Simultaneous Determination of Fenchone and Trans-Anethole in Essential Oils and Methanolic Extracts of Foeniculum vulgare Mill. Fruits Obtained from Different Geographical Regions Using GC-MS Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GC-MS Analysis
2.2.1. SCAN Method
2.2.2. Single Ion Current (SIM) Method
2.3. Calibration Curves of FCO and TOH
2.4. Extraction Procedure for F. vulgare Obtained from IND, PAK, and SA
2.5. Isolation of the Essential Oil from F. vulgare Fruits Obtained from IND, PAK, and SA
2.6. Method Validation
2.7. Application of Proposed GC-MS Approach in the Simultaneous Determination of FCO and TOH in Methanolic Extracts and Essential Oils of F. vulgare Obtained from IND, PAK, and SA
3. Results and Discussion
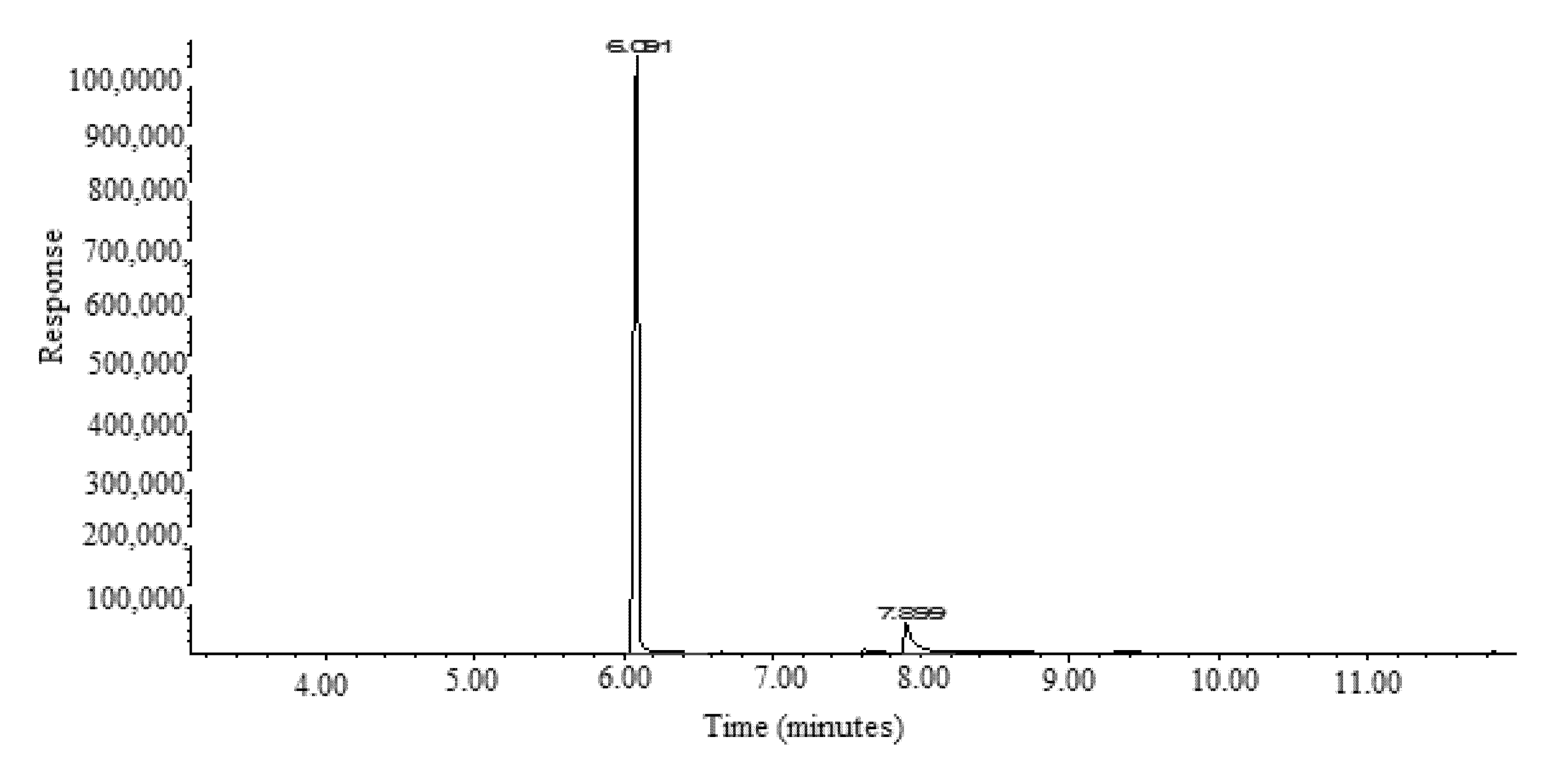
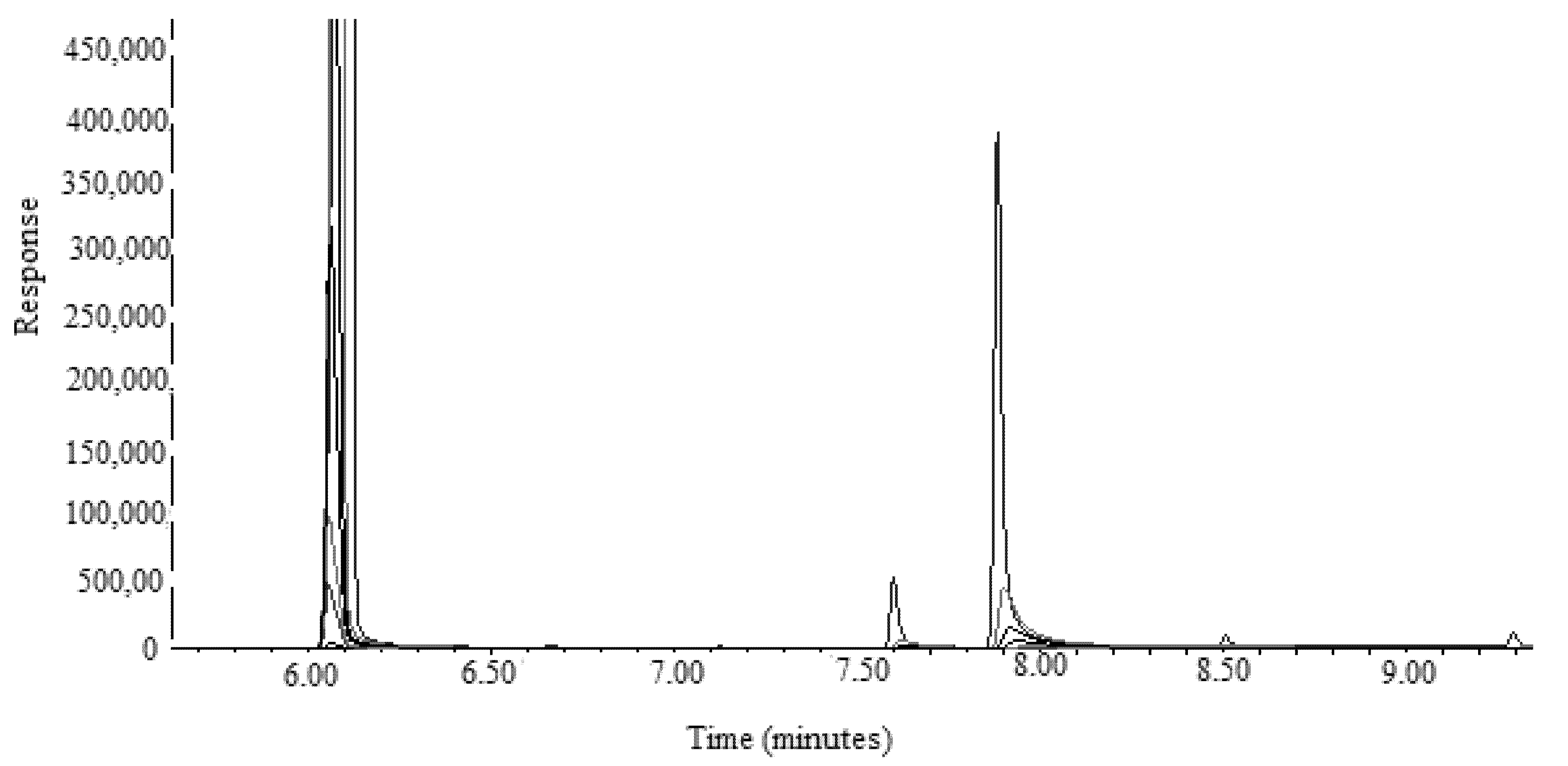
3.1. GC-MS Method Validation
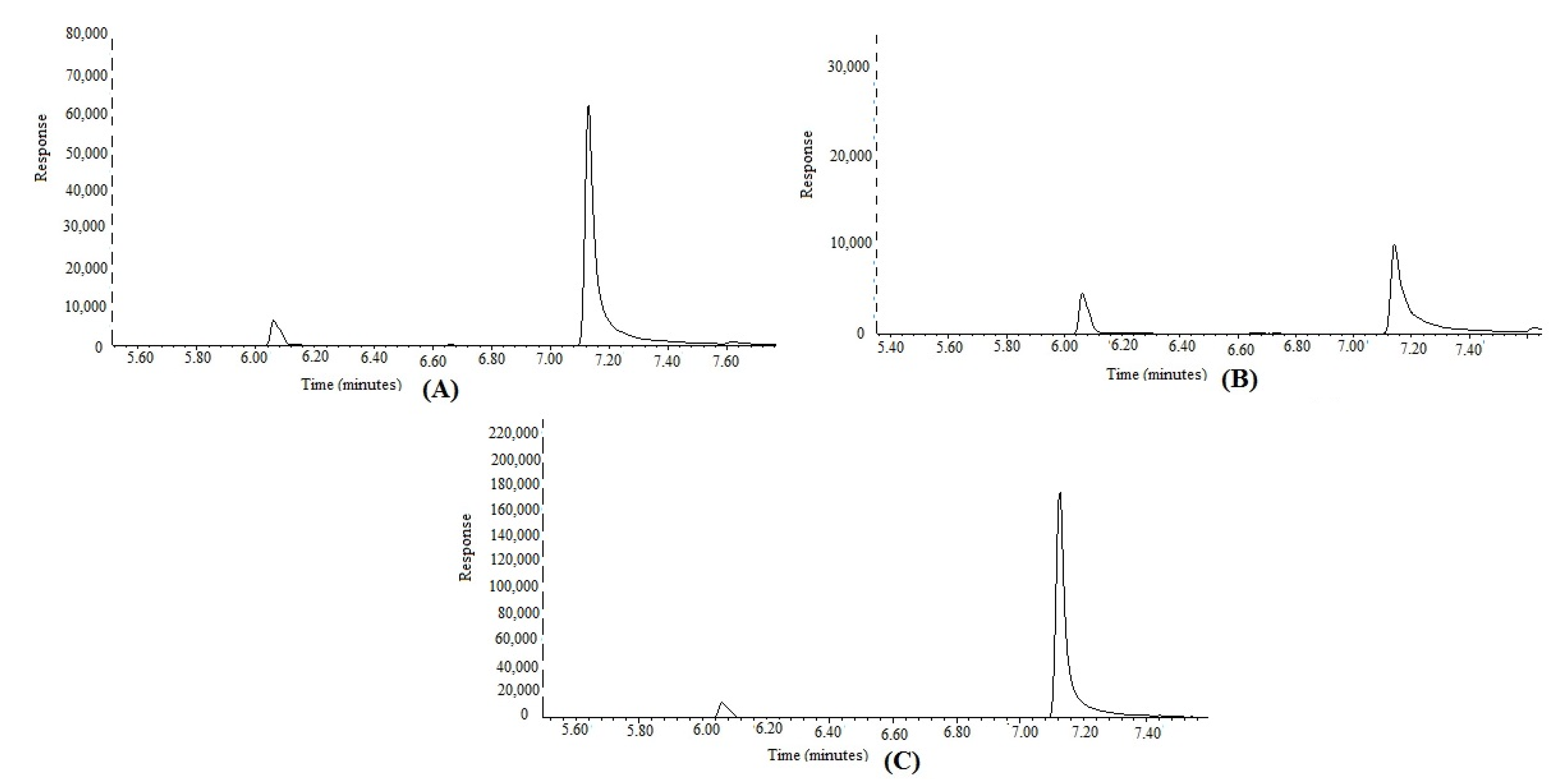
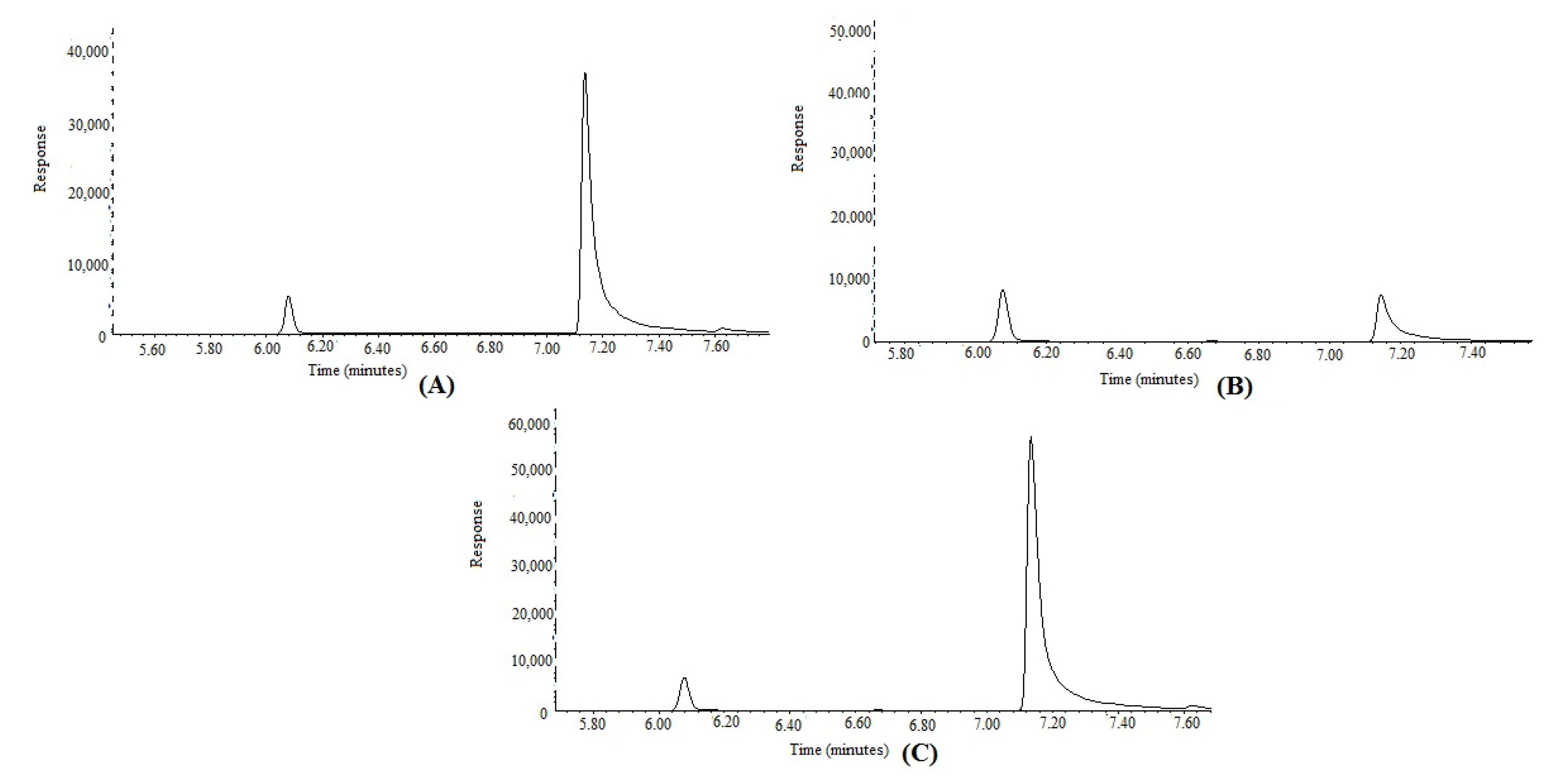
3.2. Application of Proposed GC-MS Approach in the Simultaneous Determination of FCO and TOH in Methanolic Extracts and Essential Oils of F. vulgare Obtained from IND, PAK, and SA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahmani, K.; Darbandi, A.I.; Ramshini, H.A.; Moradi, N.; Akbari, A. Agro-morphological and phytochemical diversity of various Iranian fennel landraces. Ind. Crops Prod. 2015, 77, 282–294. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M. Characterization of some Italian types of wild fennel (Foeniculum vulgare Mill.). J. Agric. Food Chem. 2001, 49, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Cosge, B.; Ipek, A.; Gurbuz, B. Gas chromatography/mass spectrometry analysis of essential oil from different vegetative organs and fruits of Foeniculum vulgare Mill. var. vulgare growing in Turky. Asian J. Chem. 2009, 21, 4081–4087. [Google Scholar]
- Renjie, L.; Zhenhong, L.; Shidi, S. GC-MS analysis of fennel essential oil and its effect on microbiology growth in rats’ intestine. Afr. J. Microbiol. Res. 2010, 4, 1319–1323. [Google Scholar]
- Anubhuti, P.; Rahul, S.; Kant, K.C. Standardization of fennel (Foeniculum vulgare), its oleoresin and marketed ayurvedic dosage forms. Int. J. Pharm. Sci. Drug Res. 2011, 3, 265–269. [Google Scholar]
- Hammouda, F.M.; Saleh, M.A.; Abdel-Azim, N.S.; Shams, K.A.; Ismail, S.I.; Shahat, A.A.; Saleh, I.A. Evaluation of the essential oil of Foeniculum vulgare Mill (fennel) fruits extracted by three different extraction methods by GC/MS. Afr. J. Trad. Compl. Alt. Med. 2013, 11, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Acimovic, M.; Tesevic, V.; Todosijevic, M.; Djisalov, J.; Oljaca, S. Compositional characteristics of the essential oil of Pimpinella anisum and Foeniculum vulgare grown in Serbia. Bot. Serb. 2015, 39, 9–14. [Google Scholar]
- Upadhyay, R.K. GC-MS analysis and in vitro antimicrobial susceptibility of Foeniculum vulgare seed essential oil. Am. J. Plant Sci. 2015, 6, 1058–1068. [Google Scholar] [CrossRef] [Green Version]
- Abdel Karm, M.; Ayda, A.; Khalid, M.S. GC-MS analysis and antimicrobial activity of Saudi Foeniculum vulgare Mill. (Apiaceae) fixed oil. Int. J. Adv. Res. 2017, 5, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Shahmokhtar, M.K.; Armand, S. Phytochemical and biological studies of fennel (Foeniculum vulgare Mill.) from the South Western region of Iran (Yasouj). Nat. Prod. Chem. Res. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Ravid, U.; Putievsky, E.; Katzir, I.; Ikan, R. Chiral gc analysis of enantiomerically pure fenchone in essential oils. Flavor Frag. J. 1992, 7, 169–172. [Google Scholar] [CrossRef]
- Alam, P.; Abdel-Kader, M.S.; Alqarni, M.H.; Zaatout, H.H.; Ahamad, S.R.; Shakeel, F. Chemical composition of fennel seed extract and determination of fenchone in commercial formulations by GC-MS method. J. Food Sci. Technol. 2019, 56, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; He, F.; Jin, K.; Wang, J.; Wang, Y.; Zhou, J.; Sun, J.; Fang, Q. Facile conversion of plant oil (anethole) to a high-performance material. Polym. Chem. 2017, 8, 2010–2015. [Google Scholar] [CrossRef] [Green Version]
- Jurado, J.M.; Alcazar, A.; Pablos, F.; Martin, M.J. LC determination of anethole in aniseed drinks. Chromatographia 2006, 64, 223–226. [Google Scholar] [CrossRef]
- Pang, X.; Bai, L.; Wang, Z.; Yang, H.; Liu, H.; Yan, H. Establishment of quantitatively analytical methods for the determination of aroma compounds in edible spices using a homemade chromatographic monolithic column. Chromatographia 2019, 82, 1345–1354. [Google Scholar] [CrossRef]
- Rajabi, M.; Haji-Esfandiari, S.; Barfi, B.; Ghanbari, H. Ultrasound-assisted temperature-controlled ionic-liquid dispersive liquid phase microextraction method for simultaneous determination of anethole, estragole, and para-anisaldehyde in different plant extracts and human urine: A comparative study. Anal. Bioanal. Chem. 2014, 406, 4501–4512. [Google Scholar] [CrossRef]
- Li, W.; Deng, J.; Qiao, J.; Li, Q.; Zhang, Y. HPLC determination of 4-hydroxy-anethole trithione in plasma via enzymatic hydrolysis and its application to bioequivalence study. J. Pharm. Biomed. Anal. 2008, 47, 612–617. [Google Scholar] [CrossRef]
- Fagundes, V.H.V.; Pinho, R.J.; Wiirzler, L.A.M.; Kimura, E.; Bersani-Amado, C.A.; Cuman, R.K.N. High performance liquid chromatography method for the determination of anethole in rat plasma. Trop. J. Pharm. Res. Anal. 2014, 13, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Najdoska-Bogdanov, M.; Bogdanov, J.B.; Stefova, M. Simultaneous determination of essential oil components and fatty acids in fennel using gas chromatography with a polar capillary column. Nat. Prod. Comm. 2015, 10, 1619–1626. [Google Scholar] [CrossRef] [Green Version]
- Leal, P.F.; Almeida, T.S.; Prado, G.H.C.; Prado, J.M.; Meireles, M.A.A. Extraction kinetics and anethole content of fennel (Foeniculum vulgare) and anise seed (Pimpenella anisum) extracts obtained by Soxhlet, ultrasound, percolation, centrifugation, and steam distillation. Sep. Sci. Technol. 2011, 46, 1848–1856. [Google Scholar] [CrossRef]
- Saini, N.; Singh, G.K. Gas chromatographic validated method for quantification of ayurvedic polyherbal formulation. Asian. J. Pharm. 2015, 9, 200–205. [Google Scholar] [CrossRef]
- Duta, D.E.; Culetu, A.; Negoita, M.; Ionescu, V. Quantification of anethole in fennel and anise essential oils using gas chromatography and 1H-NMR-spectroscopy. Bull. UASVM Food Sci. Technol. 2019, 76, 105–113. [Google Scholar]
- Najdoska, M.; Bogdanov, J.; Zdravkovski, Z. TLC and GC-MS analyses of essential oil isolated from Macedonian Foeniculi fructus. Maced. Pharm. Bull. 2010, 56, 29–36. [Google Scholar] [CrossRef]
- Quezada-Moreno, W.F.; Quezada-Torres, W.D.; Gallardo-Aguilar, I.; Cevallos-Carvajal, E.; Arias-Palma, G.; Travez-Castellano, A.; Zambrano-Ochoa, Z.; Rojas-Molina, O. Extraction and chemical characterization of the essential oil of Tagetes pusilla, in fresh and stored samples. Afinidad 2019, 76, 307–313. [Google Scholar]
- Schulz, K.; Schlenz, K.; Metasch, R.; Malt, S.; Romhild, W.; Drebler, J. Determination of anethole in serum samples by headspace solid-phase microextraction-gas chromatography-mass spectrometry for congener analysis. J. Chromatogr. A 2008, 1200, 235–241. [Google Scholar] [CrossRef]
- Bilia, A.R.; Fumarola, M.; Gallori, S.; Mazzi, G.; Vincieri, F.F. Identification by HPLC-DAD and HPLC-MS analyses and quantification of constituents of fennel teas and decoctions. J. Agric. Food Chem. 2000, 48, 4734–4738. [Google Scholar] [CrossRef]
- Kowalcze, M.; Wyrwa, J.; Dziubaniuk, M.; Jakubowska, M. Voltammetric determination of anethole on La2O3/CPE and BDDE. J. Anal. Methods Chem. 2018, 2018, E2158407. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Alqarni, M.H.; Alam, P.; Foudah, A.I.; Ghazwani, M. A HPTLC method for determination of anethole in essential oil, methanolic extract of Foeniculum vulgare and commercial herbal products. J. Pharm. Res. Int. 2020, 32, 68–75. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Yusufoglu, H.S.; Salkini, M.A.; Alam, P. Determination of trans-anethole in essential oil, methanolic extract and commercial formulations of of Foeniculum vulgare Mill. using a green RP-HPTLC-densitometry method. Separations 2020, 7, 51. [Google Scholar] [CrossRef]
- In Proceedings of the International Conference on Harmonization (ICH), Q2 (R1): Validation of Analytical Procedures–Text and Methodology, Geneva, Switzerland. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 1 April 2022).
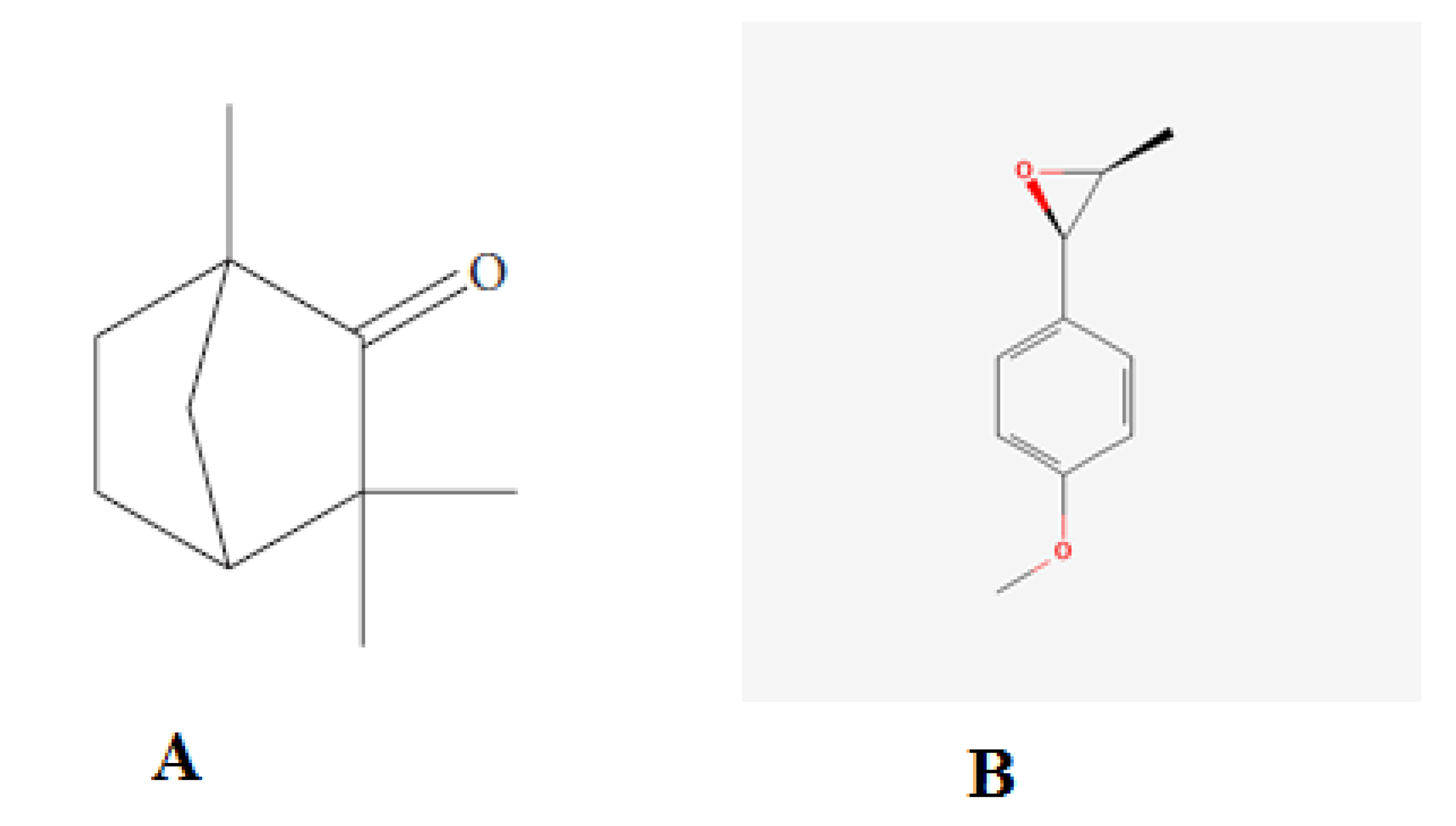
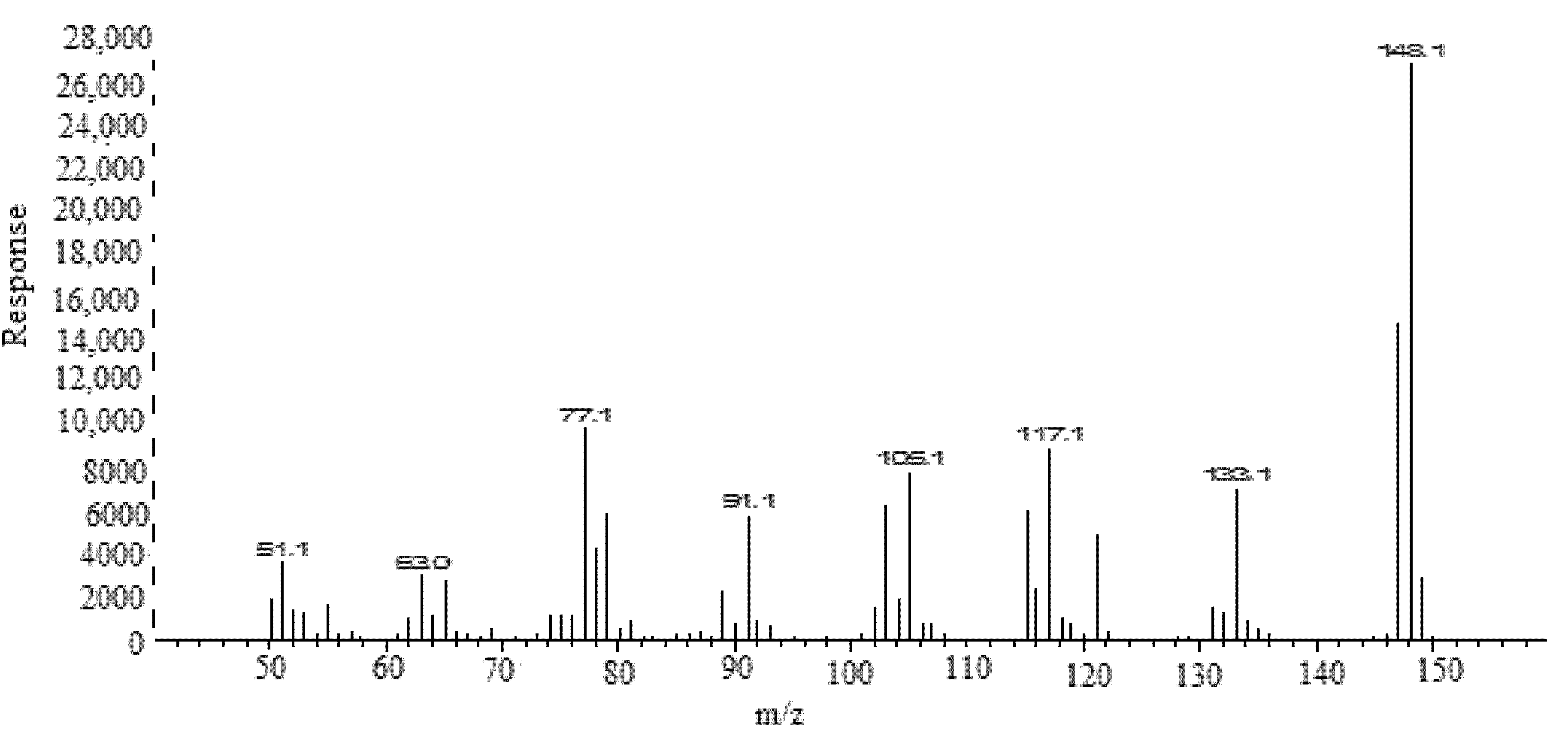
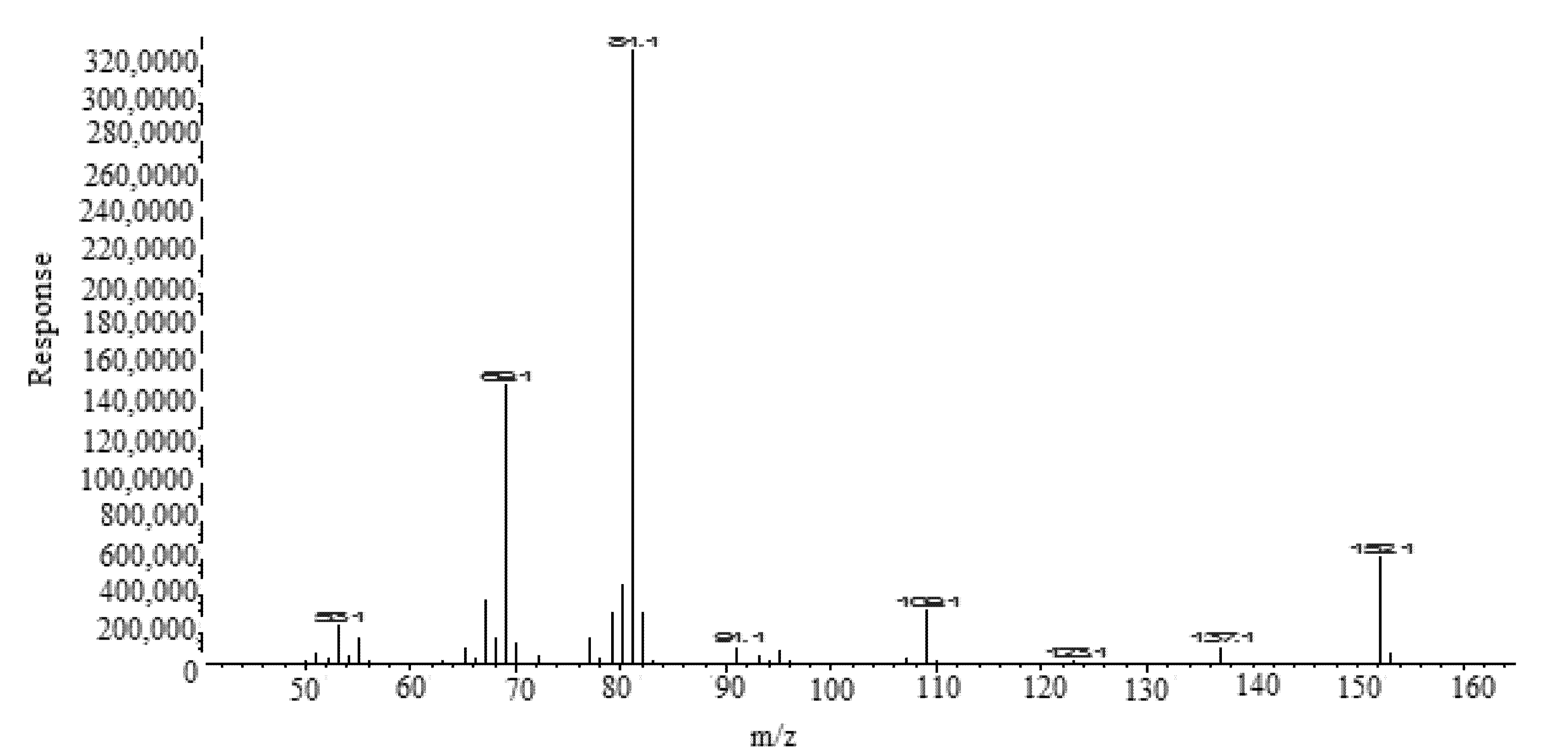
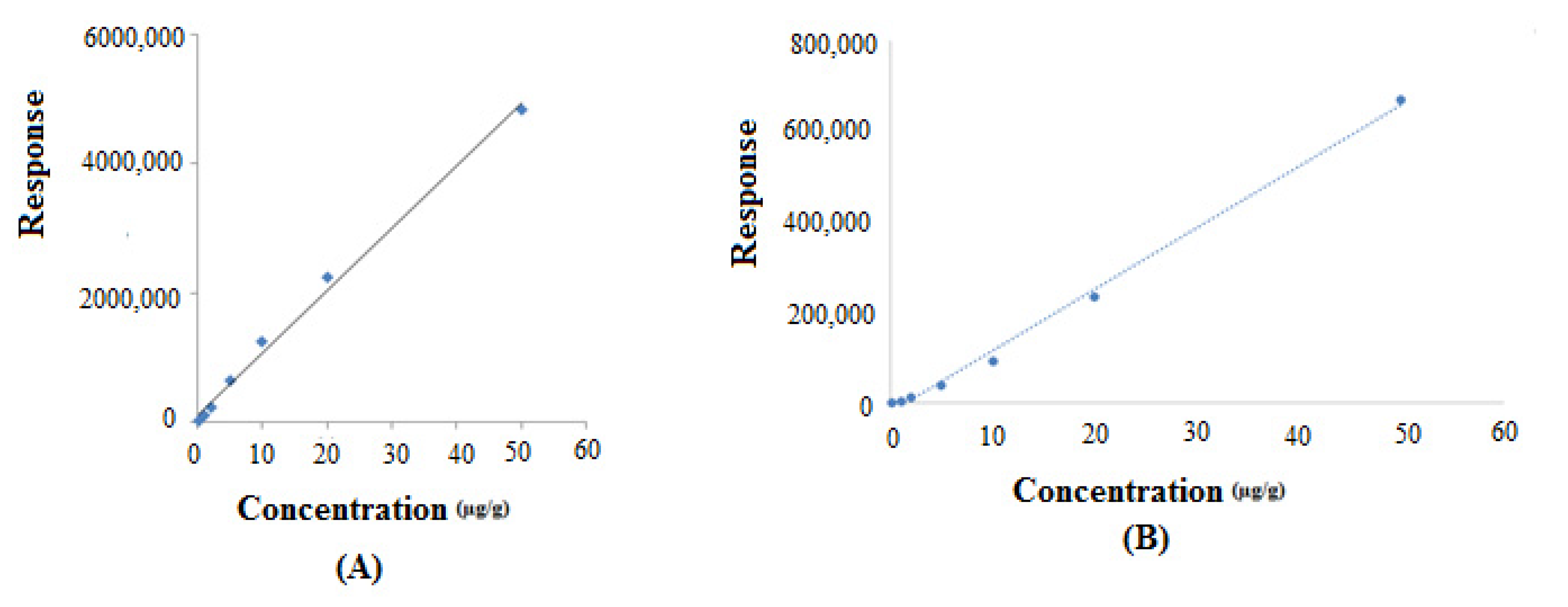
| Parameters | FCO | TOH |
|---|---|---|
| Linearity range (µg/g) | 0.1–50 | 0.1–50 |
| R2 | 0.9938 | 0.9957 |
| R | 0.9968 | 0.9978 |
| Slope ± SD | 96,653 ± 115 | 13,574 ± 38 |
| Intercept ± SD | 115,884 ± 985 | 20,084 ± 442 |
| Standard error of slope | 66.69 | 21.93 |
| Standard error of intercept | 568.70 | 255.19 |
| 95% confidence interval of slope | 96,367–96,938 | 13,479–13,668 |
| 95% confidence interval of intercept | 113,436–118,331 | 18,958–21,182 |
| LOD ± SD (µg/g) | 0.04 ± 0.00 | 0.05 ± 0.00 |
| LOQ ± SD (µg/g) | 0.12 ± 0.0 | 0.15 ± 0.00 |
| Conc. (µg/g) | Conc. Found (µg/g) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| FCO | |||
| 5 | 5.07 ± 0.10 | 101.40 | 1.97 |
| 10 | 9.84 ± 0.14 | 98.40 | 1.42 |
| 50 | 49.38 ± 0.32 | 98.76 | 0.64 |
| TOH | |||
| 5 | 4.97 ± 0.06 | 99.40 | 1.20 |
| 10 | 10.18 ± 0.10 | 101.80 | 0.98 |
| 50 | 49.92 ± 0.18 | 99.84 | 0.36 |
| Conc. (µg/g) | Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (µg/g) ± SD | Standard Error | CV (%) | Conc. (µg/g) ± SD | Standard Error | CV (%) | |
| FCO | ||||||
| 5 | 4.97 ± 0.07 | 0.04 | 1.40 | 5.09 ± 0.09 | 0.05 | 1.76 |
| 10 | 10.12 ± 0.12 | 0.06 | 1.18 | 9.63 ± 0.14 | 0.08 | 1.45 |
| 50 | 51.08 ± 0.28 | 0.16 | 0.54 | 48.86 ± 0.43 | 0.24 | 0.88 |
| TOH | ||||||
| 5 | 4.92 ± 0.05 | 0.05 | 1.01 | 5.03 ± 0.08 | 0.04 | 1.59 |
| 10 | 10.14 ± 0.09 | 0.09 | 0.88 | 10.13 ± 0.12 | 0.06 | 1.18 |
| 50 | 50.78 ± 0.22 | 0.22 | 0.43 | 49.76 ± 0.51 | 0.29 | 1.02 |
| Samples | Amount of FCO (mg/g) | Amount of TOH (mg/g) |
|---|---|---|
| F. vulgare extract (IND) | 0.031 ± 0.002 | 0.440 ± 0.010 |
| F. vulgare extract (PAK) | 0.021 ± 0.001 | 0.498 ± 0.012 |
| F. vulgare extract (SA) | 0.057 ± 0.008 | 1.74 ± 0.018 |
| F. vulgare oil (IND) | 0.021 ± 0.002 | 7.40 ± 0.342 |
| F. vulgare oil (PAK) | 0.034 ± 0.004 | 14.8 ± 0.621 |
| F. vulgare oil (SA) | 0.029 ± 0.005 | 10.2 ± 0.464 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Amir, M.; Ahamad, S.R.; Alam, P.; Alshehri, S.; Ghoneim, M.M.; Wahab, S.; Shakeel, F. Simultaneous Determination of Fenchone and Trans-Anethole in Essential Oils and Methanolic Extracts of Foeniculum vulgare Mill. Fruits Obtained from Different Geographical Regions Using GC-MS Approach. Separations 2022, 9, 132. https://doi.org/10.3390/separations9050132
Ahmad W, Amir M, Ahamad SR, Alam P, Alshehri S, Ghoneim MM, Wahab S, Shakeel F. Simultaneous Determination of Fenchone and Trans-Anethole in Essential Oils and Methanolic Extracts of Foeniculum vulgare Mill. Fruits Obtained from Different Geographical Regions Using GC-MS Approach. Separations. 2022; 9(5):132. https://doi.org/10.3390/separations9050132
Chicago/Turabian StyleAhmad, Wasim, Mohd Amir, Syed Rizwan Ahamad, Prawez Alam, Sultan Alshehri, Mohammed M. Ghoneim, Shadma Wahab, and Faiyaz Shakeel. 2022. "Simultaneous Determination of Fenchone and Trans-Anethole in Essential Oils and Methanolic Extracts of Foeniculum vulgare Mill. Fruits Obtained from Different Geographical Regions Using GC-MS Approach" Separations 9, no. 5: 132. https://doi.org/10.3390/separations9050132
APA StyleAhmad, W., Amir, M., Ahamad, S. R., Alam, P., Alshehri, S., Ghoneim, M. M., Wahab, S., & Shakeel, F. (2022). Simultaneous Determination of Fenchone and Trans-Anethole in Essential Oils and Methanolic Extracts of Foeniculum vulgare Mill. Fruits Obtained from Different Geographical Regions Using GC-MS Approach. Separations, 9(5), 132. https://doi.org/10.3390/separations9050132








