Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Myconoside Extraction
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Fluorescent Staining of F-Actin and Zonula Occludens (ZO-1)
2.5. Fluorescent Cell Staining for Spectroscopy and Microscopy Experiments
3. Results
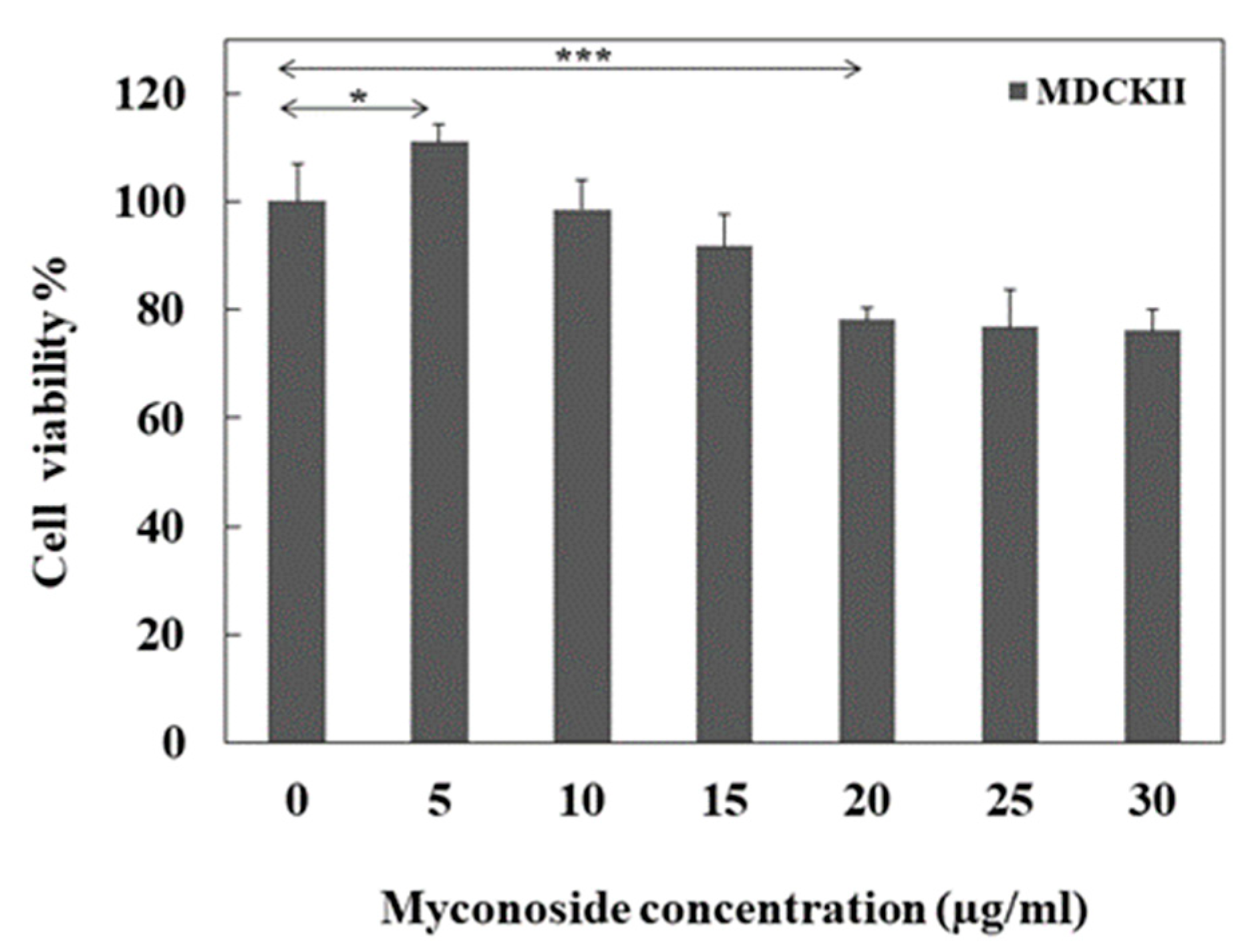
3.1. Cell Viability
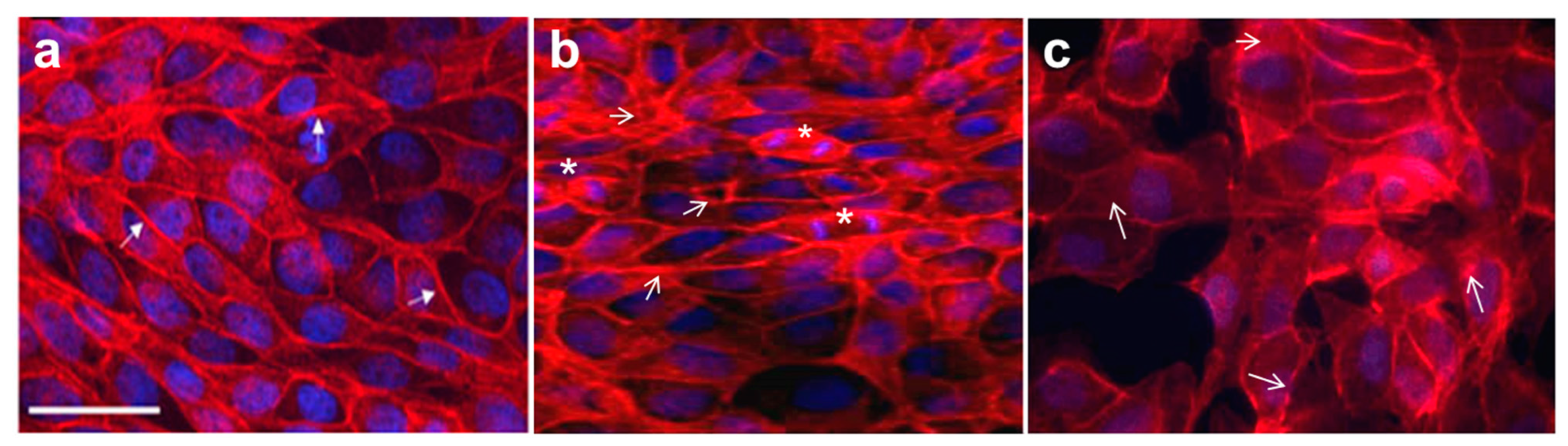
3.2. Actin Filament Cytoskeleton
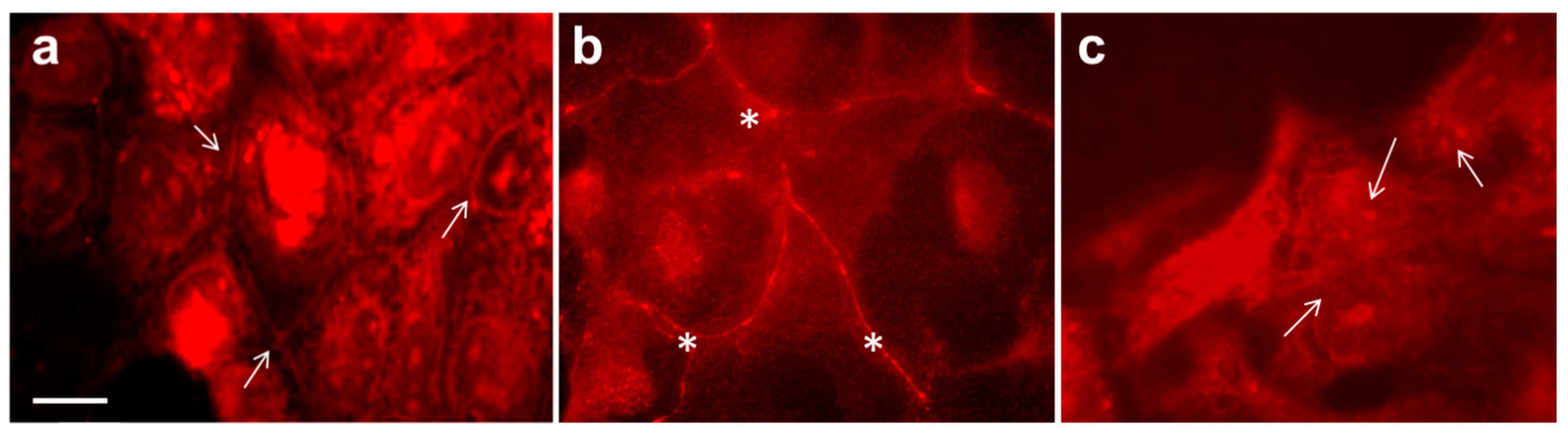
3.3. Zonula Occludens (ZO-1) Protein
3.4. Plasma Membrane Lipid Order
3.4.1. Visualization of Lipid Packing by di-4-ANEPPDHQ Fluorescence Microscopy
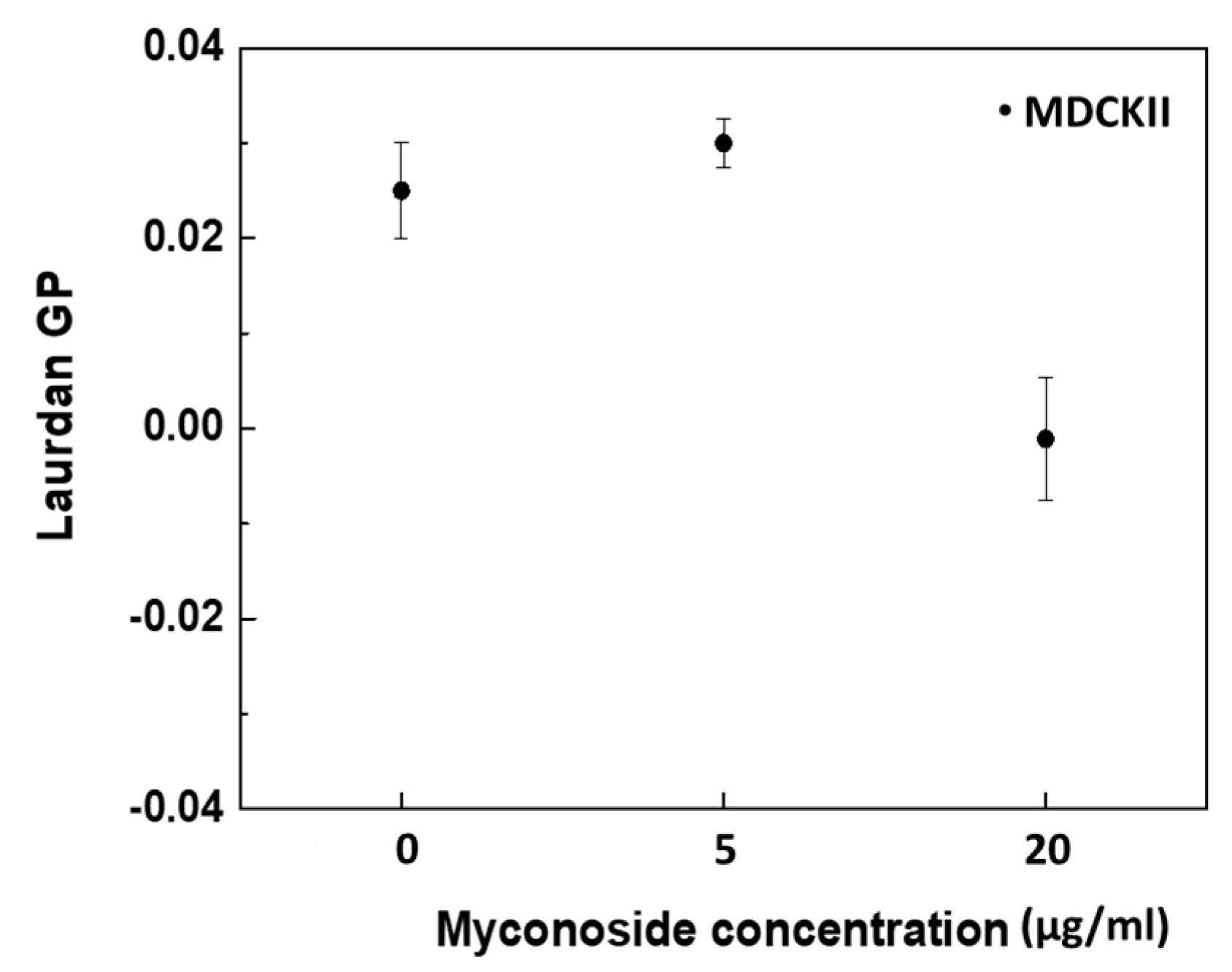
3.4.2. Membrane Order Measurements by Laurdan Fluorescence Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval
References
- Gechev, T.S.; Hille, J.; Woerdenbag, H.J.; Benina, M.; Mehterov, N.; Toneva, V.; Fernie, A.R.; Mueller-Roeber, B. Natural products from resurrection plants: Potential for medical applications. Biotechnol. Adv. 2014, 32, 1091–1101. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Ognyanov, M.H.; Denev, P.N. The ancient Thracian endemic plant Haberlea rhodopensis Friv. and related species: A review. J. Ethnopharmacol. 2020, 249, 112359. [Google Scholar] [CrossRef]
- Todorova, R.; Atanasov, A. Haberlea rhodopensis: Pharmaceutical and medical potential as a food additive. Nat. Prod. Res. 2015, 30, 1–23. [Google Scholar] [CrossRef]
- Dell’Acqua, G.; Schweikert, K. Skin benefits of a myconoside-rich extract from resurrection plant Haberlea rhodopensis. Int. J. Cosmet. Sci. 2012, 34, 132–139. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Mitcheva, M. Cytoprotective and antioxidant effects of phenolic compounds from Haberlea rhodopensis Friv. (Gesneriaceae). Pharmacogn. Mag. 2013, 9, 294–301. [Google Scholar] [CrossRef]
- Mihaylova, D.; Bahchevanska, S.; Toneva, V. Examination of the antioxidant activity of Haberlea Rhodopensis leaf extracts and their phenolic constituents. J. Food Biochem. 2013, 37, 255–261. [Google Scholar] [CrossRef]
- Popov, B.; Georgieva, S.; Gadjeva, V. Modulatory effects of total extract of Haberlea rhodopensis against the cyclophosphamide induced genotoxicity in rabbit lymphocytes in vivo. Trakia J. Sci. 2011, 9, 51–57. [Google Scholar]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Yamazaki, S.; Iwama, A.; Takayanagi, S.; Morita, Y.; Eto, K.; Ema, H.; Nakauchi, H. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006, 25, 3515–3523. [Google Scholar] [CrossRef]
- Chichili, G.R.; Rodgers, W. Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mol. Life Sci. 2009, 66, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, A.; Hazarosova, R.; Topouzova-Hristova, T.; Moyankova, D.; Yordanova, V.; Veleva, R.; Nikolova, B.; Momchilova, A.; Djilianov, D.; Staneva, G. Myconoside interacts with the plasma membranes and the actin cytoskeleton and provokes cytotoxicity in human lung adenocarcinoma A549 cells. J. Bioenerg. Biomembr. 2022, 54, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Moyankova, D.; Mladenov, P.; Berkov, S.; Peshev, D.; Georgieva, D.; Djilianov, D. Metabolic profiling of the resurrection plant Haberlea rhodopensis during desiccation and recovery. Physiol. Plant. 2014, 152, 675–687. [Google Scholar] [CrossRef]
- Saotome, K.; Morita, H.; Umeda, M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol Vitro 1989, 3, 317–321. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef]
- Maioli, E.; Torricelli, C.; Fortino, V.; Carlucci, F.; Tommassini, V.; Pacini, A. Critical Appraisal of the MTT Assay in the Presence of Rottlerin and Uncouplers. Biol. Proced. Online 2009, 11, 229. [Google Scholar] [CrossRef]
- Kostadinova, A.; Doumanov, J.; Moyankova, D.; Ivanov, S.; Mladenova, K.; Djilianov, D.; Topouzova-Hristova, T. Haberlea rhodopensis extracts affect cell periphery of keratinocytes. Comptes Rendus L’académie Bulg. Sci. 2016, 69, 439. [Google Scholar]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative imaging of membrane lipid order in cells and organisms. Nat. Protoc. 2011, 7, 24–35. [Google Scholar] [CrossRef]
- Butler, C.E.; Wheeler, G.; Graham, J.; Tyler, K.M. Visual Discrimination of Membrane Domains in Live Cells by Widefield Microscopy. In Fluorescent Methods to Study Biological Membranes; Mély, Y., Duportail, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 163–184. [Google Scholar]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Miyoshi, J.; Takai, Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug Deliv. Rev. 2005, 57, 815–855. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Millard, A.C.; Wuskell, J.P.; Clark, H.A.; Loew, L.M. Cholesterol-enriched lipid domains can be visualized by di-4-ANEPPDHQ with linear and nonlinear optics. Biophys. J. 2005, 89, L04–L06. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Conti, F.; Gratton, E. Time-resolved fluorescence emission spectra of Laurdan in phospholipid vesicles by multifrequency phase and modulation fluorometry. Cell. Mol. Biol. 1986, 1, 103–108. [Google Scholar]
- Amaro, M.; Reina, F.; Hof, M.; Eggeling, C.; Sezgin, E. Laurdan and di-4-ANEPPDHQ probe different properties of the membrane. J. Phys. Appl. Phys. 2017, 50, 134004. [Google Scholar] [CrossRef] [PubMed]
- Dilberger, B.; Weppler, S.; Eckert, G.P. Phenolic acid metabolites of polyphenols act as inductors for hormesis in C. elegans. Mech. Ageing Dev. 2021, 198, 111518. [Google Scholar] [CrossRef]
- Milackova, I.; Rackova, L.; Majekova, M.; Mrvova, N.; Stefek, M. Protection or cytotoxicity mediated by a novel quinonoid-polyphenol compound? Gen. Physiol. Biophys. 2015, 34, 51–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Mattson, M.P.; Rattan, S.I. Curcumin and hormesis with particular emphasis on neural cells. Food Chem. Toxicol. 2019, 129, 399–404. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Calabrese, V.; Tsatsakis, A.; Giordano, J.J. Hormesis and Ginkgo biloba (GB): Numerous biological effects of GB are mediated via hormesis. Aging Res. Rev. 2020, 64, 101019. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects. Molecules 2020, 25, 2719. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Tsatsakis, A.; Agathokleous, E.; Giordano, J.; Calabrese, V. Does Green Tea Induce Hormesis? Dose Response 2020, 18, 1559325820936170. [Google Scholar] [CrossRef]
- Calabrese, E.J. Cancer biology and hormesis: Human tumor cell lines commonly display hermetic (biphasic) dose responses. Crit. Rev. Toxicol. 2005, 35, 463–582. [Google Scholar] [CrossRef] [PubMed]
- Aoishi, Y.; Yoshimasu, T.; Oura, S.; Kawago, M.; Hirai, Y.; Miyasaka, M.; Ohashi, T.; Nishimura, Y. Quantitative Evaluation of Hormesis in Breast Cancer Using Histoculture Drug Response Assay. Dose Response 2019, 17, 1559325819896183. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J.; Kleinfeld, A.M.; Hoover, R.L.; Dawidowicz, E.A.; McIntyre, D.E.; Salzman, E.A.; Klausner, R.D. Lipid domains in membranes. Ann. N. Y. Acad. Sci. 1982, 401, 61–75. [Google Scholar] [CrossRef]
- Pakiet, A.; Kobiela, J. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- Pike, L.J. The challenge of lipid rafts. J. Lipid Res. 2009, 50, S323–S328. [Google Scholar] [CrossRef]
- Cereijido, M.; Robbins, E.S.; Dolan, W.J.; Rotunno, C.A.; Sabatini, D.D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 1978, 77, 853–880. [Google Scholar] [CrossRef]
- Roignot, J.; Peng, X.; Mostov, K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb. Perspect. Biol. 2013, 5, a013789. [Google Scholar] [CrossRef]
- Ikenouchi, J. Roles of membrane lipids in the organization of epithelial cells: Old and new problems. Tissue Barriers 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Yang, B.; Oo, T.N.; Rizzo, V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006, 20, 1501–1503. [Google Scholar] [CrossRef]
- Yeagle, P. Biology of Cholesterol; Philip, L., Yeagle, P., Eds.; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Song, Y.; Kenworthy, A.K.; Sanders, C.R. Cholesterol as a co-solvent and a ligand for membrane proteins. Protein Sci. 2014, 23, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hoejholt, K.L.; Mužić, T. Calcium electroporation and electrochemotherapy for cancer treatment: Importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 2019, 9, 4758. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Yarrarapu, S.N.S.; Dimri, M. Biochemistry, Cholesterol; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 4, 343–346. [Google Scholar] [CrossRef]
- Bagnat, M.; Simons, K. Lipid rafts in protein sorting and cell polarity in budding yeast Saccharomyces cerevisiae. Biol. Chem. 2002, 383, 1475–1480. [Google Scholar] [CrossRef]
- Murai, T. The role of lipid rafts in cancer cell adhesion and migration. Int. J. Cell Biol. 2012, 2012, 763283. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Liu, A.P.; Fletcher, D.A. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys. J. 2006, 91, 4064–4070. [Google Scholar] [CrossRef]
- Head, B.P.; Patel, H.H.; Roth, D.M.; Murray, F.; Swaney, J.S.; Niesman, I.R.; Farquhar, M.G.; Insel, P.A. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J. Biol. Chem. 2006, 281, 26391–26399. [Google Scholar] [CrossRef]
- Forstner, M.B.; Yee, C.K.; Parikh, A.N.; Groves, J.T. Lipid lateral mobility and membrane phase structure modulation by protein binding. J. Am. Chem. Soc. 2006, 128, 15221–15227. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Gupta, N. Tether and trap: Regulation of membrane-raft dynamics by actin-binding proteins. Nat. Rev. Immunol. 2007, 7, 889–896. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostadinova, A.; Staneva, G.; Topouzova-Hristova, T.; Moyankova, D.; Yordanova, V.; Veleva, R.; Nikolova, B.; Momchilova, A.; Djilianov, D.; Hazarosova, R. Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes. Separations 2022, 9, 239. https://doi.org/10.3390/separations9090239
Kostadinova A, Staneva G, Topouzova-Hristova T, Moyankova D, Yordanova V, Veleva R, Nikolova B, Momchilova A, Djilianov D, Hazarosova R. Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes. Separations. 2022; 9(9):239. https://doi.org/10.3390/separations9090239
Chicago/Turabian StyleKostadinova, Aneliya, Galya Staneva, Tanya Topouzova-Hristova, Daniela Moyankova, Vesela Yordanova, Ralitsa Veleva, Biliana Nikolova, Albena Momchilova, Dimitar Djilianov, and Rusina Hazarosova. 2022. "Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes" Separations 9, no. 9: 239. https://doi.org/10.3390/separations9090239
APA StyleKostadinova, A., Staneva, G., Topouzova-Hristova, T., Moyankova, D., Yordanova, V., Veleva, R., Nikolova, B., Momchilova, A., Djilianov, D., & Hazarosova, R. (2022). Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes. Separations, 9(9), 239. https://doi.org/10.3390/separations9090239







