Aqueous Extracts of Fish Roe as a Source of Several Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Standard Solutions
2.3. Aqueous Extracts of Fish Roe
2.4. Chromatographic Analysis
2.4.1. Equipment
2.4.2. Chromatographic Conditions and Analyses
2.4.3. Method Validation
3. Results and Discussion
3.1. Analytical Method for Screening and Quantification
3.2. Analysis of the Roe-Derived Aqueous Extracts Samples from the Three Fish Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domingo, J.L. Nutrients and Chemical Pollutants in Fish and Shellfish. Balancing Health Benefits and Risks of Regular Fish Consumption. Crit. Rev. Food Sci. Nutr. 2016, 56, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Tilami, S.K.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Bledsoe, G.E.; Bledsoe, C.D.; Rasco, B. Caviars and fish roe products. Crit. Rev. Food Sci. Nutr. 2003, 43, 317–356. [Google Scholar] [CrossRef]
- Arbeloa, E.M.; Uez, M.J.; Bertolotti, S.G.; Churio, M.S. Antioxidant activity of gadusol and occurrence in fish roes from Argentine Sea. Food Chem. 2010, 119, 586–591. [Google Scholar] [CrossRef]
- Guedes, M.; Costa-Pinto, A.R.; Gonçalves, V.M.F.; Moreira-Silva, J.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Sardine Roe as a Source of Lipids to Produce Liposomes. ACS Biomater. Sci. Eng. 2020, 6, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Mikellidi, A.; Nomikos, T.; Chiou, A. Screening of macro- and bioactive microconstituents of commercial finfish and sea urchin eggs. LWT Food Sci. Technol. 2012, 46, 525–531. [Google Scholar] [CrossRef]
- Rosa, A.; Scano, P.; Atzeri, A.; Deiana, M.; Falchi, A.M. Potential anti-tumor effects of Mugil cephalus processed roe extracts on colon cancer cells. Food Chem. Toxicol. 2013, 60, 471–478. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Bhaskarachary, K.; Vajreswari, A.; Kumar, R.R.; Kumar, B.D. Chemical composition and immunomodulatory effects of enzymatic protein hydrolysates from common carp (Cyprinus carpio) egg. Nutrition 2015, 31, 388–398. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Kumar, B.D. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef] [Green Version]
- Yoon, I.S.; Lee, H.J.; Kang, S.I.; Park, S.Y.; Kang, Y.M.; Kim, J.-S.; Heu, M.S. Food functionality of protein isolates extracted from Yellowfin Tuna (Thunnus albacares) roe using alkaline solubilization and acid precipitation process. Food Sci. Nutr. 2019, 7, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Shirai, N.; Suzuki, H. Effects of dietary herring roe lipids on plasma lipid, glucose, insulin, and adiponectin concentrations in mice. J. Agric. Food Chem. 2006, 54, 3750–3755. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Shirai, N.; Higuchi, T.; Suzuki, H. Effect of lipids extracted from a salted herring roe food product on maze-behavior in mice. J. Nutr. Sci. Vitaminol. 2006, 52, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease; Elsevier Science Ltd.: London, UK, 2016; pp. 35–58. [Google Scholar]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W. Chemical compositions of the roes from skipjack, tongol and bonito. Food Chem. 2011, 124, 1328–1334. [Google Scholar] [CrossRef]
- EUMOFA. The EU Fish Market. 2018. Available online: https://www.eumofa.eu/documents/20178/132648/EN_The+EU+fish+market+2018.pdf (accessed on 10 October 2019).
- Caponio, F.; Lestingi, A.; Summo, C.; Bilancia, M.T.; Laudadio, V. Chemical characteristics and lipid fraction quality of sardines (Sardina pilchardus W.): Influence of sex and length. J. Appl. Ichthyol. 2004, 20, 530–535. [Google Scholar] [CrossRef]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M. Seasonal variation in the chemical composition of horse-mackerel (Trachurus trachurus). Eur. Food Res. Technol. 2001, 212, 535–539. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Zubcov, E.; Shahidi, F.; Alexis, M. Differentiation of cultured and wild sea bass (Dicentrarchus labrax): Total lipid content, fatty acid and trace mineral composition. Food Chem. 2002, 79, 145–150. [Google Scholar] [CrossRef]
- Perrone, D.; Monteiro, M.; Castelo-Branco, V.N. The Chemistry of Imidazole Dipeptides. Imidazole Dipeptides: Chemistry, Analysis, Function and Effects 2015, Volume 8, 43–60. [Google Scholar]
- ICH Steering Committee; European Agency for the Evaluation of Medicinal Products; ICOH. Q2B Validation of Analytical Procedures: Methodology. 1996. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2b-validation-analytical-procedures-methodology (accessed on 10 October 2019).
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)-a powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [Green Version]
- Hamed, R.R.; Saleh, N.S.M.; Shokeer, A.; Guneidy, R.A.; Abdel-Ghany, S.S. Glutathione and its related enzymes in the gonad of Nile Tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2016, 42, 353–364. [Google Scholar] [CrossRef]
- Krizek, M.; Vacha, F.; Pelikanova, T. Biogenic amines in carp roe (Cyprinus carpio) preserved by four different methods. Food Chem. 2011, 126, 1493–1497. [Google Scholar] [CrossRef]
- Mori, M.; Mizuno, D.; Konoha-Mizuno, K.; Sadakane, Y.; Kawahara, M. Quantitative analysis of carnosine and anserine in foods by performing high performance liquid chromatography. Biomed. Res. Trace Elem. 2015, 26, 147–152. [Google Scholar]
- Chen, Z.; Chen, B.; Yao, S.Z. High-performance liquid chromatography/electrospray ionization-mass spectrometry for simultaneous determination of taurine and 10 water-soluble vitamins in multivitamin tablets. Anal. Chim. Acta 2006, 569, 169–175. [Google Scholar] [CrossRef]
- De la Torre, M.P.D.; Priego-Capote, F.; de Castro, M.D.L. Tentative identification of polar and mid-polar compounds in extracts from wine lees by liquid chromatography-tandem mass spectrometry in high-resolution mode. J. Mass Spectrom. 2015, 50, 826–837. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Wang, X.; Li, H.M.; Yang, B.; Yang, H.J.; Huang, L.Q. Characterization of Nucleosides and Nucleobases in Natural Cordyceps by HILIC-ESI/TOF/MS and HILIC-ESI/MS. Molecules 2013, 18, 9755–9769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedes, M.; Vieira, S.F.; Reis, R.L.; Ferreira, H.; Neves, N.M. Fishroesomes as carriers with antioxidant and anti-inflammatory bioactivities. Biomed. Pharmacother. 2021, 140, 111680. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bandarra, N.M.; Calhau, M.A.; Oliveira, L.; Ramos, M.; Dias, M.G.; Bártolo, H.; Faria, M.R.; Fonseca, M.C.; Gonçalves, J.B.; Nunes, I.M.L. Composição e valor nutricional dos produtos da pesca mais consumidos em Portugal. IPIMAR 2005. [Google Scholar]
- Baki, B.; Gonener, S.; Kaya, D. Comparison of Food, Amino Acid and Fatty Acid Compositions of Wild and Cultivated Sea Bass (Dicentrarchus labrax L., 1758). Turk. J. Fish. Aquat. Sci. 2015, 15, 175–179. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.; Mæhre, H.; Jensen, I.; Elvevoll, E.O. Taurine Content in Marine Foods: Beneficial Health Effects. In Bioactive Compounds from Marine Foods; Herrero, M., Hernandez-Ledesma, B., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2014. [Google Scholar]
- Chatterjee, N.S.; Kumar, K.A.; Ajeeshkumar, K.K.; Kumari, K.R.R.; Vishnu, K.V.; Anandan, R.; Mathew, S.; Ravishankar, C.N. Screening Natural Content of Water-Soluble B Vitamins in Fish: Enzymatic Extraction, HILIC Separation, and Tandem Mass Spectrometric Determination. J. AOAC Int. 2017, 100, 579–585. [Google Scholar] [CrossRef]
- Ball, G. Vitamins In Foods; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Santos, J.; Mendiola, J.A.; Oliveira, M.B.; Ibanez, E.; Herrero, M. Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J. Chromatogr. A 2012, 1261, 179–188. [Google Scholar] [CrossRef]
- Li, T.T.; Ren, L.K.; Wang, D.F.; Song, M.J.; Li, Q.Y.; Li, J.R. Optimization of extraction conditions and determination of purine content in marine fish during boiling. Peer J. 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wamser, M.N.; Leite, E.F.; Ferreira, V.V.; Delwing-de Lima, D.; da Cruz, J.G.P.; Wyse, A.T.S.; Delwing-Dal Magro, D. Effect of hypoxanthine, antioxidants and allopurinol on cholinesterase activities in rats. J. Neural Transm. 2013, 120, 1359–1367. [Google Scholar] [CrossRef]
- Virag, L.; Szabo, C. Purines inhibit poly(ADP-ribose) polymerase activation and modulate oxidant-induced cell death. Faseb. J. 2001, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chioccara, F.; Delia Gala, A.; De Rosa, M.; Novellino, E.; Prota, G. Mycosporine aminoacids and related compounds from the eggs of fishes. Bull. Soc. Chim. Belg. 1980, 89, 1101–1106. [Google Scholar] [CrossRef]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. eLife 2015, 4, e05919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plack, P.A.; Fraser, N.W.; Grant, P.T.; Middleton, C.; Mitchell, A.I.; Thomson, R.H. Gadusol, an enolic derivative of cyclohexane-1,3-dione present in the roes of cod and other marine fish. Isolation, properties and occurrence compared with ascorbic acid. Biochem. J. 1981, 199, 741–747. [Google Scholar] [CrossRef] [PubMed]
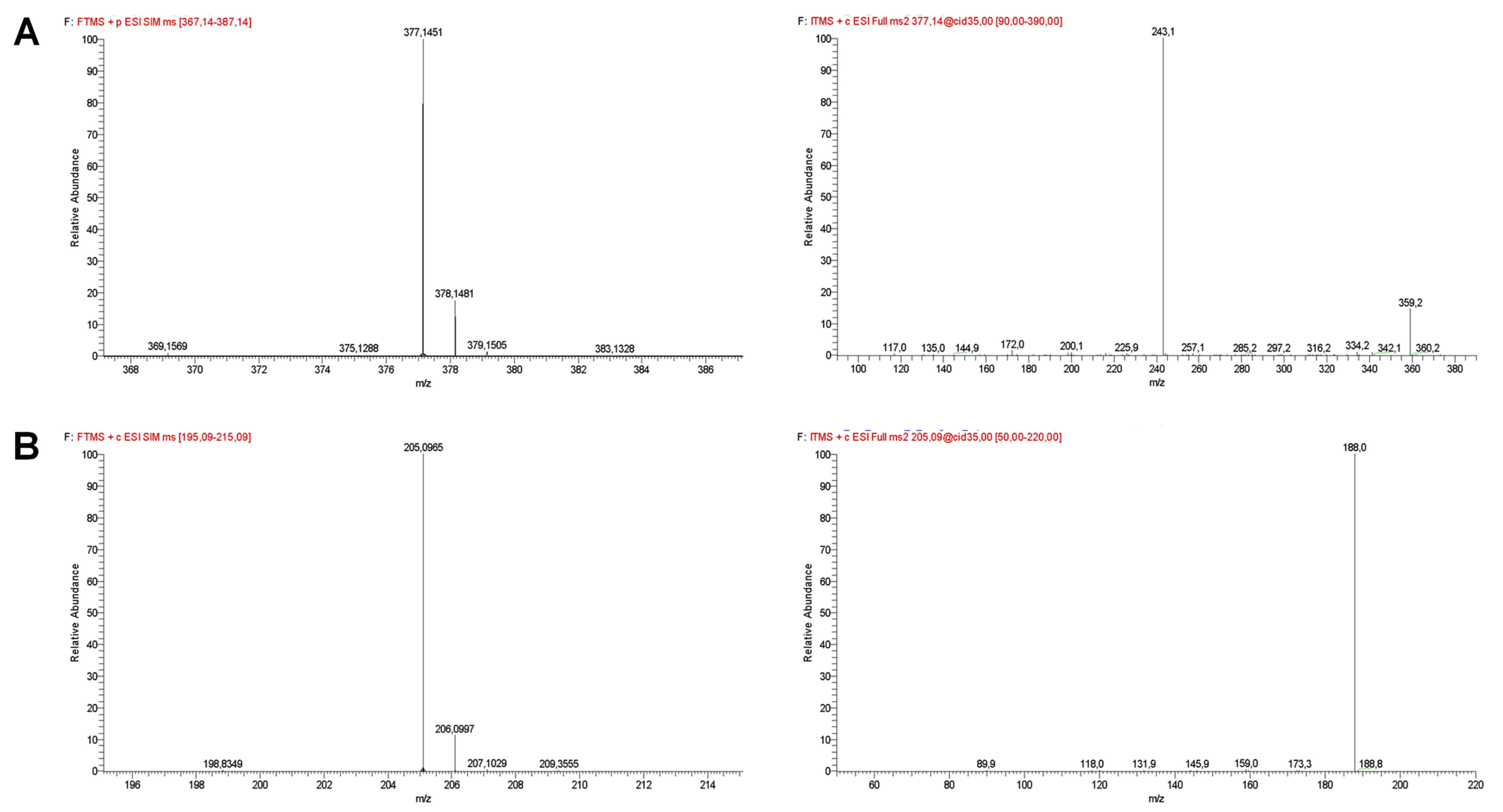
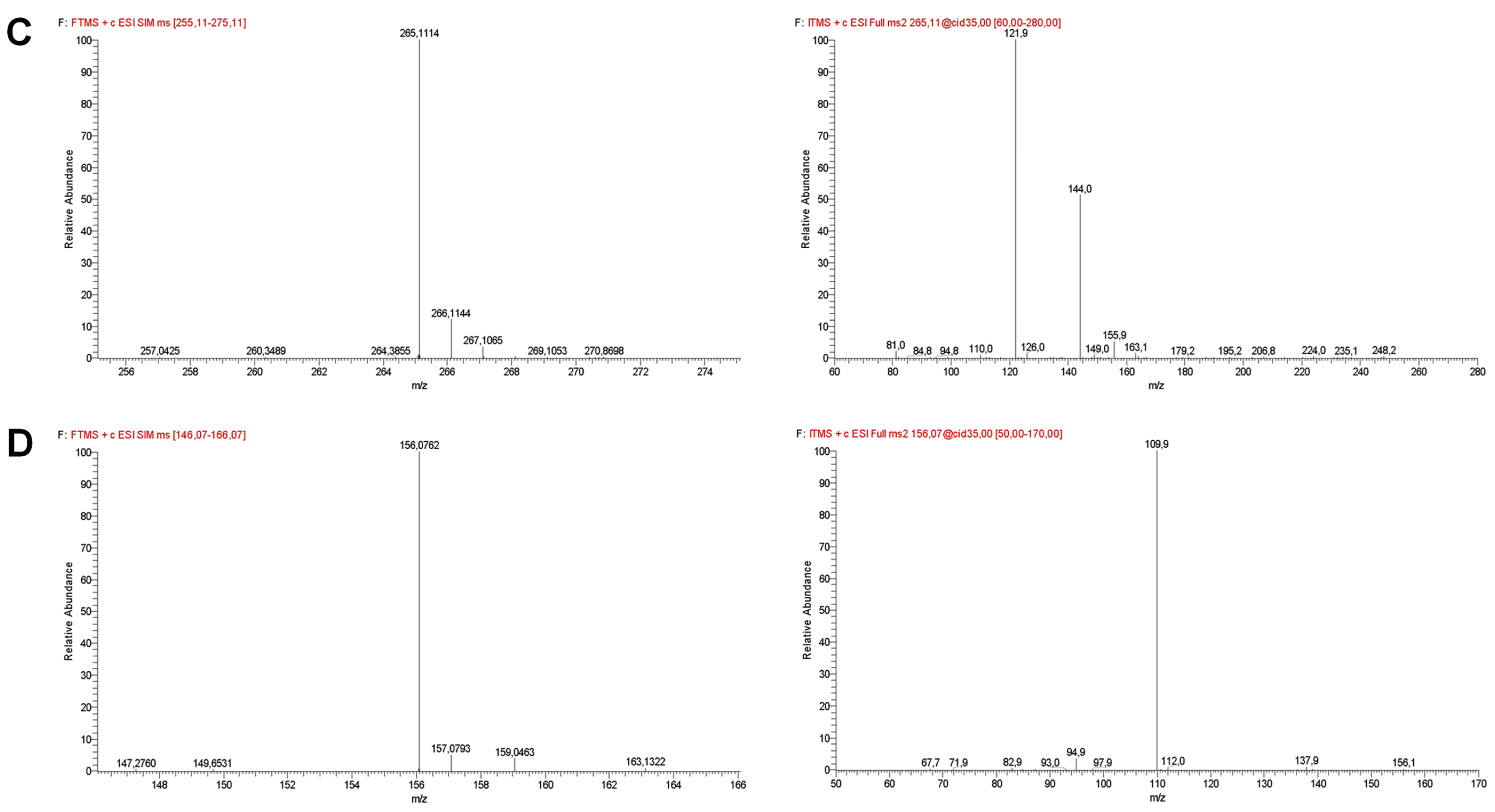


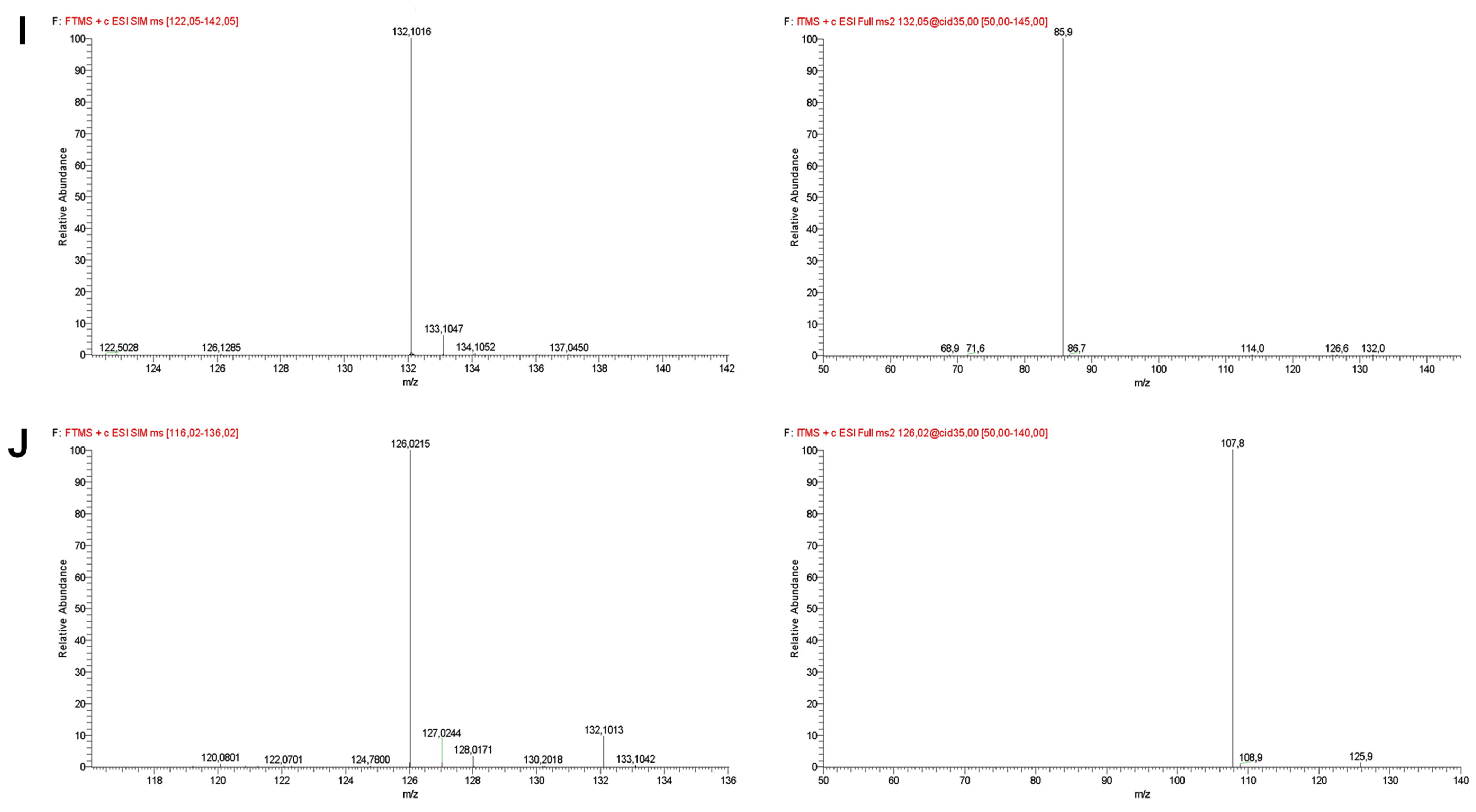

| Compound | Retention Time (min) | Selected Ion Monitoring (SIM) | [M−H]+ (m/z) | Error (ppm) | MS2 Fragment Ions |
|---|---|---|---|---|---|
| Riboflavin | 7.81 ± 0.09 | 367–387 | 377.1451 ± 0.0003 | −1.19 | 243.1 (100), 359.2 (14) |
| Tryptophan | 13.20 ± 0.08 | 195–215 | 205.0965 ± 0.0002 | −1.12 | 188.0 (100) |
| Thiamine | 13.63 ± 0.30 | 256–276 | 265.1114 ± 0.0004 * | −0.59 | 121.9 (100), 144.0 (51), 155.9 (5) |
| Histidine | 17.65 ± 0.38 | 146–166 | 156.0762 ± 0.0001 | −3.33 | 109.9 (100), 94.9 (3) |
| Anserine | 17.92 ± 0.06 | 231–251 | 241.1290 ± 0.0003 | −2.28 | 170.0 (100), 224.1 (76), 197.1 (36), 108,9 (27) |
| Nicotinamide | 5.05 ± 0.06 | 113–133 | 123.0549 ± 0.0001 | −2.84 | 122.9 (100), 79.8 (48), 106.0 (22), 95.8 (17), 95.2 (3) |
| Hypoxanthine | 8.06 ± 0.04 | 127–147 | 137.0455 ± 0.0001 | −1.97 | 137.0 (100), 109.9 (58), 93.8 (37), 119.0 (36) |
| Phenylalanine | 12.93 ± 0.04 | 156–176 | 166.0859 ± 0.0002 | −2.19 | 119.9 (100), 148.9 (4) |
| Isoleucine | 13.53 ± 0.03 | 122–142 | 132.1016 ± 0.0002 | −2.02 | 85.9 (100) |
| Taurine | 13.96 ± 0.04 | 116–136 | 126.0215 ± 0.0001 | −3.13 | 107.8 (100) |
| Carnosine | 18.12 ± 0.02 | 217–237 | 227.1134 ± 0.0002 | −2.04 | 210.1 (100), 209.3 (55), 109.9 (14), 179.9 (11), 156.0 (10) |
| Compound | Range (µg/mL) | Calibration Curve Equation | r2 | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Riboflavin | 5–50 | y = 2,597,970.9083x + 22,883,398.7348 | 0.9987 | 1.65 | 5.00 |
| Tryptophan | 6.7–100 | y = 1,419,395.4086x + 21,252,564.7864 | 0.9934 | 2.21 | 6.70 |
| Thiamine | 1.3–33.3 | y = 4,397,787.5153x − 270,937.1986 | 0.9941 | 0.43 | 1.30 |
| Histidine | 20–150 | y = 1,975,845.0061x + 2,836,204.2581 | 0.9998 | 3.14 | 9.50 |
| Anserine | 5–75 | y = 955,314.8167x + 9,065,014.7500 | 0.9987 | 1.65 | 5.00 |
| Nicotinamide | 10–75 | y = 2,817,664.2995x + 7,778,762.8222 | 0.9995 | 2.19 | 6.63 |
| Hypoxanthine | 6.7–46.7 | y = 3,640,970.0000x + 46,010,702.0000 | 0.9920 | 2.21 | 6.70 |
| Phenylalanine | 6.7–46.7 | y = 2,861,270.2246x + 16,386,226.9065 | 0.9970 | 2.21 | 6.70 |
| Isoleucine | 13.3–100 | y = 1,525,464.4712x + 40,827,232.0979 | 0.9955 | 4.39 | 13.30 |
| Taurine | 13.3–80 | y = 629,499.1431x + 9,232,958.8045 | 0.9941 | 4.39 | 13.30 |
| Carnosine | 1–25 | y = 1,594,248.8705x + 1,402,639.7461 | 0.9990 | 0.33 | 1.00 |
| Compound | Concentration (µg/100 mg Dry Extracts) | |||
|---|---|---|---|---|
| Sardine | Horse Mackerel | Sea Bass | ||
| Vitamins | Nicotinamide | 18.32 ± 0.61 | 16.66 ± 2.69 | <LOQ |
| Riboflavin | <LOQ | <LOQ | <LOQ | |
| Thiamine | <LOQ | 1.72 ± 0.32 | <LOQ | |
| Amino acids | Tryptophan | 100.96 ± 7.83 | 49.72 ± 8.63 | 27.41 ± 5.69 |
| Histidine | 130.77 ± 6.72 | 118.92 ± 5.00 | <LOQ | |
| Phenylalanine | 275.49 ± 12.28 | 70.22 ± 6.59 | 47.89 ± 7.75 | |
| Isoleucine | 427.01 ± 19.92 | 643.48 ± 97.29 | 380.11 ± 2.64 | |
| Taurine | 1131.08 ± 150.16 | 1306.43 ± 124.52 | 596.54 ± 54.43 | |
| Peptides | Anserine | not detected | <LOQ | <LOQ |
| Carnosine | not detected | 10.86 ± 1.91 | <LOQ | |
| Purine derivative | Hypoxanthine | 200.97 ± 9.47 | 158.28 ± 25.55 | 54.48 ± 5.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedes, M.; Gonçalves, V.M.F.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Aqueous Extracts of Fish Roe as a Source of Several Bioactive Compounds. Separations 2022, 9, 210. https://doi.org/10.3390/separations9080210
Guedes M, Gonçalves VMF, Tiritan ME, Reis RL, Ferreira H, Neves NM. Aqueous Extracts of Fish Roe as a Source of Several Bioactive Compounds. Separations. 2022; 9(8):210. https://doi.org/10.3390/separations9080210
Chicago/Turabian StyleGuedes, Marta, Virgínia M. F. Gonçalves, Maria Elizabeth Tiritan, Rui L. Reis, Helena Ferreira, and Nuno M. Neves. 2022. "Aqueous Extracts of Fish Roe as a Source of Several Bioactive Compounds" Separations 9, no. 8: 210. https://doi.org/10.3390/separations9080210
APA StyleGuedes, M., Gonçalves, V. M. F., Tiritan, M. E., Reis, R. L., Ferreira, H., & Neves, N. M. (2022). Aqueous Extracts of Fish Roe as a Source of Several Bioactive Compounds. Separations, 9(8), 210. https://doi.org/10.3390/separations9080210











