Extraction and Physicochemical Composition of Irvingiagabonensis Almond Oil: A Potential Healthy Source of Lauric-Myristic Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Proximate Composition of Almonds
2.3. Microscopic Cryo-MEB Analysis
2.4. Oil Extraction by Soxhlet and Hot-Pressing
2.5. Determination of the Fatty Acids Profile
2.6. Determination of the Triglyceride Profile of the Butter
2.7. Thermogravimetric Analysis (TGA)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microscopic Characterization of I. gabonensis Almonds
3.2. Composition of I. gabonensis Almonds
3.3. Extraction Yield of I. gabonensis Oil
3.4. Triglyceride Profile of I. gabonensis by Statistical Analysis
3.5. Fatty Acid Profile of I. gabonensis in Butter
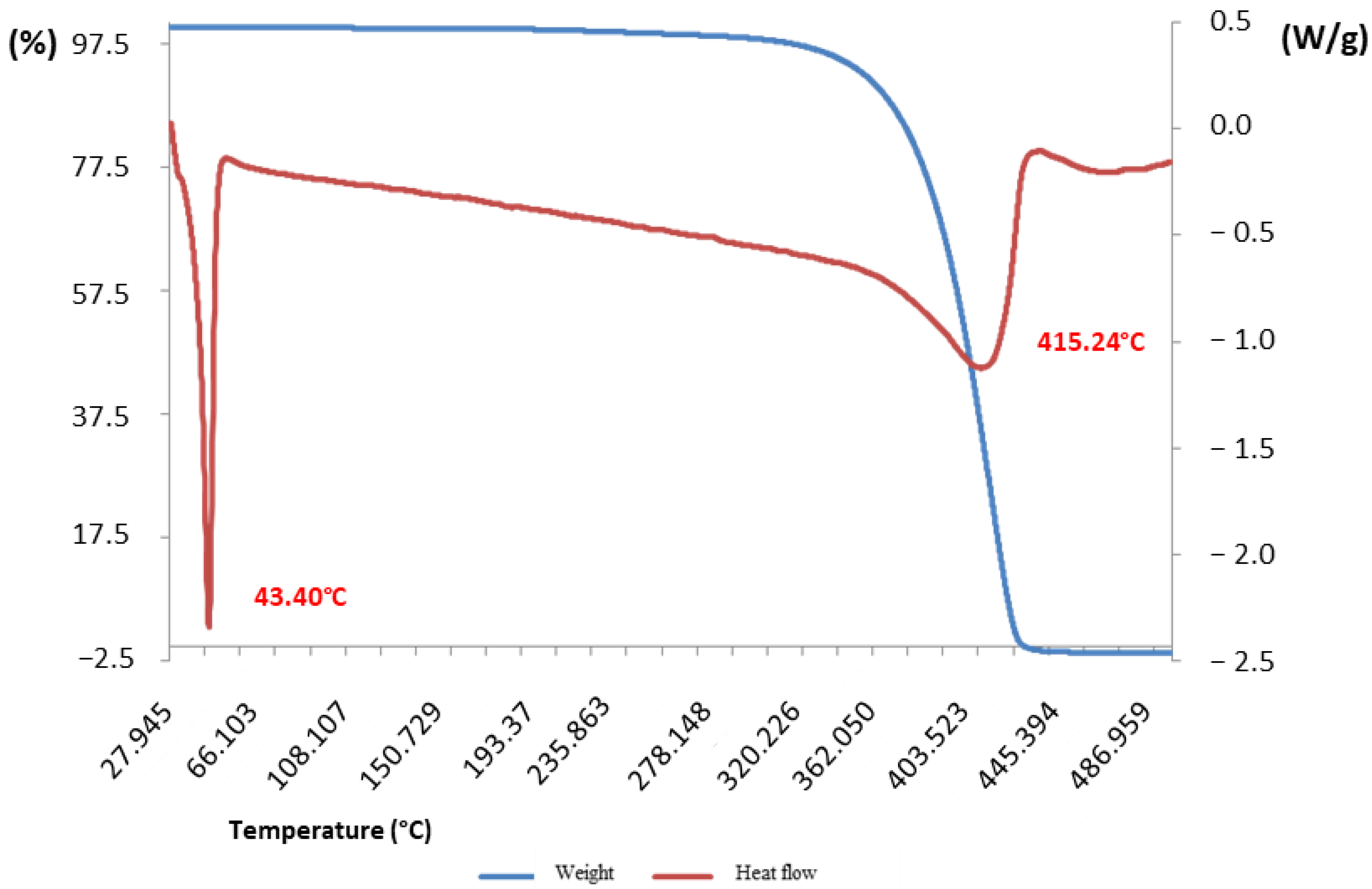
3.6. Thermogravimetric and Melting Point Analyses of I. gabonensis Butter
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matos, L.; Nzikou, J.M.; Matouba, E.; Pandzou-Yembe, V.N.; Guembot Mapepoulou, T.; Linder, M.; Desobry, S. Studies of Irvingia gabonensis Seed Kernels: Oil Technological Applications. Pak. J. Nutr. 2009, 8, 151–157. [Google Scholar] [CrossRef]
- Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring Extra-Virgin Olive Oil with Aromatic and Medicinal Plants Essential Oils Stabilizes Oleic Acid Composition during Photo-Oxidative Stress. Agriculture 2021, 11, 266. [Google Scholar] [CrossRef]
- Beauchamp, E.; Rioux, V.; Legrand, P. Acide Myristique: Nouvelles Fonctions de Régulation et de Signalisation. Med. Sci. 2009, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rioux, V. Fatty Acid Acylation of Proteins: Specific Roles for Palmitic, Myristic and Caprylic Acids. OCL 2016, 23, D304. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L. Occurrence and Characterization of Oils and Fats. In Lipid Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 37–141. ISBN 978-1-4200-0967-5. [Google Scholar]
- Silou, T.; Biyoko, S.; Heron, S.; Tchapla, A.; Maloumbi, M.G. Caractéristiques Pysico-Chimiques et Potentialités Technologiques Des Amandes de Irvingia gabonensis. Riv. Ital. Sostanze Gr. 2004, 81, 49–57. [Google Scholar]
- Emejulu, A.A.; Alisi, C.S.; Asiwe, E.S.; Iheanacho, K.M.; Onwuliri, V.A. Hypolipidemic Effect of Irvingia gabonensis Fruits Juice on Sodium Fluoride Induced Dyslipidemia in Rats. Afr. J. Biochem. Res. 2014, 8, 151–157. [Google Scholar] [CrossRef][Green Version]
- Emejulu, A.A.; Alisi, C.S.; Asiwe, E.S.; Nwaogu, L.A.; Igwe, C.U.; Onwuliri, V.A. Renal and Hepato-Protective Effect of Irvingia gabonensis Juice on Sodium Fluoride-Induced Toxicity in Wistar Rats. J. Clin. Toxicol. 2015, 6, 1000296. [Google Scholar] [CrossRef]
- Sahoré, A.D.; Koffi, L.B.; Kouamé, A.M. Technical Sheet of Irvingia gabonensis Seeds Preparation in Ivory Coast. Am. J. Food Technol. 2013, 1, 42–44. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Greenwell, P.; Hall, M.N.; Atangana, A.R.; Usoro, C.; Anegbeh, P.O.; Fondoun, J.-M.; Tchoundjeu, Z. Domestication of Irvingia gabonensis: 4. Tree-to-Tree Variation in Food-Thickening Properties and in Fat and Protein Contents of Dika Nut. Food Chem. 2005, 90, 365–378. [Google Scholar] [CrossRef]
- FAO. Domestication and Commercialization of Non-Timber Forest Products in Agroforestry Systems. In Proceedings of the International Conference on Domestication and Commercialization of Non-Timber Forest Products in Agroforestry Systems, Nairobi, Kenya, 19–26 February 1996. [Google Scholar]
- CBFP. The Forests of the Congo Basin: A Preliminary Assessment; Maryland University Press: Baltimore, MD, USA, 2005; p. 33. [Google Scholar]
- Silou, T. Corps gras non conventionnels du Bassin du Congo: Caractérisation, biodiversité et qualité. OCL 2014, 21, D209. [Google Scholar] [CrossRef][Green Version]
- Loumouamou, B.W.; Gomoufatan, J.P.M.; Silou, T.; Nzikou, J.M.; Mbaya Gindo, G.V.; Figueredo, G.; Chalard, J.P. Extraction and Chemical Composition of Seed Kernel Oil from Irvingia Smithii of Congo Basin. Adv. J. Food Sci. Technol. 2013, 5, 506–513. [Google Scholar] [CrossRef]
- Silou, T.; Loumouamou, B.W.; Nsikabaka, S.; Kinkela, T.; Nzikou, M.; Chalard, P.; Figueredo, G. Contribution to the Varietal Delimitation of Irvingia Gabonensis and Irvingia wombulu Chemical Composition Variability of Fats Extracted from Kernels. J. Food Technol. 2011, 9, 36–42. [Google Scholar] [CrossRef][Green Version]
- Etong, D.I.; Mustapha, A.O.; Taleat, A.A. Physicochemical Properties and Fatty Acid Composition of Dikanut (Irvingia gabonensis) Seed Oil. Res. J. Chem. Sci. 2014, 4, 70–74. [Google Scholar]
- Sonwai, S.; Ponprachanuvut, P. Characterization of Physicochemical and Thermal Properties and Crystallization Behavior of Krabok (Irvingia malayana) and Rambutan Seed Fats. J. Oleo Sci. 2012, 61, 671–679. [Google Scholar] [CrossRef][Green Version]
- Sonwai, S.; Ornla-ied, P.; Aneknun, T. Lauric Fat Cocoa Butter Replacer from Krabok (Irvingia malayana) Seed Fat and Coconut Oil. J. Oleo Sci. 2015, 64, 357–365. [Google Scholar] [CrossRef][Green Version]
- Ogunsina, B.S.; Bhatnagar, A.; Indira, T.; Radha, C. The Proximate Composition of African Bush Mango Kernels (Irvingia gabonensis) and Characteristics of Its Oil. Ife J. Sci. 2012, 14, 177–183. [Google Scholar]
- Moore, S.; Stein, W.H. Photometric Ninhydrin Method for Use in the Chromatography of Amino Acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of almonds (Prunus dulcis) geographical origin by minerals and fatty acids profiling. Nat. Prod. Res. 2016, 30, 2107–2110. [Google Scholar] [CrossRef]
- Yamoneka, J.; Malumba, P.; Blecker, C.; Gindo, M.; Richard, G.; Fauconnier, M.-L.; Lognay, G.; Danthine, S. Physicochemical Properties and Thermal Behaviour of African Wild Mango (Irvingia gabonensis) Seed Fat. LWT-Food Sci. Technol. 2015, 64, 989–996. [Google Scholar] [CrossRef]
- Ifeduba, E.A.; Awachie, M.N.; Sabir, J.S.M.; Akoh, C.C. Fatty Acid Composition of Irvingia gabonensis and Treculia africana Seed Lipids and Phospholipids. J. Am. Oil Chem. Soc. 2013, 90, 517–528. [Google Scholar] [CrossRef]
- Ogodo, A.C. Physical and Chemical Characteristics of the African Bush Mango (Irvingia gabonensis Var. garbonensis) Seed Oil. Int. J. Adv. Eng. Manag. 2014, 1, 28–31. [Google Scholar]
- Tzen, J.; Cao, Y.; Laurent, P.; Ratnayake, C.; Huang, A. Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiol. 1993, 101, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.H.E.; Sanchez, J.; Millan, F.; Murphy, D.J. Differential Presence of Oleosins in Oleogenic Seed and Mesocarp Tissues in Olive (Olea europaea) and Avocado (Persea Americana). Plant Sci. 1993, 93, 203–210. [Google Scholar] [CrossRef]
- Sánchez-Albarrán, F.; Salgado-Garciglia, R.; Molina-Torres, J.; López-Gómez, R. Oleosome Oil Storage in the Mesocarp of Two Avocado Varieties. J. Oleo Sci. 2019, 68, 87–94. [Google Scholar] [CrossRef]
- Ogunsina, B.S.; Olatunde, G.A.; Adeleye, O. Effect of Pre-Treatments on Mechanical Oil Expression from Dika Kernels. Niger. Food J. 2014, 32, 1–9. [Google Scholar] [CrossRef][Green Version]
- Yamoneka, J.; Malumba, P.; Lognay, G.; Béra, F.; Blecker, C.; Danthine, S. Enzymatic Inter-Esterification of Binary Blends Containing Irvingia gabonensis Seed Fat to Produce Cocoa Butter Substitute. Eur. J. Lipid Sci. Technol. 2018, 120, 1700423. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.M.; Omar, A.K.M. Cocoa Butter Fats and Possibilities of Substitution in Food Products Concerning Cocoa Varieties, Alternative Sources, Extraction Methods, Composition, and Characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]


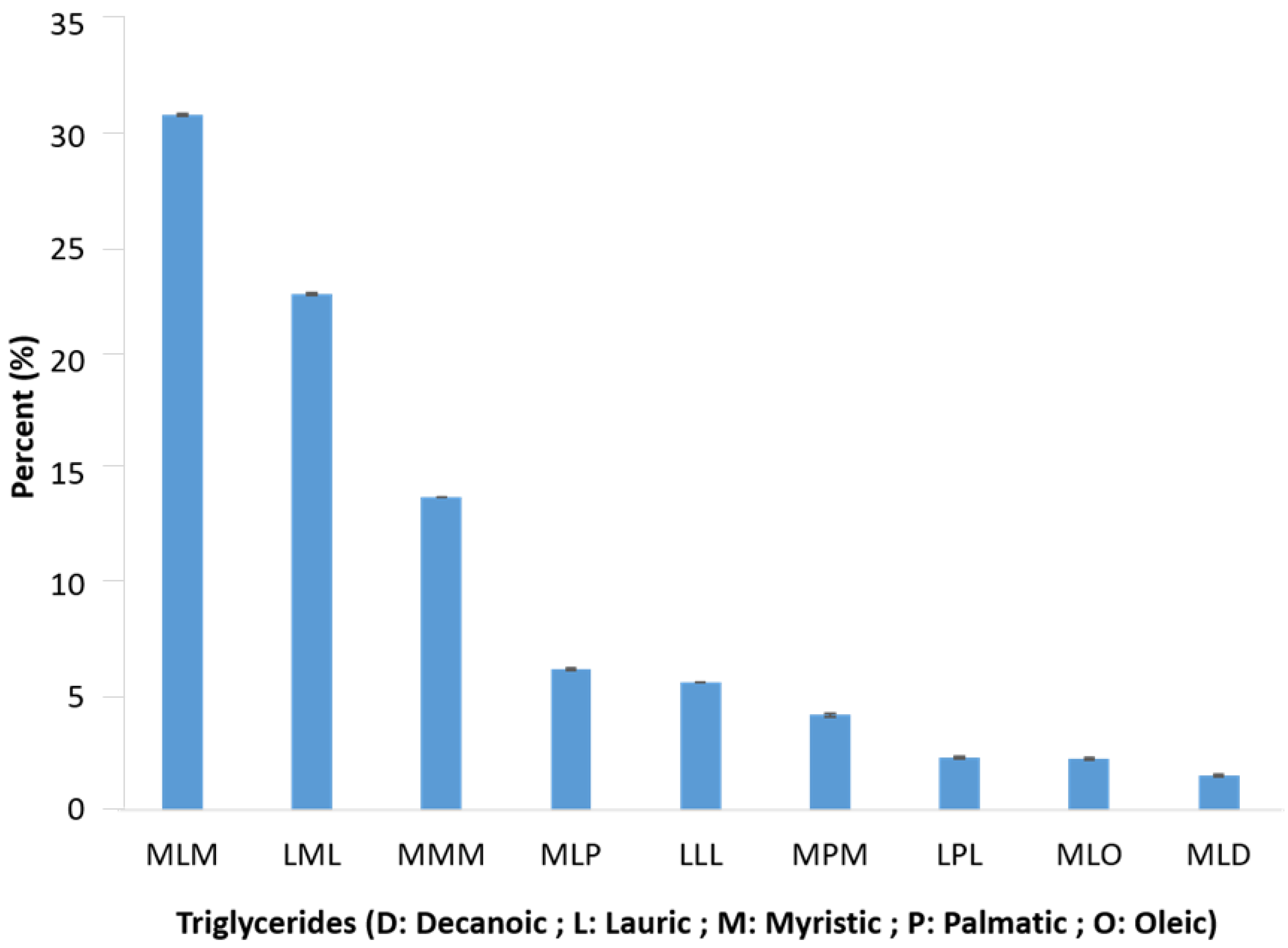
| Species | Triglyceride Profile | Reference |
|---|---|---|
| I. smithii | LLM > LMM > LLL > MMM | [14] |
| LLL > MMM > LLM > LMM | [13] | |
| I. wombulu | LLL > MMM > LLM > LMM | [13] |
| LLM > LMM > LLL > MMM | [15] | |
| I. gabonensis | LLL > MMM > LLM > LMM | [13] |
| LMM > LLM > MMM > LLL | [15] |
| Fatty Acids (%) | C10:0 | C12:0 | C14:0 | C16:0 | C18:0 | C18:n1−9 | C18:2n−6 | Refs. |
|---|---|---|---|---|---|---|---|---|
| Ig | 1.34 | 39.37 | 50.92 | 4.97 | 0.73 | 1.82 | 0.49 | [3] |
| Ig | 27.63 | 61.68 | 7.49 | 0.81 | 2.12 | 0.27 | [19] | |
| Ig | 39.40 | 20.50 | 10.30 | 11.40 | 6.90 | 6.40 | [16] | |
| Is | 3.67 | 51.85 | 33.84 | 3.78 | 0.44 | 3.18 | 0.51 | [14] |
| Is | 2.23 | 42.74 | 41.63 | 5.77 | 0.61 | 4.35 | 0.70 | [14] |
| Iw | 2.05 | 48.54 | 43.43 | 3.37 | 0.59 | 1.71 | 0.31 | [15] |
| Im | 46.91 | 40.28 | 3.18 | 1.62 | 2.26 | 0.01 | [17,18] |
| Amino Acids | Content (%) |
|---|---|
| Aspartic acid | 10.05 ± 0.33 |
| Threonine | 3.93 ± 0.12 |
| Serine | 5.45 ± 0.01 |
| Glutamic acid | 19.93 ± 1.02 |
| Glycine | 5.20 ± 0.01 |
| Alanine | 4.72 ± 0.01 |
| Cysteine | 1.89 ± 0.26 |
| Valine | 5.24 ± 0.08 |
| Methionine | 2.70 ± 1.63 |
| Isoleucine | 5.20 ± 0.53 |
| Leucine | 8.54 ± 0.75 |
| Tyrosine | 2.58 ± 0.24 |
| Phenylalanine | 3.54 ± 0.33 |
| Lysine | 5.10 ± 0.12 |
| Histidine | 2.52 ± 0.24 |
| Proline | 4.40 ± 0.41 |
| Arginine | 9.05 ± 0.54 |
| Tryptophan | ND 1 |
| Fatty Acids | Length of Carbon Chain | % Relative Mass |
|---|---|---|
| Decanoic | C10:0 | 1.29 ± 0.03 |
| Lauric | C12:0 | 38.48 ± 0.09 |
| Myristic | C14:0 | 51.87 ± 0.18 |
| Palmitic | C16:0 | 5.25 ± 0.01 |
| Stearic | C18:0 | 0.73 ± 0.02 |
| Oleic | C18:n1−9 | 1.89 ± 0.04 |
| Linoleic | C18:2n−6 | 0.57 ± 0.01 |
| SFA 1 | 97.60 ± 0.05 | |
| MUFA 2 | 1.89 ± 0.04 | |
| PUFA 3 | 0.57 ± 0.01 | |
| SFA/MUFA | 51.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koumba Ibinga, S.K.; Cerny, M.; Lacroux, E.; Fabre, J.-F.; Valentin, R.; Merah, O.; Bikanga, R.; Mouloungui, Z. Extraction and Physicochemical Composition of Irvingiagabonensis Almond Oil: A Potential Healthy Source of Lauric-Myristic Oil. Separations 2022, 9, 207. https://doi.org/10.3390/separations9080207
Koumba Ibinga SK, Cerny M, Lacroux E, Fabre J-F, Valentin R, Merah O, Bikanga R, Mouloungui Z. Extraction and Physicochemical Composition of Irvingiagabonensis Almond Oil: A Potential Healthy Source of Lauric-Myristic Oil. Separations. 2022; 9(8):207. https://doi.org/10.3390/separations9080207
Chicago/Turabian StyleKoumba Ibinga, Sidrine Kerthy, Muriel Cerny, Eric Lacroux, Jean-François Fabre, Romain Valentin, Othmane Merah, Raphaël Bikanga, and Zéphirin Mouloungui. 2022. "Extraction and Physicochemical Composition of Irvingiagabonensis Almond Oil: A Potential Healthy Source of Lauric-Myristic Oil" Separations 9, no. 8: 207. https://doi.org/10.3390/separations9080207
APA StyleKoumba Ibinga, S. K., Cerny, M., Lacroux, E., Fabre, J.-F., Valentin, R., Merah, O., Bikanga, R., & Mouloungui, Z. (2022). Extraction and Physicochemical Composition of Irvingiagabonensis Almond Oil: A Potential Healthy Source of Lauric-Myristic Oil. Separations, 9(8), 207. https://doi.org/10.3390/separations9080207







