CO2 Capture over Activated Carbon Derived from Pulverized Semi-Coke
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Demineralization of Pulverized Semi-Coke
2.3. Activated Carbon Preparation
2.4. Activated Carbon Characterization
2.5. CO2 Uptake Measurement
3. Results and Discussion
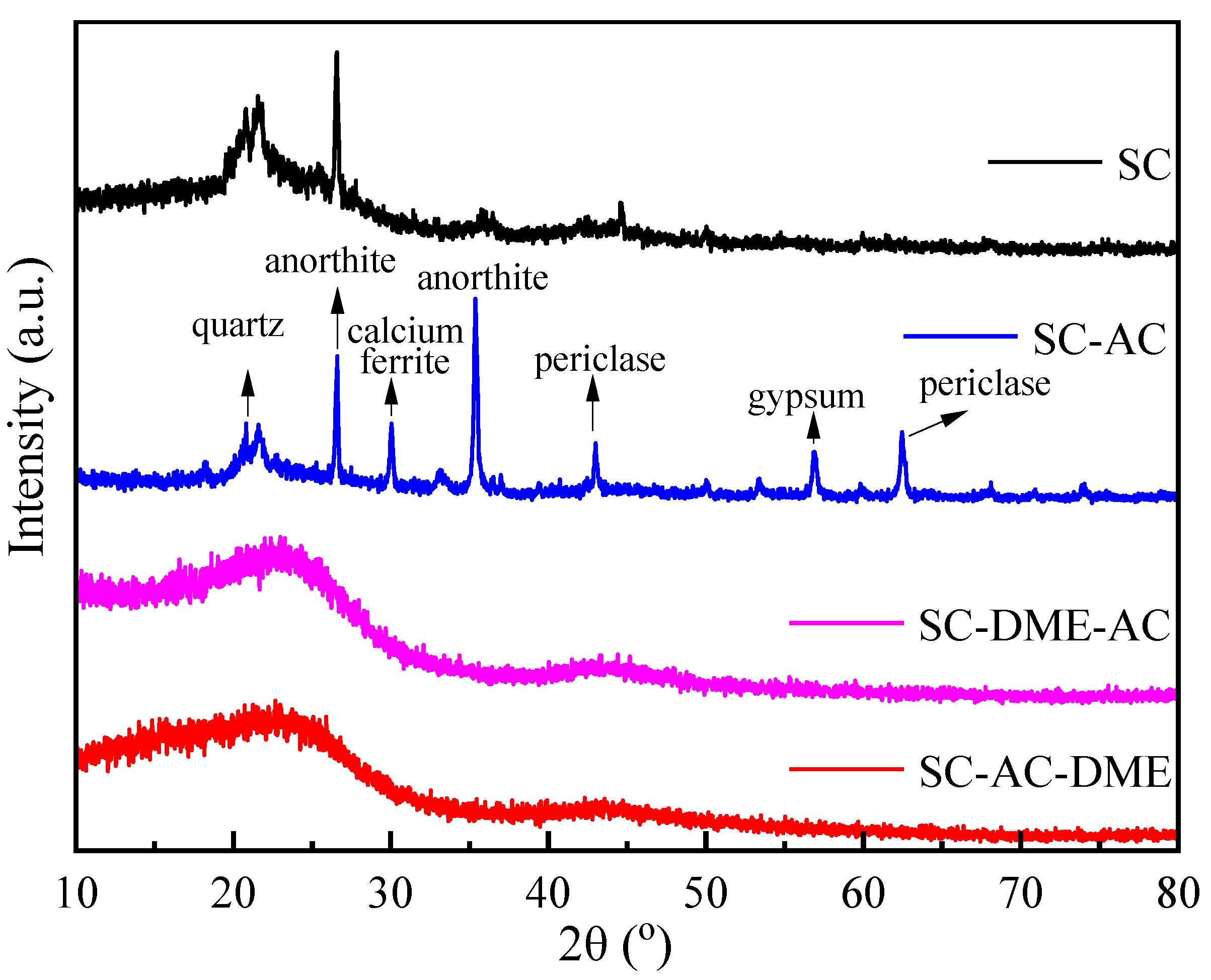
3.1. Influence of Demineralization on the Properties of the Activated Carbon
3.2. Optimization of Activated Carbon Preparation Parameters
3.2.1. Optimization of Activation Temperature
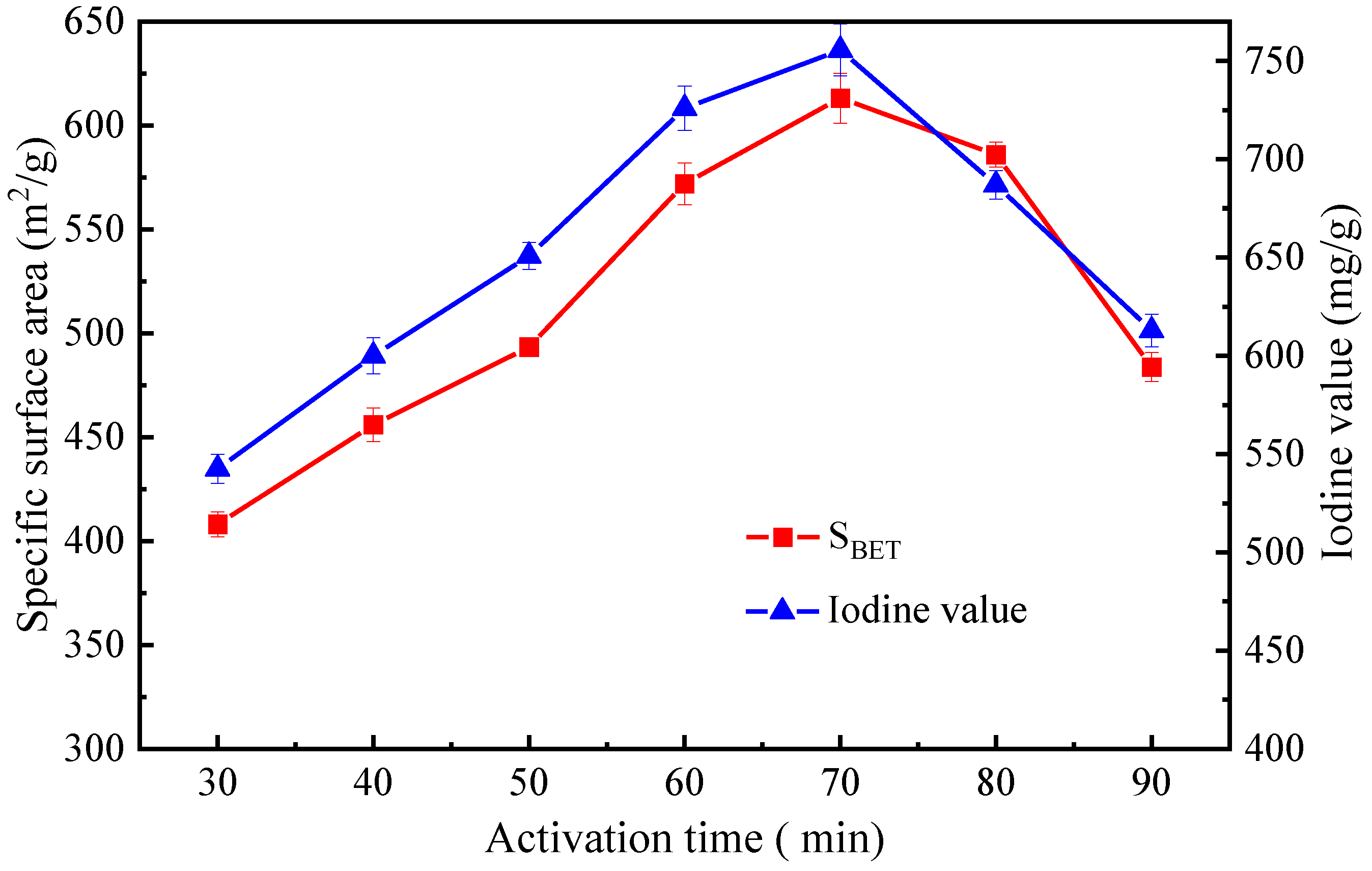
3.2.2. Optimization of Activation Time
3.2.3. Optimization of Steam Flow
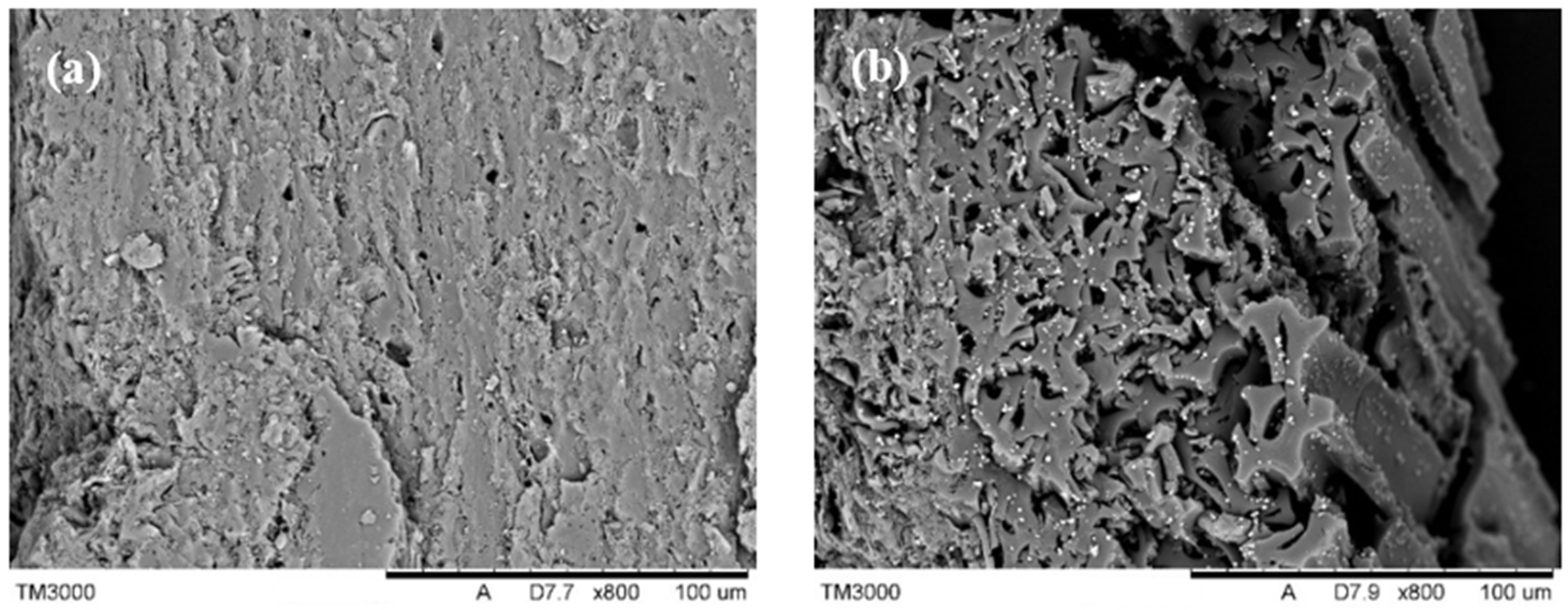
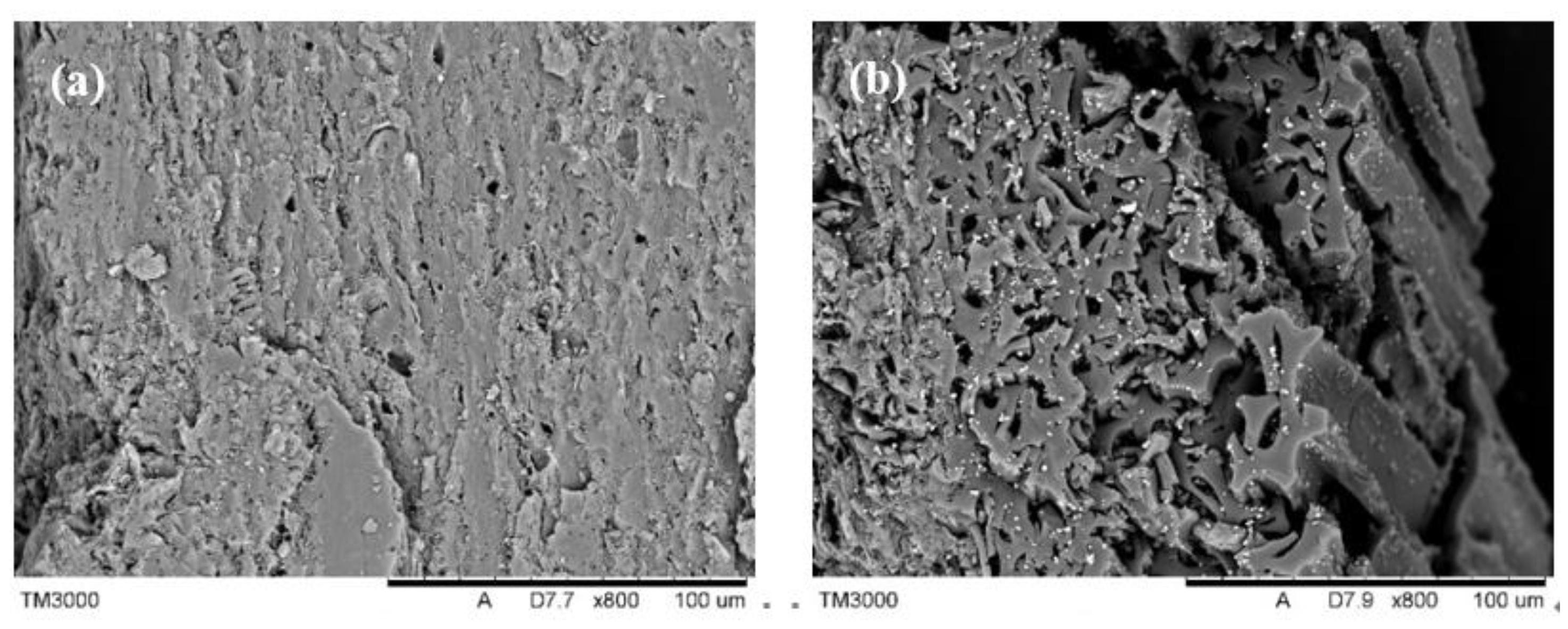
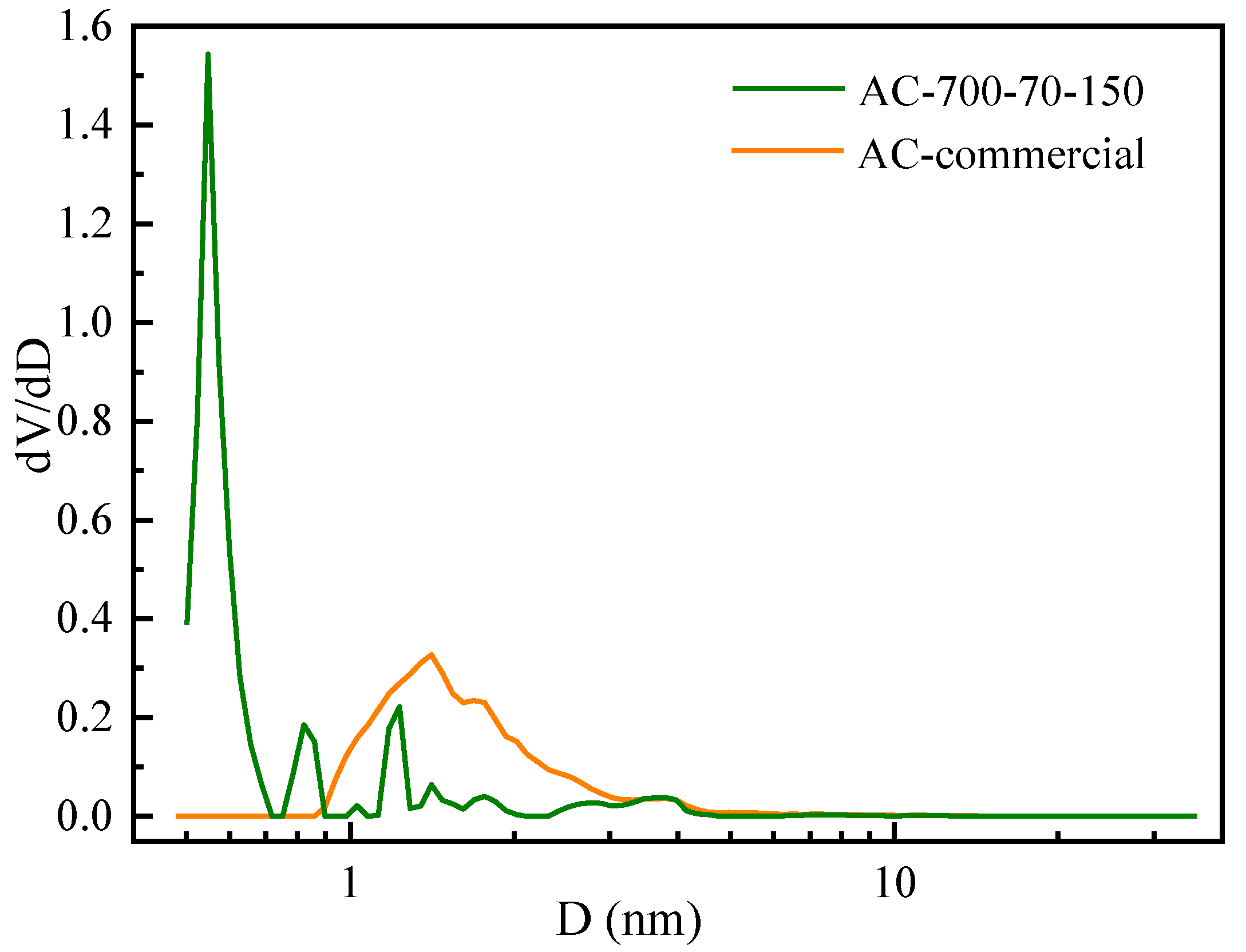
3.2.4. Surface Properties of the Obtained Activated Carbon
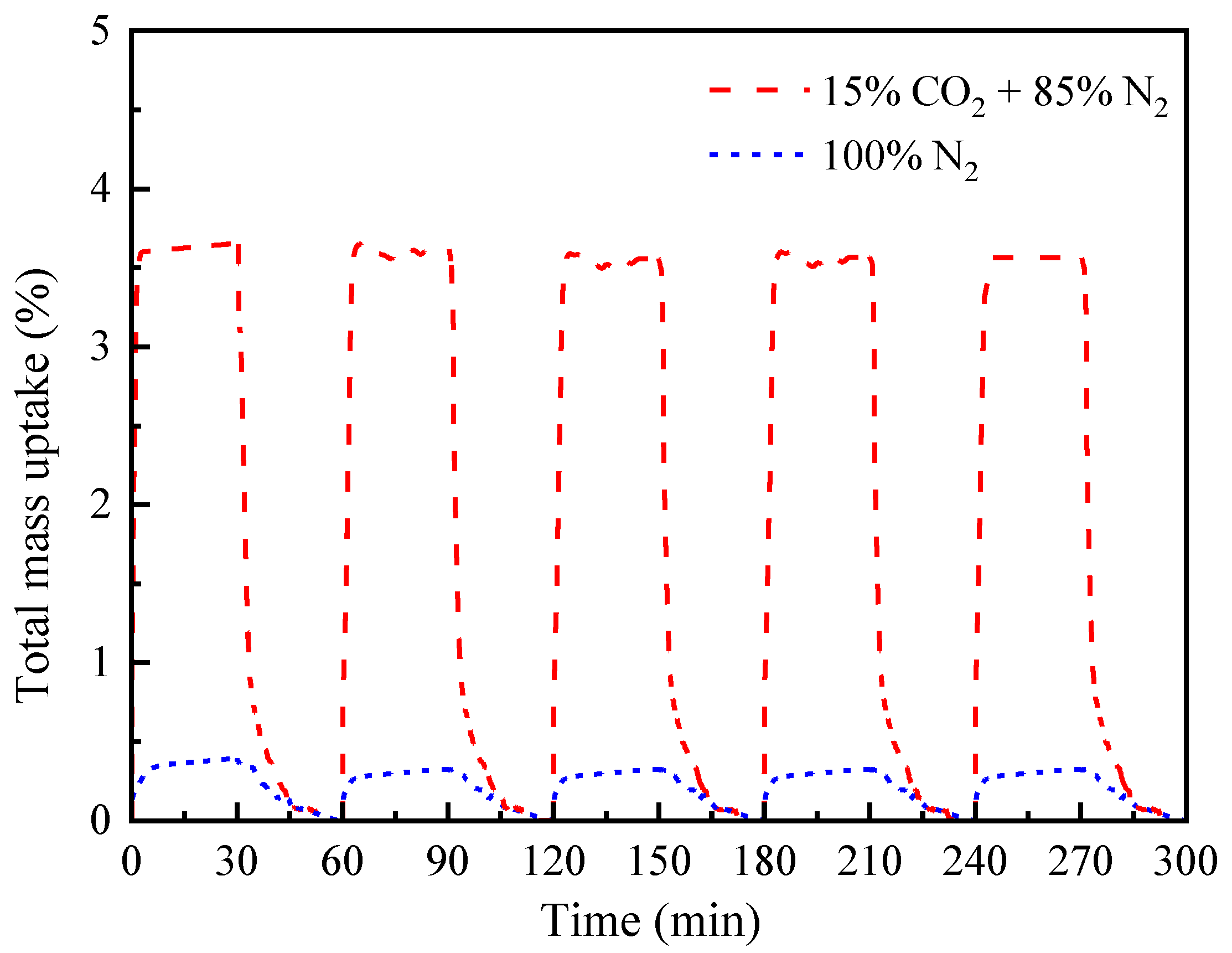
3.3. CO2 Sorption Performance of the Obtained Activated Carbon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, S.Y.W.; Ngu, L.H.; How, B.S. Review of carbon capture absorbents for CO2 utilization. Greenh. Gases 2022, 12, 394–427. [Google Scholar] [CrossRef]
- Dunstan, M.T.; Donat, F.; Bork, A.H.; Grey, C.P.; Muller, C.R. CO2 capture at medium to high temperature using solid oxide-based sorbents: Fundamental aspects, mechanistic insights, and recent advances. Chem. Rev. 2021, 121, 12681–12745. [Google Scholar] [CrossRef] [PubMed]
- Aghel, B.; Janati, S.; Alobaid, F.; Almoslh, A.; Epple, B. Application of nanofluids in CO2 absorption: A review. Appl. Sci. 2022, 12, 3200. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Plaza, M.G.; Thurecht, K.J.; Pevida, C.; Rubiera, F.; Pis, J.J.; Snape, C.E.; Drage, T.C. Influence of oxidation upon the CO2 capture performance of a phenolic-resin-derived carbon. Fuel Process. Technol. 2013, 110, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Guo, Y.F.; Zhao, C.W.; Yan, J.J.; Lu, P. Biomass derived wood ash with amine modification for post-combustion CO2 capture. Appl. Energy 2017, 201, 34–44. [Google Scholar] [CrossRef]
- Chan, W.H.; Mazlee, M.N.; Ahmad, Z.A.; Ishak, M.A.M.; Shamsul, J.B. The development of low cost adsorbents from clay and waste materials: A review. J. Mater. Cycles Waste Manag. 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Cont. 2022, 119, 103715. [Google Scholar] [CrossRef]
- Rozaiddin, S.A.M.; Lau, K.K. A review on enhancing solvent regeneration in CO2 absorption process using nanoparticles. Sustainability 2022, 14, 4750. [Google Scholar] [CrossRef]
- Ooi, Z.L.; Tan, P.Y.; Tan, L.S.; Yeap, S.P. Amine-based solvent for CO2 absorption and its impact on carbon steel corrosion: A perspective review. Chin. J. Chem. Eng. 2020, 28, 1357–1367. [Google Scholar] [CrossRef]
- Bravo, J.; Drapanauskaite, D.; Sarunac, N.; Romero, C.; Jesikiewicz, T.; Baltrusaitis, J. Optimization of energy requirements for CO2 post-combustion capture process through advanced thermal integration. Fuel 2021, 283, 118940. [Google Scholar] [CrossRef]
- Dong, B.X.; Zhang, S.Y.; Liu, W.L.; Wu, Y.C.; Ge, J.; Song, L.; Teng, Y.L. Gas storage and separation in water-stable [CuI5BTT3]4– anion framework comprising giant multi-prismatic nanoscale cage. Chem. Commun. 2015, 51, 5691–5694. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Wang, J.; Pu, Q.; Ning, P.; Lu, S. Activated carbon-based composites for capturing CO2: A review. Greenh. Gases 2021, 11, 377–393. [Google Scholar] [CrossRef]
- Karami, F.; Aghel, B.; Moradi, P.; Karimi, M. Stripping of hydrogen sulfide from crude oil desalter effluent via different adsorbents. Int. J. Environ. Sci. Technol. 2022, 19, 5119–5130. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.W.; Morawski, A.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Wang, J.; Lei, S.; Liang, L. Preparation of porous activated carbon from semi-coke by high temperature activation with KOH for the high-efficiency adsorption of aqueous tetracycline. Appl. Surf. Sci. 2020, 530, 147187. [Google Scholar] [CrossRef]
- Tian, H.; Pan, J.; Zhu, D.; Guo, Z.; Yang, C.; Xue, Y.; Li, S.; Wang, Y. Innovative one-step preparation of activated carbon from low-rank coals activated with oxidized pellets. J. Clean. Prod. 2021, 313, 127877. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Plaza, M.G.; González, A.S.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Li, B.F.; Li, X.H.; Li, W.Y.; Feng, J. Co-pyrolysis performance of coal and its direct coal liquefaction residue with solid heat carrier. Fuel Process. Technol. 2017, 166, 69–76. [Google Scholar] [CrossRef]
- Ye, C.P.; Yang, Z.J.; Li, W.Y.; Rong, H.L.; Feng, J. Effect of adjusting coal properties on HulunBuir lignite pyrolysis. Fuel Process. Technol. 2017, 156, 415–420. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Sandle, N.K. Effect of different oxidizing agent treatments on the surface properties of activated carbons. Carbon 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- Bjornerback, F.; Hedin, N. Microporous humins prepared from sugars and bio-based polymers in concentrated sulfuric acid. ACS Sust. Chem. Eng. 2019, 7, 1018–1027. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass, A review. Renew. Sust. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ngu, L.H.; Hashim, S.S. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh. Gases 2021, 11, 1076–1117. [Google Scholar] [CrossRef]
- Martín, C.F.; Plaza, M.G.; Pis, J.J.; Rubiera, F.; Pevida, C.; Centeno, T.A. On the limits of CO2 capture capacity of carbons. Sep. Purif. Technol. 2010, 74, 225–229. [Google Scholar] [CrossRef]
- Murakami, T.; Xu, G.; Suda, T.; Matsuzawa, Y.; Tani, H.; Fujimori, T. Some process fundamentals of biomass gasification in dual fluidized bed. Fuel 2007, 86, 244–255. [Google Scholar] [CrossRef]
- Alauddin, Z.A.B.Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review. Renew. Sust. Energy Rev. 2010, 14, 2852–2862. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Sun, J.; Zheng, Z.; Fu, K. Pyrolysis polygeneration of pine nut shell: Quality of pyrolysis products and study on the preparation of activated carbon from biochar. Bioresour. Technol. 2016, 216, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.; Senanayake, G.; Shah, N.; Thile, X.; Parkinson, G.; Fawcett, D. Adsorption of the aurocyanide, Au(CN)2− complex on granular activated carbons derived from macadamia nut shells: A preliminary study. Miner. Eng. 2011, 24, 1694–1702. [Google Scholar] [CrossRef] [Green Version]
- Campoy, M.; Gómez-Barea, A.; Vidal, F.B.; Ollero, P. Air–steam gasification of biomass in a fluidised bed: Process optimisation by enriched air. Fuel Process. Technol. 2009, 90, 677–685. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, B.; Zhang, Z.; Wang, T.; Cheng, X.; Lin, L.; Ma, C. One-step rapid pyrolysis activation method to prepare nanostructured activated coke powder. Fuel 2020, 262, 116514. [Google Scholar] [CrossRef]
- Marco-Lozar, J.P.; Kunowsky, M.; Suárez-García, F.; Linares-Solano, A. Sorbent design for CO2 capture under different flue gas conditions. Carbon 2014, 72, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Valencia, L.; Abdelhamid, H.N. Nanocellulose leaf-like zeolitic imidazolate framework (ZIF-L) foams for selective capture of carbon dioxide. Carbohydr. Polym. 2019, 213, 338–345. [Google Scholar] [CrossRef]



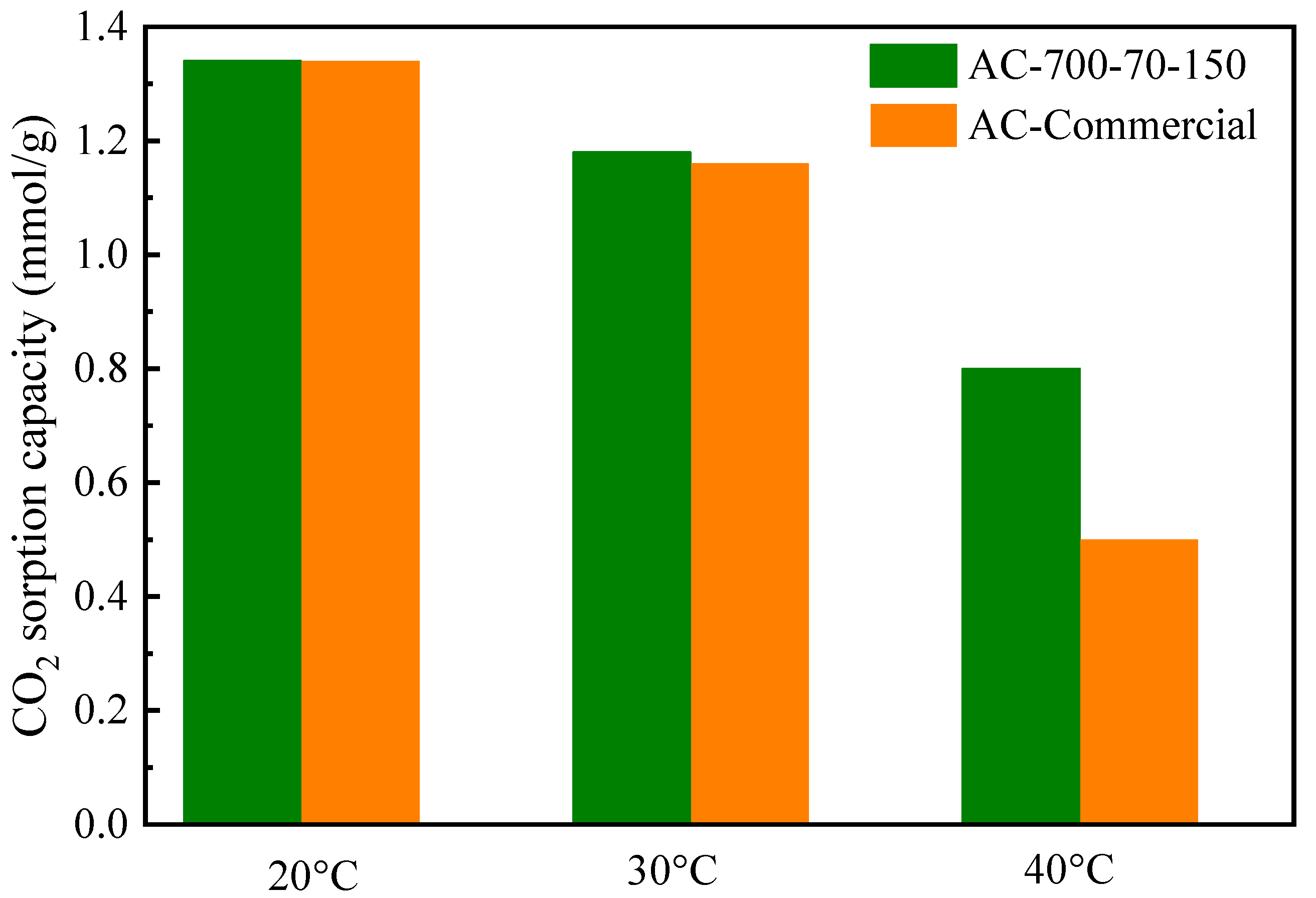
| Samples | Proximate Analysis wad (%) | Ultimate Analysis wdaf (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC * | C | H | O * | N | S | |
| Hulunbel Lignite | 11.44 | 13.11 | 33.08 | 42.37 | 74.01 | 4.11 | 19.97 | 1.26 | 0.65 |
| Pulverized semi-coke | 4.77 | 18.95 | 17.04 | 59.24 | 81.50 | 3.03 | 14.22 | 0.82 | 0.43 |
| Samples | SBET (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Ratio Vmic:Vt | Iodine Value (mg/g) |
|---|---|---|---|---|---|
| SC | 120.5 | 0.11 | 0.07 | 0.64 | 250.6 |
| SC-AC | 531.4 | 0.37 | 0.21 | 0.57 | 621.2 |
| SC-AC-DME | 620.6 | 0.44 | 0.27 | 0.61 | 705.2 |
| SC-DME-AC | 590.2 | 0.29 | 0.24 | 0.83 | 755.6 |
| Sample | CO2 Sorption Capacity (mmol/g) | ||
|---|---|---|---|
| 40 °C | 50 °C | ||
| 15 kPa | 90 kPa | 90 kPa | |
| AC-650-50-150 | 0.24 | 1.09 | 0.74 |
| AC-680-50-150 | 0.47 | 1.41 | 1.26 |
| AC-700-50-150 | 0.62 | 1.65 | 1.32 |
| AC-750-50-150 | 0.4 | 1.33 | 1.18 |
| AC-800-50-150 | 0.35 | 1.28 | 1.07 |
| AC-700-30-150 | 0.25 | 1.13 | 0.85 |
| AC-700-70-150 | 0.85 | 1.80 | 1.54 |
| AC-700-90-150 | 0.38 | 1.31 | 1.14 |
| AC-700-70-50 | 0.33 | 1.25 | 1.07 |
| AC-700-70-100 | 0.51 | 1.49 | 1.25 |
| AC-700-70-200 | 0.47 | 1.42 | 1.26 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Ratio Vmic:Vt | Pore Size (nm) |
|---|---|---|---|---|---|
| AC-Commercial | 870.4 | 0.48 | 0.22 | 0.46 | 1.41 |
| AC-700-70-150 | 590.2 | 0.29 | 0.24 | 0.83 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, J.; Zhao, Z.; Zhang, X.; Feng, J.; Li, W. CO2 Capture over Activated Carbon Derived from Pulverized Semi-Coke. Separations 2022, 9, 174. https://doi.org/10.3390/separations9070174
Jing J, Zhao Z, Zhang X, Feng J, Li W. CO2 Capture over Activated Carbon Derived from Pulverized Semi-Coke. Separations. 2022; 9(7):174. https://doi.org/10.3390/separations9070174
Chicago/Turabian StyleJing, Jieying, Zemin Zhao, Xuewei Zhang, Jie Feng, and Wenying Li. 2022. "CO2 Capture over Activated Carbon Derived from Pulverized Semi-Coke" Separations 9, no. 7: 174. https://doi.org/10.3390/separations9070174
APA StyleJing, J., Zhao, Z., Zhang, X., Feng, J., & Li, W. (2022). CO2 Capture over Activated Carbon Derived from Pulverized Semi-Coke. Separations, 9(7), 174. https://doi.org/10.3390/separations9070174







