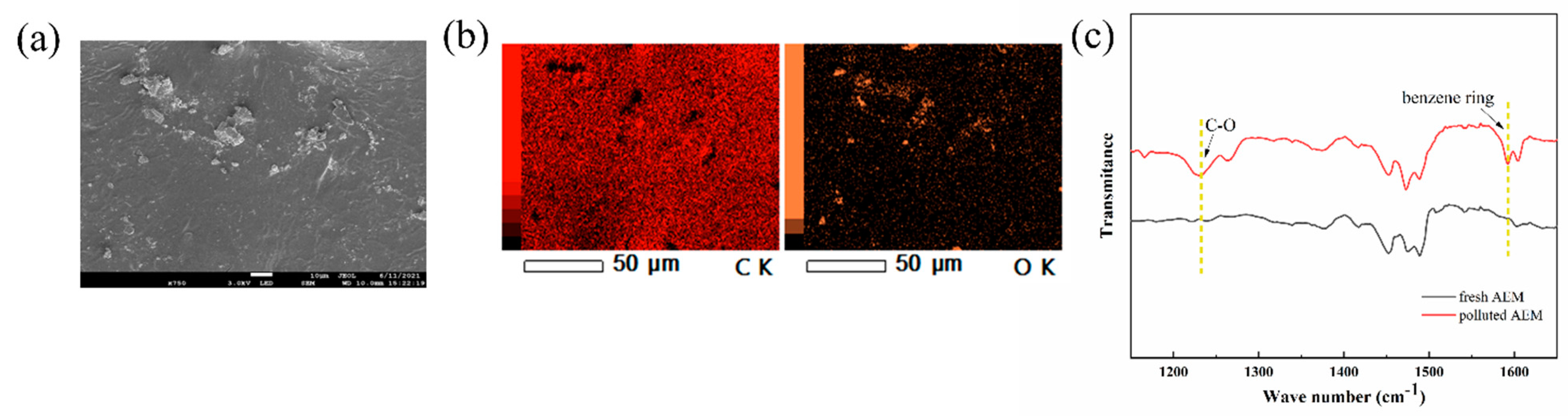
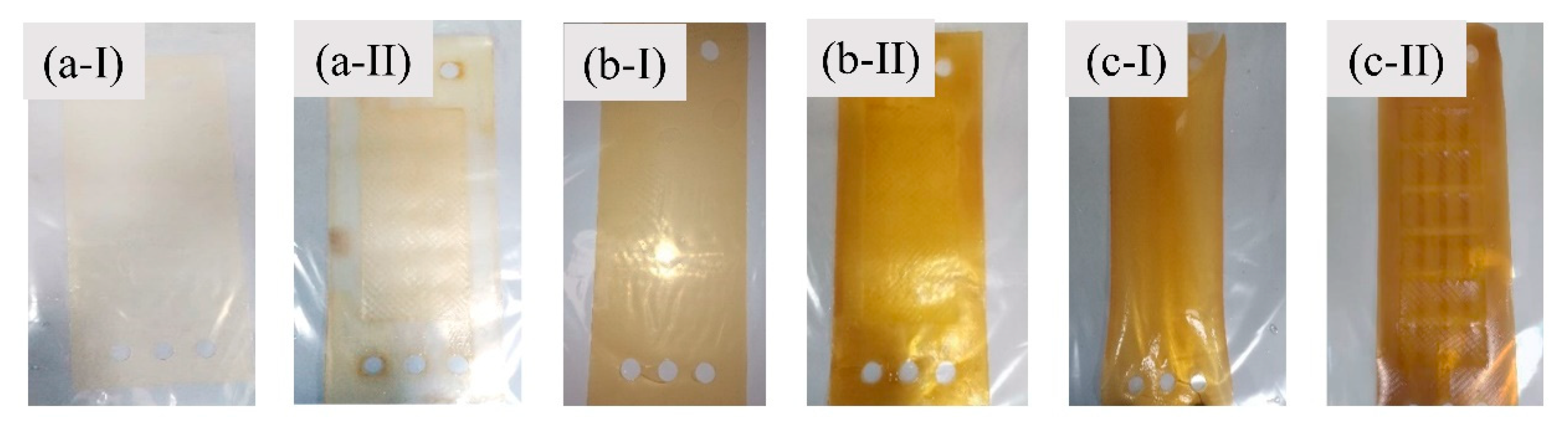
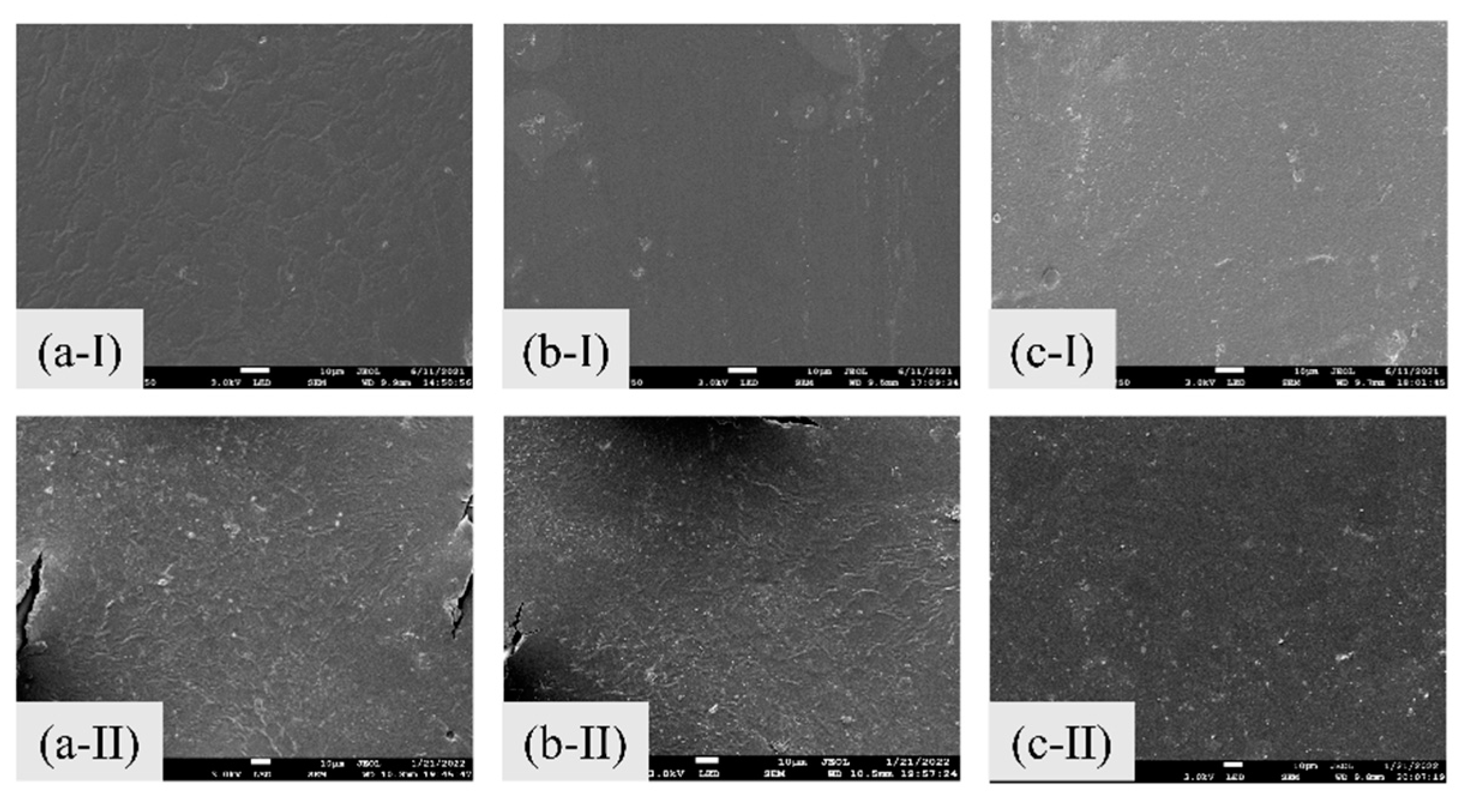
3.1.2. The Effect of Phenol Concentration on the IEMs
3.1.3. The Study of Fouling Mechanism by Soaking Membranes
3.1.4. Membrane Cleaning Exploration
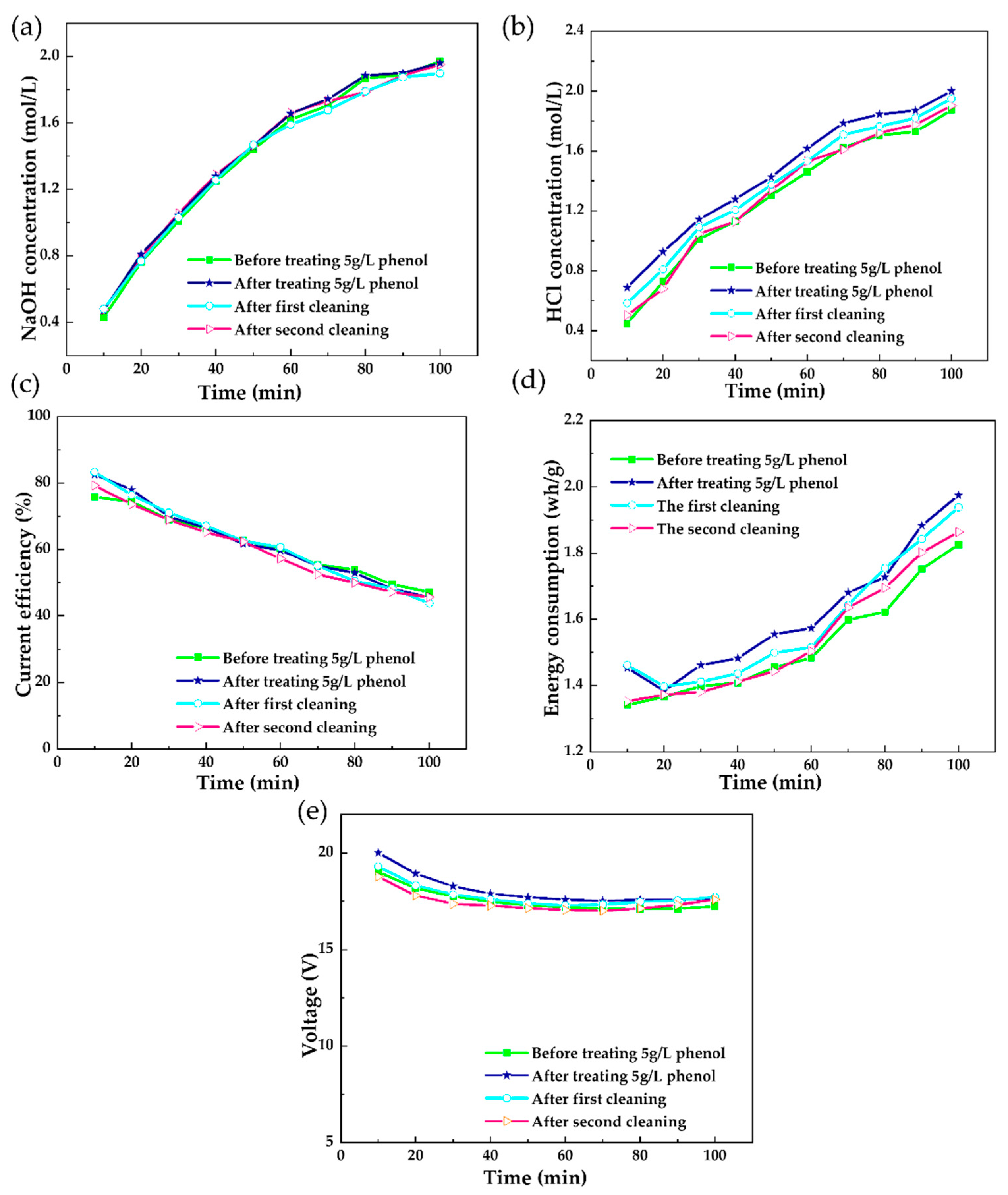
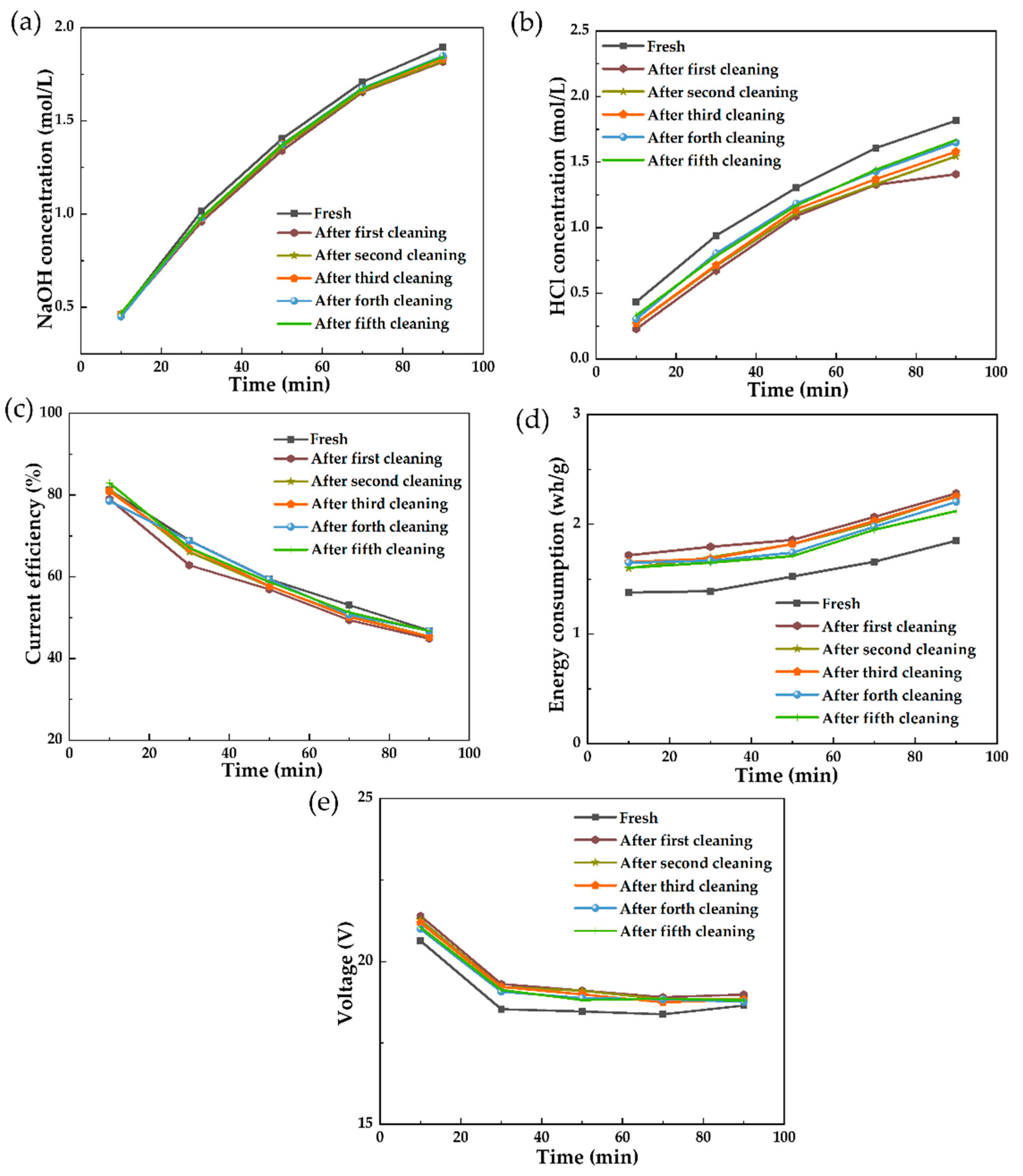
3.1.5. In Situ Chemical Cleaning on the Polluted Membrane Stack after Short-Running Processes
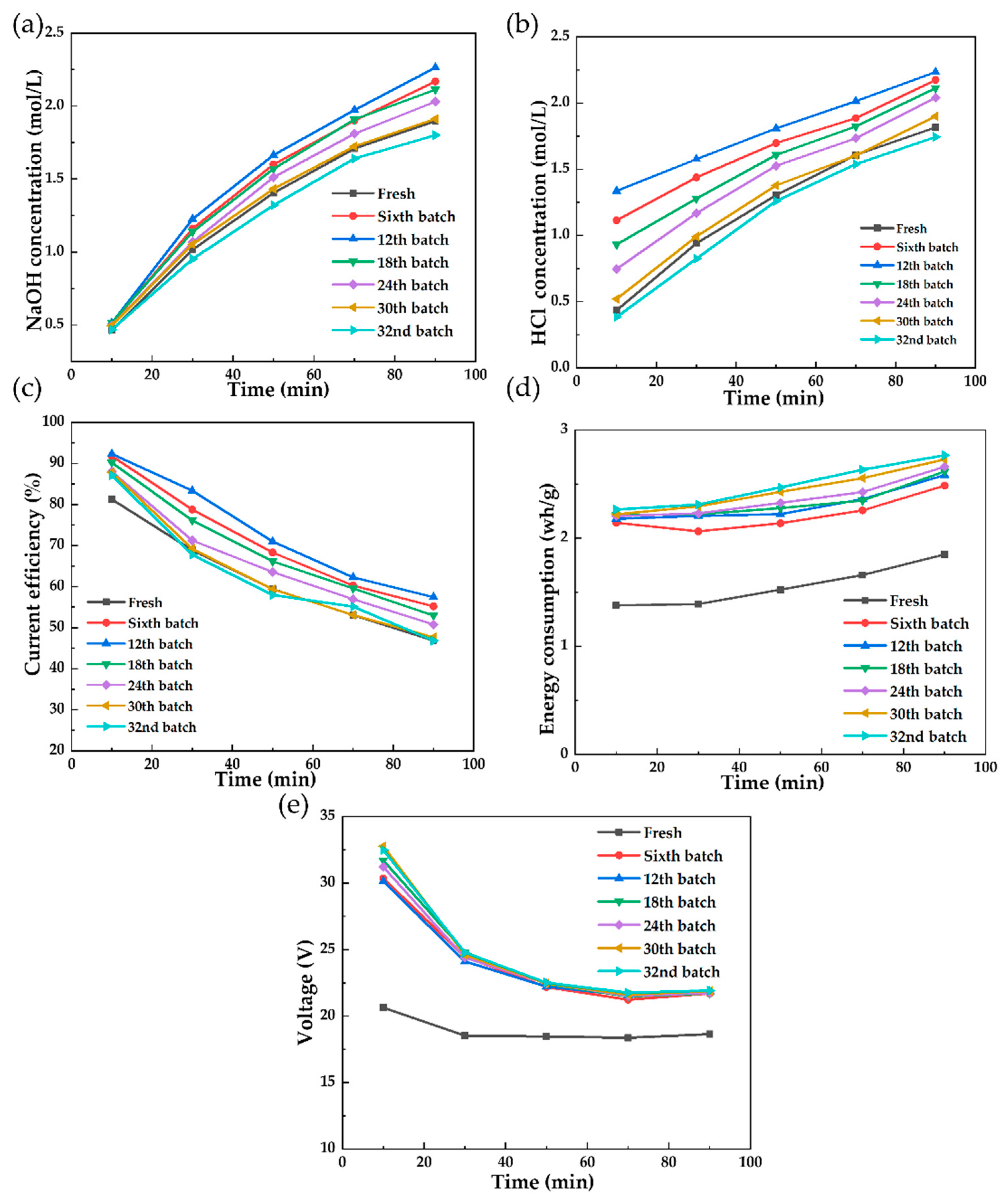
3.2. The Effect of Concentrated Phenol in 160 g/L NaCl Solution on the BMED Stack for a Long-Running Process
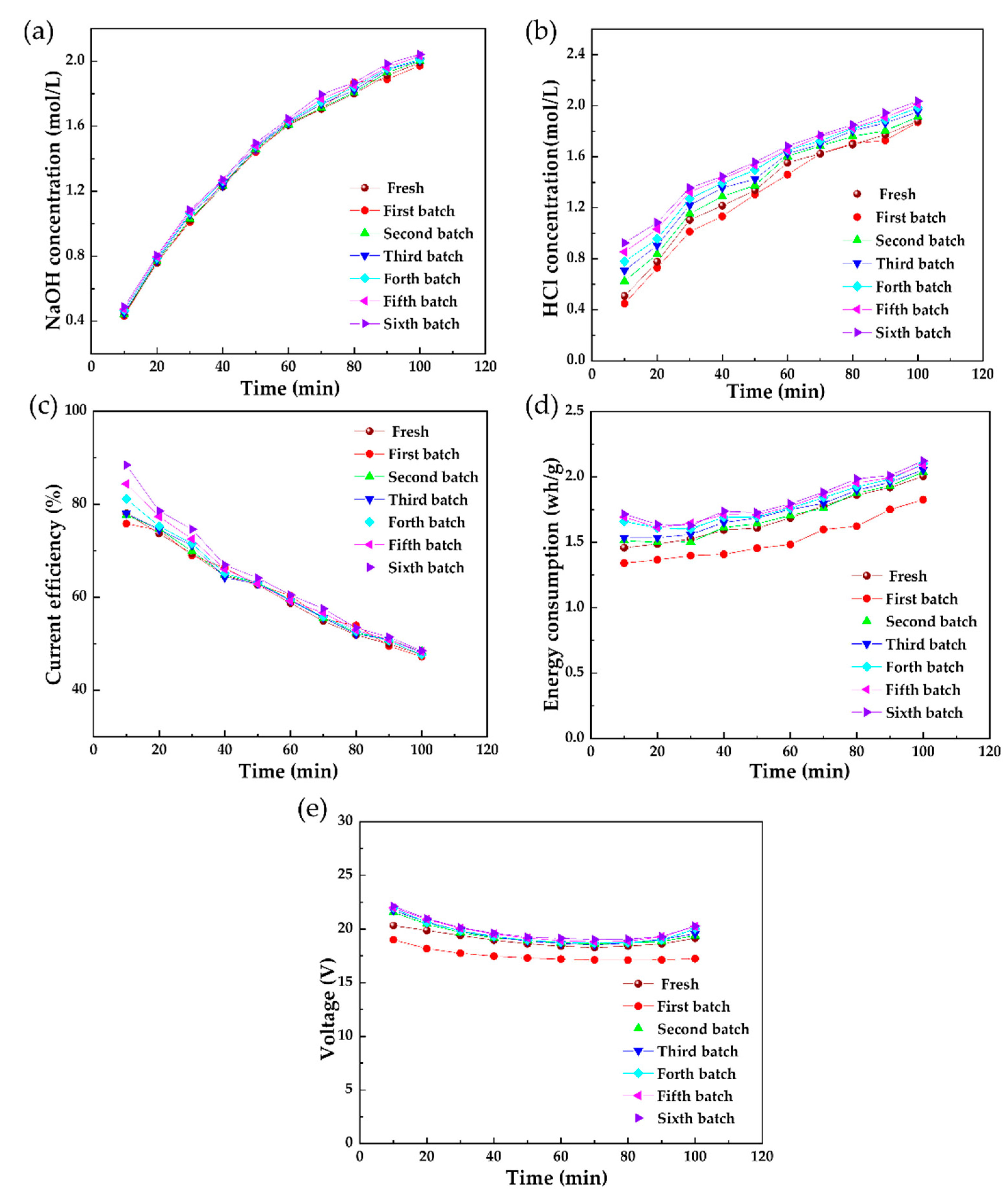
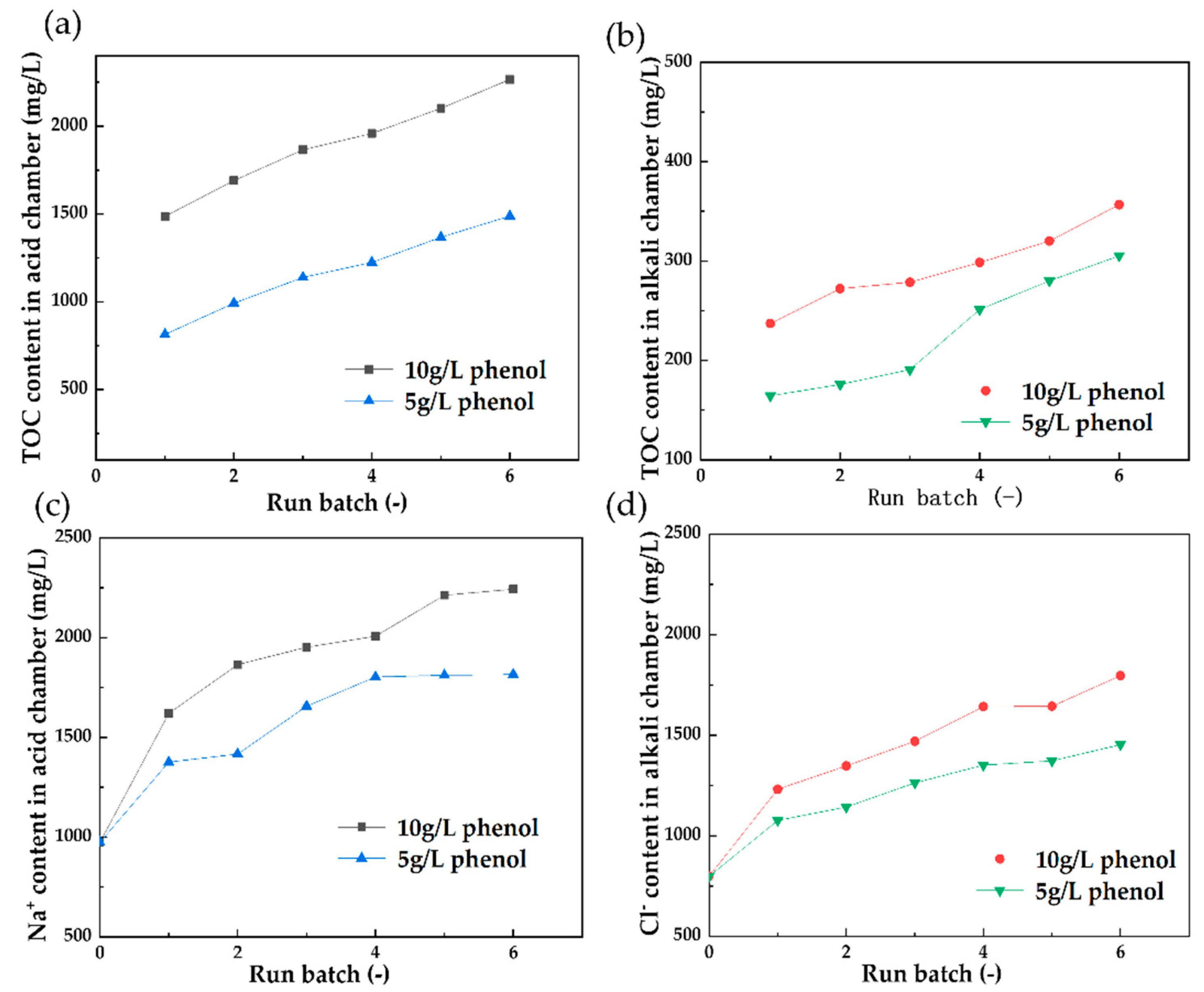
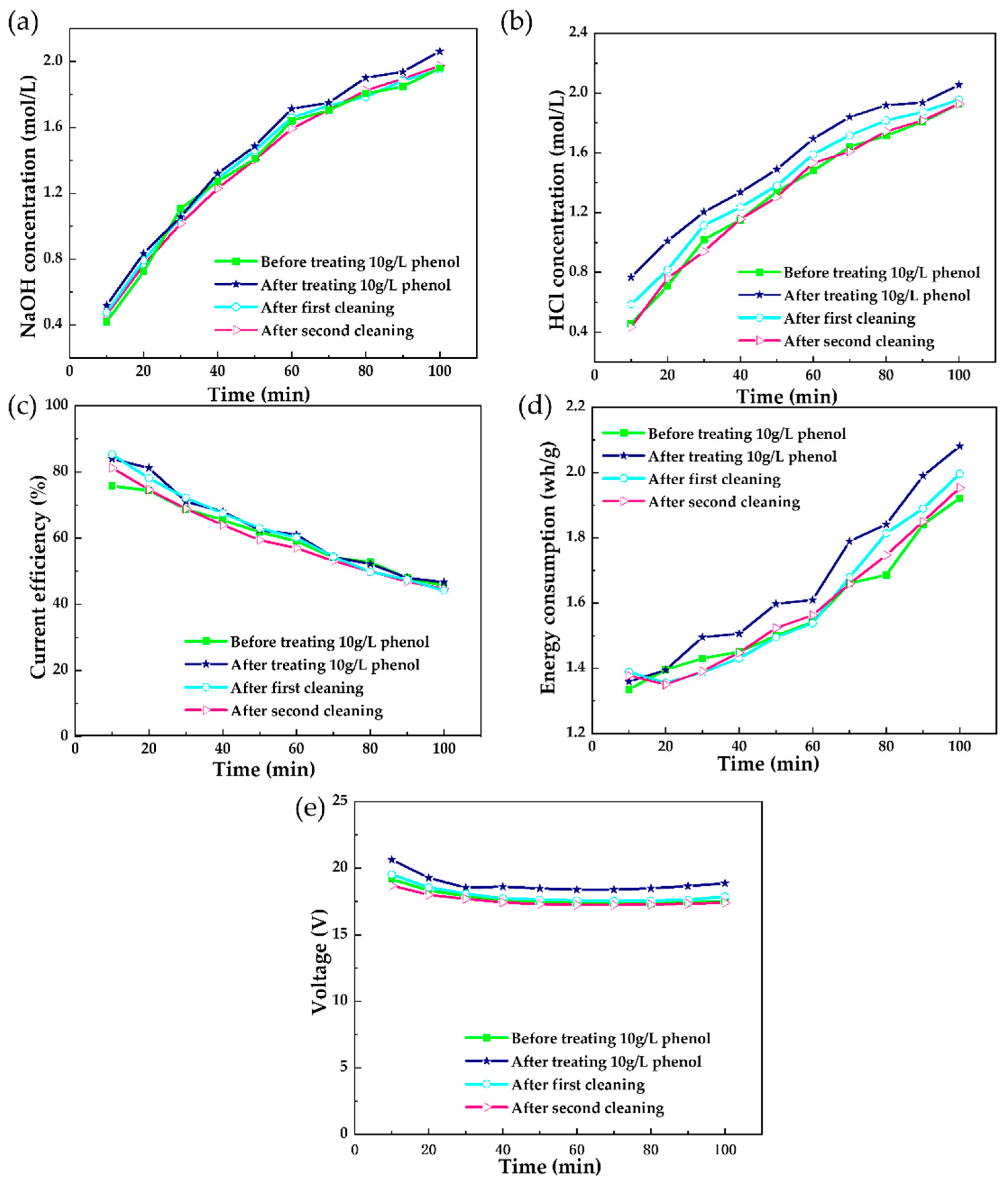
3.2.1. The Effect of 10 g/L Phenol on the BMED Performance
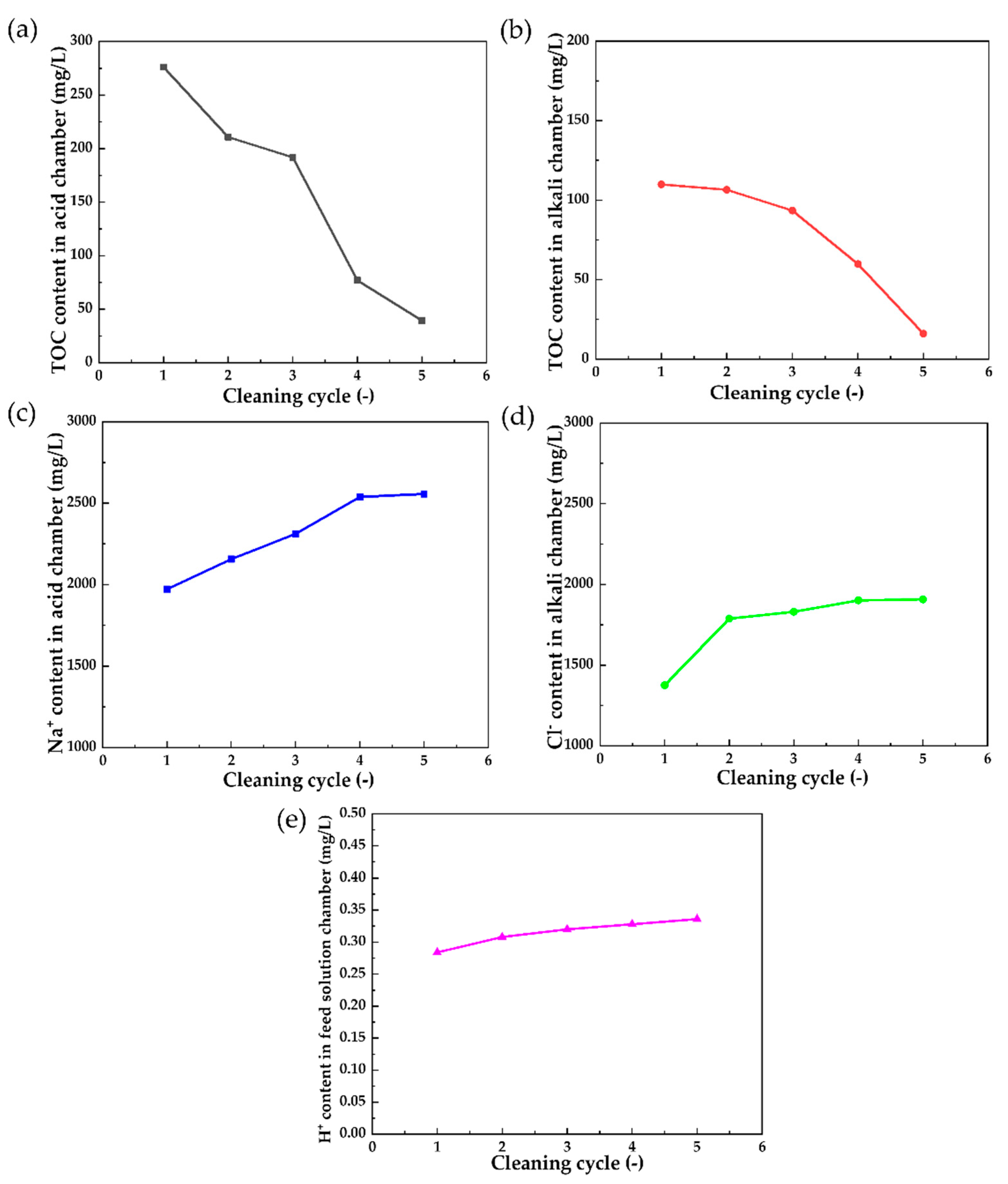
3.2.2. In Situ Chemical Cleaning on the Polluted Membrane Stack
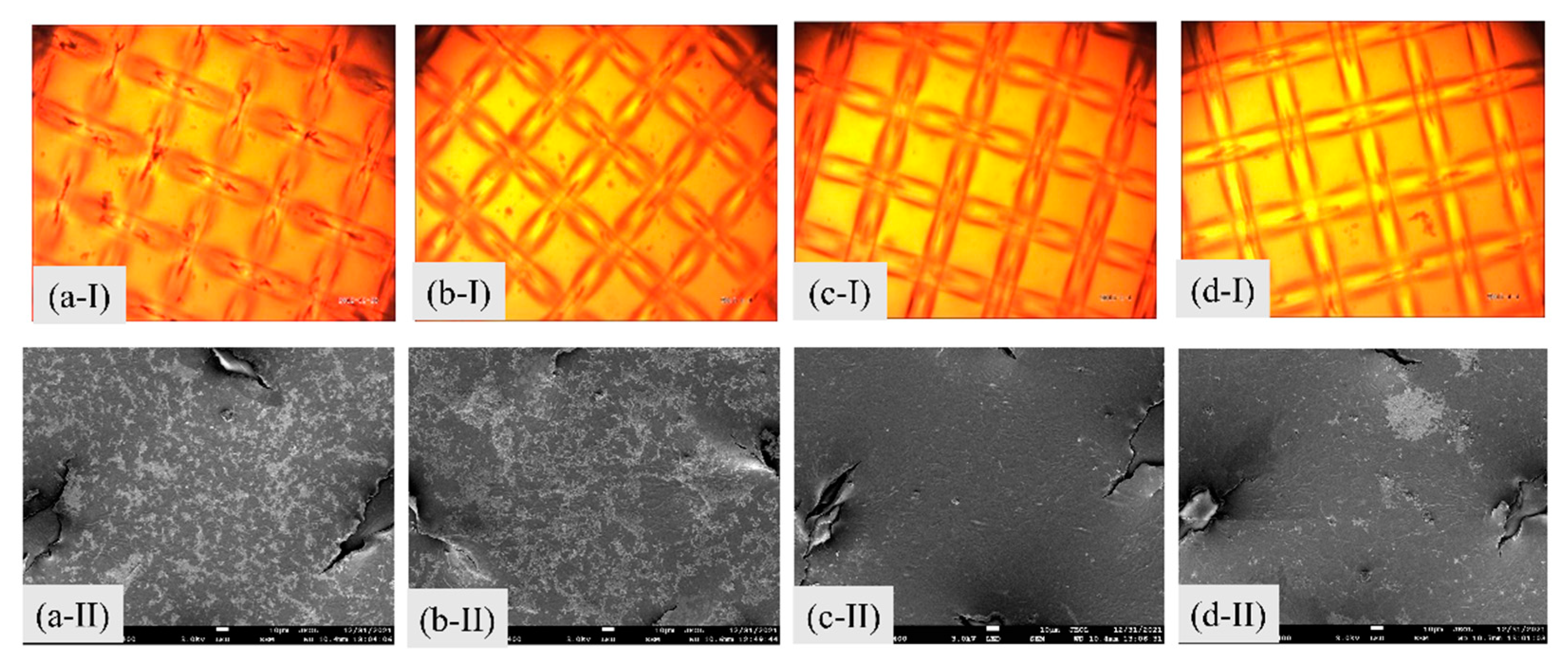
3.2.3. Analysis of the IEMs after Cleaning
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Research, P.M. Market Study on Pesticides: Popularity of Bio-Pesticides to Rise Rapidly over Coming Years. 2021. Available online: https://www.reportlinker.com/p06194129/Market-Study-on-Pesticides-Popularity-of-Bio-pesticides-to-Rise-Rapidly-Over-Coming-Years.html (accessed on 1 August 2022).
- Shan, M. China pesticide industry watch 2021: Shouldering the responsibility as ‘Manufacturing Giant’. China Pestic. Ind. Watch. 2022, 1, 26–37. [Google Scholar]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Devault, D.A.; Karolak, S. Wastewater-based epidemiology approach to assess population exposure to pesticides: A review of a pesticide pharmacokinetic dataset. Environ. Sci. Pollut. Res. 2020, 27, 4695–4702. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Gonzalez, M.; Diaz-Guridi, P.; Dominguez-Ramos, A.; Irabien, A.; Ibañez, R. Highly concentrated HCl and NaOH from brines using electrodialysis with bipolar membranes. Sep. Purif. Technol. 2020, 242, 116785. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Ahmad, T.; Rafatullah, M.; Ghazali, A.; Sulaiman, O.; Hashim, R.; Ahmad, A. Removal of pesticides from water and wastewater by different adsorbents: A review. J. Environ. Sci. Health C 2010, 28, 231–271. [Google Scholar] [CrossRef]
- Diya’uddeen, B.H.; Daud, W.M.A.W.; Abdul Aziz, A.R. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Prot. 2011, 89, 95–105. [Google Scholar] [CrossRef]
- Yuanhui, T.; Wenwen, S.; Taiyu, L.; Peng, M.; Yifan, J.; Lin, W.; Yakai, L.; Xiaolin, W. Reuse of wastewater from dicamba production by bipolar membrane electrodialysis. CIESC J. 2021, 72, 6328–6339. [Google Scholar]
- Xu, T. Electrodialysis processes with bipolar membranes (EDBM) in environmental protection—A review. Resour. Conserv. Recycl. 2002, 37, 1–22. [Google Scholar]
- Ye, W.; Huang, J.; Lin, J.; Zhang, X.; Shen, J.; Luis, P.; Van der Bruggen, B. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: Conditioning NaOH as a CO2 absorbent. Sep. Purif. Technol. 2015, 144, 206–214. [Google Scholar] [CrossRef]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of phenolic compounds and their fouling mechanisms in ion-exchange membranes used at an industrial scale for wine tartaric stabilization by electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Frilet, V.J. Preparation and characterization of bipolar ion exchange membranes. J. Phys. Chem. 1956, 60, 435–439. [Google Scholar] [CrossRef]
- Xia, M. An Innovative Beneficial Reclamation of Flue Gas Desulfurization Brine Using Bipolar Membrane Electrodialysis Technique. Int. J. Electrochem. Sci. 2018, 13, 5382–5395. [Google Scholar] [CrossRef]
- Szczygiełda, M.; Prochaska, K. Effective separation of bio-based alpha-ketoglutaric acid from post-fermentation broth using bipolar membrane electrodialysis (EDBM) and fouling analysis. Biochem. Eng. J. 2021, 166, 107883. [Google Scholar] [CrossRef]
- Ilhan, F.; Kabuk, H.A.; Kurt, U.; Avsar, Y.; Gonullu, M.T. Recovery of mixed acid and base from wastewater with bipolar membrane electrodialysis—A case study. Desalination Water Treat. 2015, 57, 5165–5173. [Google Scholar] [CrossRef]
- Wang, X.-X.; Wang, M.; Jia, Y.-X.; Yao, T.-T. The feasible study on the reclamation of the glyphosate neutralization liquor by bipolar membrane electrodialysis. Desalination 2012, 300, 58–63. [Google Scholar] [CrossRef]
- Lin, J.; Lin, F.; Chen, X.; Ye, W.; Li, X.; Zeng, H.; Van der Bruggen, B. Sustainable Management of Textile Wastewater: A Hybrid Tight Ultrafiltration/Bipolar-Membrane Electrodialysis Process for Resource Recovery and Zero Liquid Discharge. Ind. Eng. Chem. Res. 2019, 58, 11003–11012. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Mulyati, S.; Takagi, R.; Matsuyama, H. Improved antifouling of anion-exchange membrane by polydopamine coating in electrodialysis process. Desalination 2014, 332, 126–133. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Larchet, C.; Hellal, F.; Porozhnyy, M.; Nevakshenova, E.; Pismenskaya, N.; Nikonenko, V. Characterization and cleaning of anion-exchange membranes used in electrodialysis of polyphenol-containing food industry solutions; comparison with cation-exchange membranes. Sep. Purif. Technol. 2019, 210, 636–650. [Google Scholar] [CrossRef]
- Tanaka, N.; Nagase, M.; Higa, M. Organic fouling behavior of commercially available hydrocarbon-based anion-exchange membranes by various organic-fouling substances. Desalination 2012, 296, 81–86. [Google Scholar] [CrossRef]
- Lv, Y.; Yan, H.; Yang, B.; Wu, C.; Zhang, X.; Wang, X. Bipolar membrane electrodialysis for the recycling of ammonium chloride wastewater: Membrane selection and process optimization. Chem. Eng. Res. Des. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Shen, J.; Huang, J.; Liu, L.; Ye, W.; Lin, J.; Van der Bruggen, B. The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge. J. Hazard. Mater. 2013, 260, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Sarapulova, V.; Nevakshenova, E.; Nebavskaya, X.; Kozmai, A.; Aleshkina, D.; Pourcelly, G.; Nikonenko, V.; Pismenskaya, N. Characterization of bulk and surface properties of anion-exchange membranes in initial stages of fouling by red wine. J. Membr. Sci. 2018, 559, 170–182. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Effects of acid–base cleaning procedure on structure and properties of anion-exchange membranes used in electrodialysis. J. Membr. Sci. 2016, 507, 12–23. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, P.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]






| Reagent Name | Molecular Weight | Purity | Provider |
|---|---|---|---|
| Sodium chloride | 58.5 | AR | Shanghai Titan Scientific Co., Ltd., Shanghai, China |
| Phenol (PhOH) | 94.11 | AR | Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China |
| Sodium hydroxide | 40 | AR | Shanghai Titan Scientific Co., Ltd., Shanghai, China |
| Sodium carbonate | 105.99 | AR | Shanghai Titan Scientific Co., Ltd., Shanghai, China |
| Hydrogen potassium phthalate | 204.22 | AR | Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China |
| Phenolphthalein | 318.32 | AR | Shanghai Meryer Chemical Technology Co., Ltd., Shanghai, China |
| Methyl orange | 327.33 | AR | Shanghai Meryer Chemical Technology Co., Ltd., Shanghai, China |
| Hydrochloric acid | 36.5 | AR | Beijing Modern Oriental Fine Chemicals Co., Ltd., Beijing, China |
| Number | Composition of the Feed Solution | Operation Parameters | ||
|---|---|---|---|---|
| NaCl (g/L) | Phenol (g/L) | Current Density (mA/cm2) | Initial Acid/Alkali Compartment Concentration (mol/L) | |
| 1 | 160 | 1 | 70 | 0.075 |
| 2 | 160 | 5 | 70 | 0.075 |
| 3 | 160 | 10 | 70 | 0.075 |
| Membrane | Carbon Content (wt.%) | Oxygen Content (wt.%) |
|---|---|---|
| AEM (fresh) | 94.14 | 2.5 |
| AEM (polluted) | 87.83 | 8.52 |
| CEM (fresh) | 66.38 | 17.91 |
| CEM (polluted) | 63.72 | 19.99 |
| The smooth side of BPM (fresh) | 94.40 | 2.59 |
| The smooth side of BPM (polluted) | 93.49 | 3.82 |
| The rough side of BPM (fresh) | 66.38 | 15.53 |
| The rough side of BPM (polluted) | 65.86 | 15.20 |
| Membrane | Carbon Content (wt.%) | Oxygen Content (wt.%) |
|---|---|---|
| The surface of AEM soaked in water | 85.06 | 3.34 |
| The surface of AEM soaked in 2 mol/L HCl solution | 85.54 | 3.26 |
| The surface of AEM soaked in 2 mol/L NaOH solution | 85.87 | 3.59 |
| The surface of AEM soaked in 10 g/L phenol solution | 81.23 | 9.93 |
| The cross-section of AEM soaked in water | 84.24 | 5.17 |
| The cross-section of AEM soaked in 10 g/L phenol solution | 83.82 | 8.66 |
| Membrane | Carbon Content (wt.%) | Oxygen Content (wt.%) |
|---|---|---|
| The surface of AEM soaked in water | 81.30 | 9.66 |
| The surface of AEM soaked in 0.1 wt.% HCl solution | 82.84 | 8.69 |
| The surface of AEM soaked in 0.1 wt.% NaOH solution | 83.17 | 4.33 |
| The surface of AEM soaked in 1 wt.% NaOH solution | 82.58 | 6.91 |
| The cross-section of AEM soaked in water | 82.82 | 8.56 |
| The cross-section of AEM soaked in 0.1 wt.% HCl solution | 82.75 | 8.54 |
| The cross-section of AEM soaked in 0.1 wt.% NaOH solution | 83.26 | 6.12 |
| The cross-section of AEM soaked in 1 wt.% NaOH solution | 83.07 | 7.08 |
| Membrane | Carbon Content (wt.%) | Oxygen Content (wt.%) |
|---|---|---|
| The surface of AEM (fresh) | 94.14 | 2.5 |
| The surface of AEM (after cleaning) | 93.68 | 2.63 |
| The surface of CEM (fresh) | 66.38 | 17.91 |
| the surface of CEM (after cleaning) | 65.65 | 18.11 |
| The smooth side of BPM (fresh) | 94.40 | 2.59 |
| The smooth side of BPM (after cleaning) | 93.41 | 3.3 |
| The cross-section of AEM (fresh) | 75.99 | 13.57 |
| The cross-section of AEM (after cleaning) | 76.51 | 13.82 |
| The cross-section of CEM (fresh) | 79.45 | 12.78 |
| The cross-section of CEM (after cleaning) | 78.17 | 13.05 |
| The cross-section of the smooth side of BPM (fresh) | 83.06 | 9.63 |
| The cross-section of the smooth side of BPM (after cleaning) | 82.84 | 10.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Sun, W.; Lin, Y.; Wang, L.; Chen, H.; Wu, H.; Yu, L.; Wang, X. Impact of Phenol on Membranes during Bipolar Membrane Electrodialysis for High Salinity Pesticide Wastewater Treatment. Separations 2022, 9, 241. https://doi.org/10.3390/separations9090241
Tang Y, Sun W, Lin Y, Wang L, Chen H, Wu H, Yu L, Wang X. Impact of Phenol on Membranes during Bipolar Membrane Electrodialysis for High Salinity Pesticide Wastewater Treatment. Separations. 2022; 9(9):241. https://doi.org/10.3390/separations9090241
Chicago/Turabian StyleTang, Yuanhui, Wenwen Sun, Yakai Lin, Lin Wang, Hedi Chen, Huanhuan Wu, Lixin Yu, and Xiaolin Wang. 2022. "Impact of Phenol on Membranes during Bipolar Membrane Electrodialysis for High Salinity Pesticide Wastewater Treatment" Separations 9, no. 9: 241. https://doi.org/10.3390/separations9090241
APA StyleTang, Y., Sun, W., Lin, Y., Wang, L., Chen, H., Wu, H., Yu, L., & Wang, X. (2022). Impact of Phenol on Membranes during Bipolar Membrane Electrodialysis for High Salinity Pesticide Wastewater Treatment. Separations, 9(9), 241. https://doi.org/10.3390/separations9090241





