Abstract
Cottonseed hull is a livestock feed with large daily consumption. If pesticide residues exceed the standard, it is easy for them to be introduced into the human body through the food chain, with potential harm to consumer health. A method for multi-residue analysis of 237 pesticides and their metabolites in cottonseed hull was developed by gas-chromatography and liquid-chromatography time-of-flight mass spectrometry (GC-QTOF/MS and LC-QTOF/MS). After being hydrated, a sample was extracted with 1% acetic acid in acetonitrile, then purified in a clean-up tube containing 400 mg MgSO4, 100 mg PSA, and 100 mg C18. The results showed that this method has a significant effect in removing co-extracts from the oily matrix. The screening detection limit (SDL) was in the range of 0.2–20 μg/kg, and the limit of quantification (LOQ) was in the range of 0.2–20 μg/kg. The recovery was verified at the spiked levels of 1-, 2-, and 10-times LOQ (n = 6), and the 237 pesticides were successfully verified. The percentages of pesticides with recovery in the range of 70–120% were 91.6%, 92.8%, and 94.5%, respectively, and the relative standard deviations (RSDs) of all pesticides were less than 20%. This method was successfully applied to the detection of real samples. Finally, this study effectively reduced the matrix effect of cottonseed hull, which provided necessary data support for the analysis of pesticide residues in oil crops.
1. Introduction
The composition of cottonseed hull is similar to that of soybean concentrate, with a high content of cellulose that can enhance the digestive systems of ruminants. Cottonseed hull has been widely used as an alternative feed for ruminants, due to its low price, easy availability, and excellent mixing performance [1,2,3]. The excessive and illegal use of pesticides during forage planting makes it easy for pesticides to enter the food chain and accumulate in animal adipose tissue [4], and human consumers may indirectly experience food safety problems through contact with livestock products. The composition of the oily matrix is complex: in addition to fat, it contains polysaccharides, proteins, pigments, and other substances. In the process of residue analysis, problems such as matrix enhancement, matrix inhibition, and retention-time shifts may occur in the detection of pesticides, which will hinder the detection of target compounds [5,6]. Therefore, it is urgent to develop a detection technique for the oily matrix to solve these problems.
The analysis of pesticide residue usually includes the following steps: (1) extraction of the target compound; (2) removal of interference from the extract; and (3) qualitative and quantitative detection of the target compound [4]. Lipophilic pesticides tend to be concentrated in fat. Improper pretreatment will affect the detection sensitivity, recovery, and sample throughput [7]. The current pretreatment methods for plant-derived oil substrates mainly include dispersion liquid-liquid micro-extraction (DLLME) [8], matrix solid phase dispersion (MSPD) [9,10], low temperature fat precipitation (LTFP) [11], solid phase extraction (SPE) [5], and QuEChERS [12,13,14,15,16]. The QuEChERS method requires fewer reagent consumables and short pretreatment time, so it is accepted by more and more experimenters [17]. Theurillat et al. established the QuEChERS method to determine the residues of various pesticides and verified the method for 176 pesticides in six oily matrices [12]. Rutkowska et al. investigated the matrix effect and recovery of four seed samples of cress, fennel, flax, and hemp. The final method verified 248 pesticides, and the LOQs reached 0.005 mg/kg [14]. Banerjee et al. used the QuEChERS method to analyze more than 220 pesticide residues in sesame seeds. This method can effectively reduce the interference of the matrix effect by freezing and degreasing at −80 °C and then purifying the oil.
The current trend of separation science is to develop new chromatographic mass spectrometry methods that can detect multiple compounds at the same time after a single injection, thereby reducing analysis time and cost [18]. The current detection technology for the detection of pesticide residues in oily matrices is mainly triple quadrupole mass spectrometry (MS/MS) [13,19,20,21]. The data was collected according to the specific nucleo-cytoplasmic ratio of the specified compound, but other compounds that were not in the list could not be identified. When analyzing a large number of compounds, the sensitivity and selectivity are limited. Due to their high resolution, precise mass accuracy, outstanding full-scan sensitivity, and complete mass spectrometry information, high-resolution mass spectrometry (HRMS), such as time-of-flight mass spectrometry (TOF/MS) and quadrupole Orbitrap mass spectrometry (Obitrap/MS), can be used without additional sample injection. Under retrospective analysis, with these advantages, HRMS has been widely used in the field of food analysis [22,23]. Lehotay et al. used GC-TOF to analyze 34 pesticides in flaxseed, dough, and peanuts [15]. Amadeo et al. used GC-QTOF to verify 166 pesticide residues in avocados and almonds [24].
To ensure the safety of livestock feed and to prevent pesticide residues from being introduced into the human body through the food chain, this work established a QuEChERS multi-residue analysis method, and used GC- and LC-QTOF/MS techniques to verify 237 pesticides in cottonseed hull. By optimizing the hydration volume, extraction solvent, salting-out agent, and clean-up sorbents, the influence of the matrix effect was reduced and the pesticide recovery was optimized. Finally, this method was successfully applied to the analysis of actual samples, providing data support for the risk of pesticide residues in oily substrate monitoring.
2. Materials and Methods
2.1. Chemicals and Reagents
Pesticide standards (purity ≥ 98%) were obtained from Tianjin Alta Scientific (Tianjin, China). Sodium chloride, magnesium sulfate, and sodium sulfate (analytical purity) were obtained from Tianjin Fuchen Chemical Reagent Ltd. (Tianjin, China). Primary secondary amine (PSA) and C18 were purchased from Agilent Technologies (Santa Clara, CA, USA). Methanol, acetonitrile, and toluene (chromatographic purity) were obtained from Anpel Laboratory Technology (Shanghai, China). Formic acid and ammonium acetate (mass spectrometry grade) were obtained from Honeywell (Muskegon, MI, USA).
2.2. Apparatus
HPLC-QTOF/MS Agilent 1290 and Agilent 6550 equipped with Agilent Dual Jet Stream ESI and GC-QTOF/MS Agilent 7890B and Agilent 7200 were obtained from Agilent Technologies (Santa Clara, CA, USA). A Milli-QTM Ultrapure Water System was obtained from Millipore (Milford, MA, USA). An N-EVAP112 Nitrogen Blowing Concentrator was obtained from Organomation Associates (Worcester, MA, USA). An AH-30 Automatic homogenizer was obtained from RayKol Group Corp., Ltd. (Xiamen, China). An MS204S Electronic Analytical Balance was obtained from Mettler Toledo (Shanghai, China).
2.3. Standard Solution
Ten mg of the standard substance was accurately weighed into a 10 mL brown volumetric flask. a suitable reagent was selected according to the solubility of the compound in the organic reagent. It was dissolved by ultrasound and diluted to the mark to a standard solution of 1 mg/L. The standard solution was stored at −18 °C in the dark. As needed, a pipette with an appropriate amount of the standard stock solution was diluted with methanol to prepare a working solution of appropriate concentration, and stored at 4 °C in the dark.
2.4. Sample Preparation Method
Based on other oily matrix sample preparation methods [12,16], a modified QuEChERS method was used for the detection of cottonseed hull. Two g (accurate to ±0.01 g) of sample were transferred into a 50 mL centrifuge tube; 2 mL of ultrapure water were added for hydration and then extracted with 10 mL of 1% acetic acid in acetonitrile. The homogenizer was used to homogenize the sample for 1 min at 13,500× g; then, 4 g MgSO4, 1 g NaCl and a ceramic homoproton were added. The mixture was shaken for 10 min and centrifuged at 3155× g for 5 min; then, 3 mL of supernatant was transferred to a clean-up tube containing 400 mg MgSO4, 100 mg PSA, and 100 mg C18. After shaking for 10 min and being centrifuged at 3155× g for 5 min, 1 mL of supernatant was dried under nitrogen, then ultrasonically redissolved with ethyl acetate containing internal heptachlor-exo-epoxide for GC-QTOF/MS analysis, and ultrasonically redissolved with acetonitrile aqueous solution (2:3, v/v) containing internal standard atrazine D5 for LC-QTOF/MS analysis.
2.5. Instrument Parameters
The instrument parameters of LC-QTOF/MS and GC-QTOF/MS were configurated according to a previous paper published by our laboratory [25].
An LC-QTOF/MS: ZORBAX SB-C18 column (100 mm × 2.1 mm, 3.5 μm, Agilent Technologies) was used for separation at 40 °C; 5 mmol/L ammonium acetate with 0.1% (v/v) formic acid aqueous solution and acetonitrile were applied as phase A and phase B. The flow rate was set at 0.4 mL/min. The gradient program was set as follows: 0 min, 1% B; 3 min, 30% B; 6 min, 40% B; 9 min, 40% B; 15 min, 60% B; 19 min, 90% B; 23 min, 90% B; 23.01 min, 1% B. The equilibrium time was 4 min. The injection volume was 5 μL.
The Agilent Dual Jet Stream (AJS) ESI source (Agilent Technologies) was set in positive full scan (m/z 50–1000) mode; the capillary voltage was 4 kV; nitrogen was used as the nebulizer gas at 0.14 MPa; the sheath gas temperature was set at 375 °C with 11.0 L/min; the drying gas flow rate was 12.0 L/min; the drying gas temperature was 225 °C; the fragmentation voltage was 345 V. In all ions Mass/Mass mode, the collision energy was 0 V at 0 min, and 0, 15, and 35 V at 0.5 min, respectively. The total program duration was 27.01 min.
GC-QTOF/MS: HP-5 MS UI (30 m × 0.25 mm, 0.25 μm, Agilent Technologies) was used for separation at 40 °C. The oven temperature gradient was started at 40 °C for 1 min, increased at 30 °C/min to 130 °C, heated at 5 °C/min to 250 °C, ramped to 300 °C at 10 °C/min, and maintained for 7 min. Helium (purity > 99.999%) was used as the carrier gas with a constant flow rate of 1.2 mL/min. The injection temperature was set to 270 °C and the injection volume was 1 µL. The injection mode was not split injection, and the purge valve was opened after 1 min.
The ion source was an electronic ionization source (70 eV, 280 °C), and the temperatures of the transfer line and the quadrupole were 250 °C and 180 °C, respectively. Solvent delay was set to 3 min; the ion monitoring mode was full scan; scanning ranged (m/z) from 45 to 550; the scan rate was 5 Hz. The total program duration was 42 min.
Mass calibration was required before sample acquisition, and the instrument was tuned at intervals to ensure stability.
2.6. Method Validation
The screening method of high-resolution mass spectrometry can be validated through screening detection limits (SDL), and the quantitative method can be validated through limit of quantitation (LOQ). The SDL, LOQ, linearity, recovery, and precision of this experiment were verified by SANTE/12682/2019 guidelines. SDL is the minimum concentration at which more than 95% of a series of concentration levels meets the detection requirements (20 additional experiments were conducted in parallel for each concentration). When the SDL and recovery were validated, all the target pesticides were spiked to the sample and the spiked samples were placed at room temperature for 30 min, then treated according to the above method. After the 10-point matrix matching calibration was constructed, its linearity was evaluated with the coefficient of determination (R2). The recovery and precision were investigated in three different levels of spiked blank samples with 1-, 2-, and 10-times LOQ.
The matrix effect (ME) is the interference of other components in the matrix with the target compounds. The formula is:
where bm is the slope of the matrix standard curve and bs is the slope of the solvent standard curve.
ME (%) = (bm − bs)/bs × 100%
Based on previous studies, we established several hundred kinds of pesticide databases on gas and liquid high resolution mass spectrometry, respectively [25]. According to the recovery and precision, 237 pesticides were divided into pesticides suitable for GC or LC detection.
3. Results
3.1. Optimization of Hydration Volume
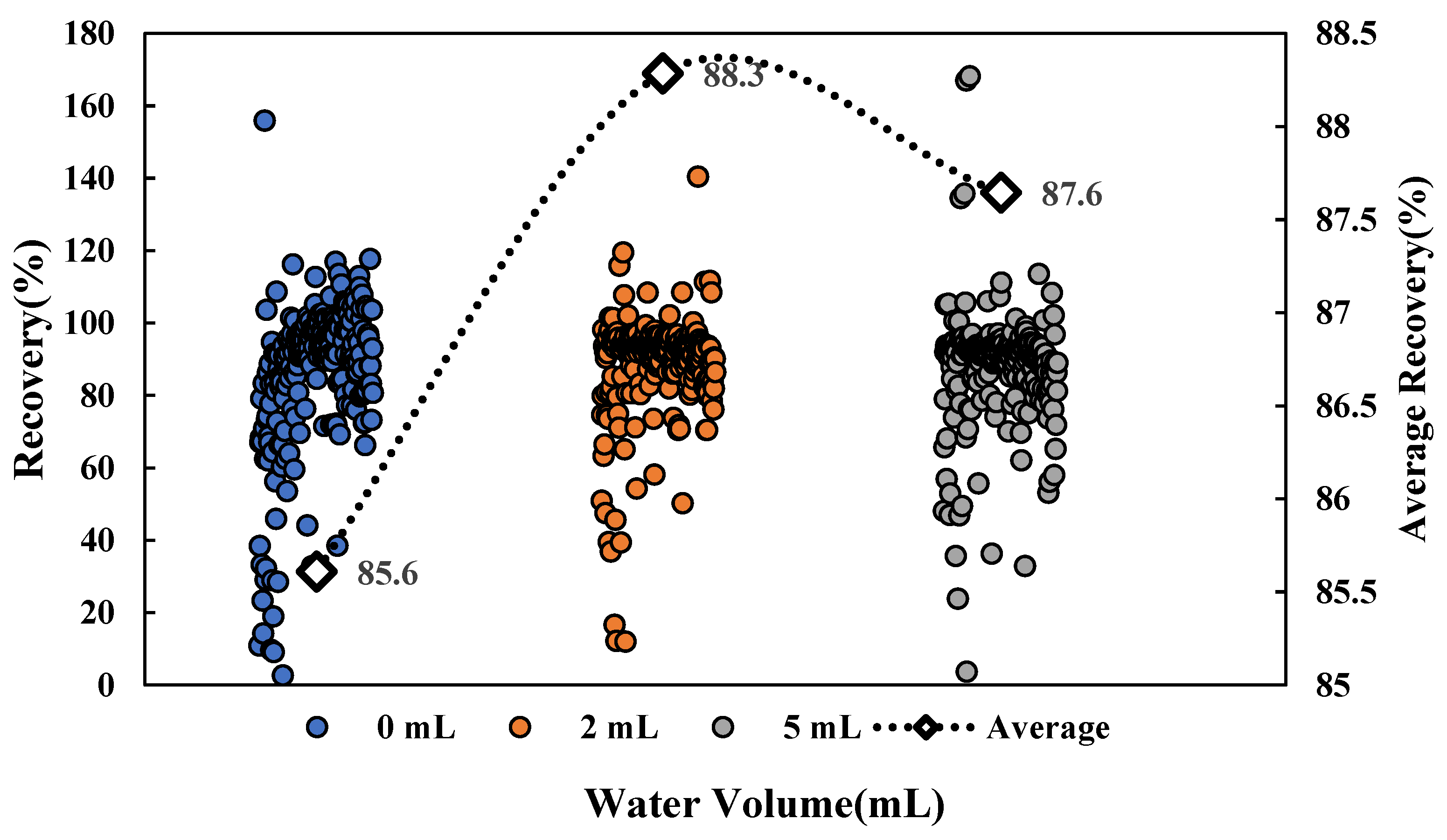
For the oily matrix, adding an appropriate amount of water for hydration during sample pretreatment was conducive to the softening of the matrix epidermis, making it easier for residual pesticides in the matrix to be extracted. This experiment explored the effect of different hydration volumes on the recovery of multiple pesticides. The experiment results show that the proportion of pesticides that met the recovery requirements (70–120%) under a non-hydration condition was 74.9%, which was less than under the conditions with water additions of 2 mL and 5 mL. Under the condition of a 2 mL water addition, the number of pesticides meeting the recovery requirements was the most numerous, accounting for 83.5%. As shown in Figure 1, the average recovery under the 2 mL condition was 88.3%, which was higher than that under the other two conditions. The results were in line with our expectations. The oil-water partition coefficient (logP) is an important parameter for the solubility of compounds, which is a simulated value based on the soil sorption coefficient normalized to organic carbon content (log Koc) [26]. The smaller the logP value, the better the water solubility of the compound. The effect of hydration volume on recovery with different logP was investigated, showing that hydration had a great impact on recovery with a low logP. The overall recovery of 54 pesticides with hydrophilic compounds (logP < 2.0) was low under a non-hydration condition, with the pesticides meeting the requirements accounting for 42.6%. When the hydration volume was 5 mL, the pores were opened due to the increase in the hydration volume, and multiple interferents in the matrix could be extracted together. The matrix promotion effect was enhanced, so that the overall recovery of pesticides with logP < 2.0 was higher than the recovery under the other two conditions. When the hydration volume was 2 mL, the pesticides that met the requirements of recovery were most numerous, accounting for 70.4%; therefore, 2 mL was finally selected as the optimal hydration volume.
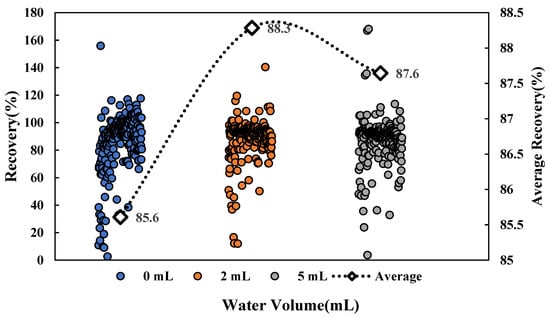
Figure 1.
Effects of hydration volumes on pesticide recovery.
3.2. Optimization of Extraction Solvent Volume
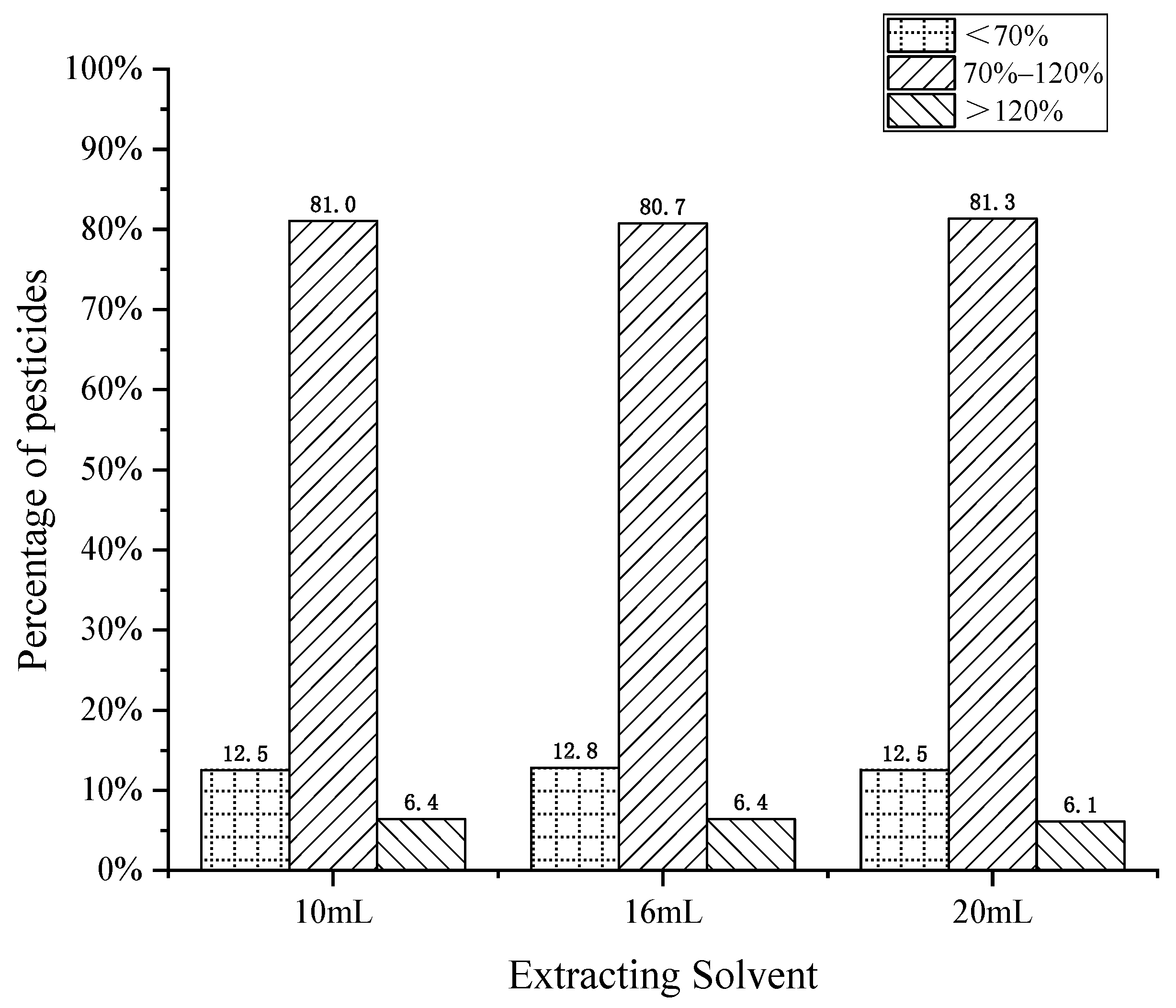
The extraction of target compounds is a critical step in pesticide residue analysis. Mol et al. [27] tested a series of solvents for extraction and found that methanol usually extracts too many compounds in the matrix, and further matrix removal steps were required. Acetonitrile has low solubility in fat and a low matrix effect when extracting from complex matrices. Therefore, acetonitrile was selected as the extraction solvent of cottonseed hull in this experiment. Three different extraction volumes of 10 mL, 16 mL, and 20 mL (i.e., a hydration volume and extraction volume ratio of 1:5, 1:8, and 1:10) were compared to explore the effect of different extraction volumes on the recovery of pesticide residues. The results are shown in Figure 2. It was found that when the extract volume was 10 mL, 16 mL, and 20 mL, the proportion of pesticides meeting the recovery requirements was similar, at 81.0%, 80.7% and 81.3% respectively. However, at the spiked level, the volume of the extraction solution decreased, the pesticide concentration per unit volume increased, and more pesticide compounds had better peak shapes. In addition, a lower organic reagent amount was recommended from the perspective of green environmental protection, so the final extraction volume was 10 mL.
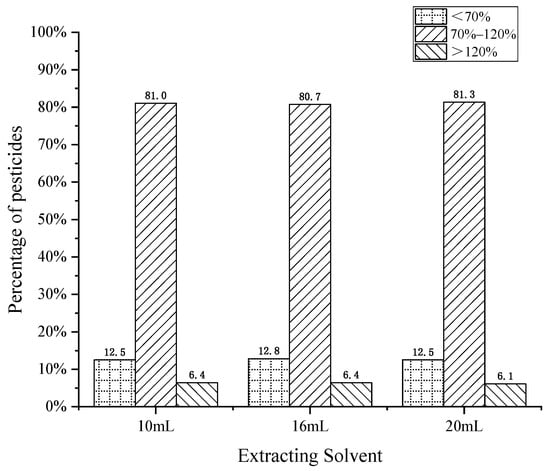
Figure 2.
Effect of extraction solvent volume on pesticide recovery.
3.3. Optimization of Salting-Out Agent
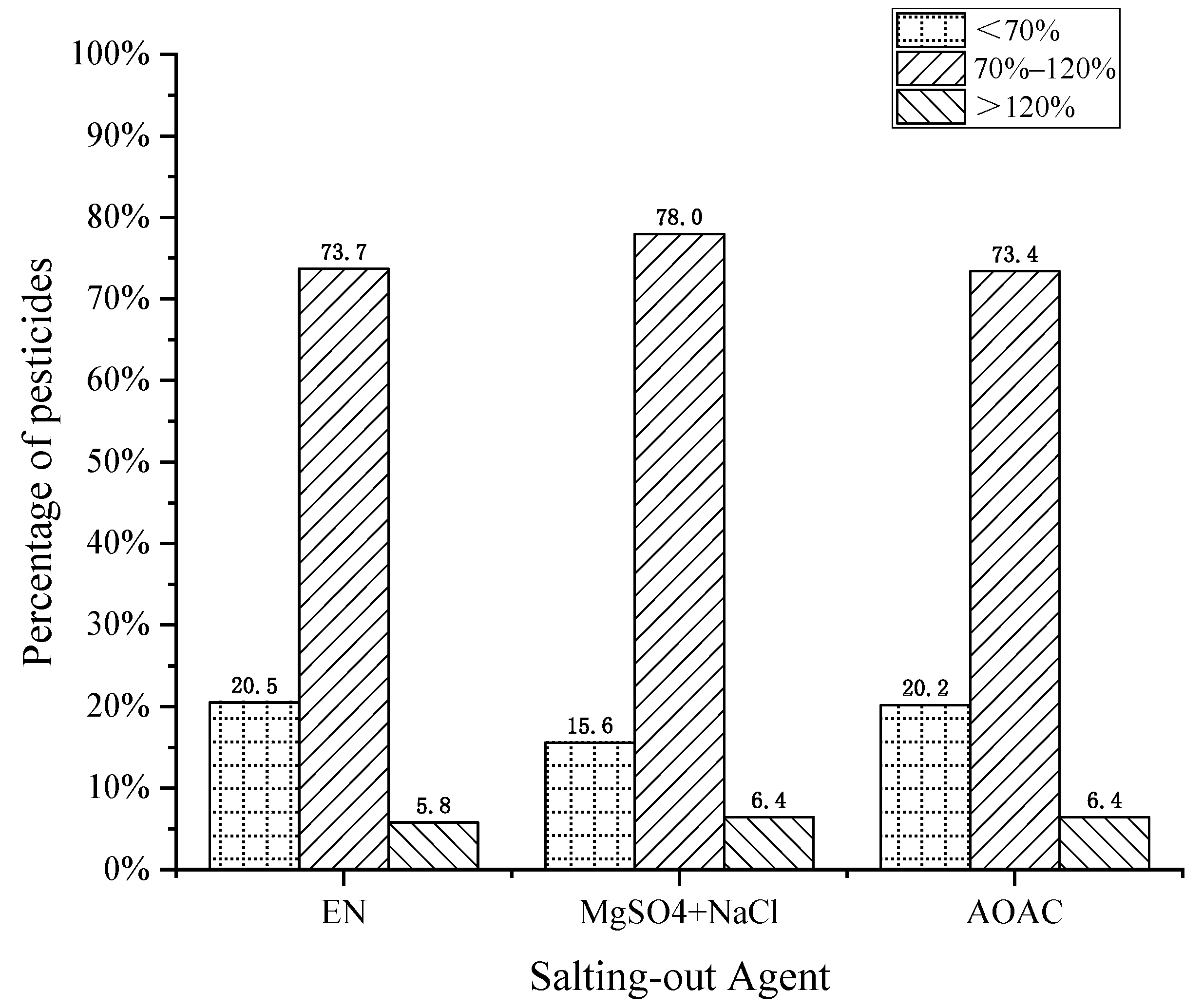
The salting-out agents commonly used in pesticide residue screening were EN buffer salt (4 g MgSO4, 1 g NaCl, 0.5 g disodium hydrogen citrate, and 1 g sodium citrate), the QuEChERS method for fruits and vegetables (4 g MgSO4 and 1 g NaCl), and AOAC buffer salt (6 g MgSO4 and 1.5 g NaAc). In this work, the effects of the above three salting-out agents on the recovery of pesticides were compared. As shown in Figure 3, although EN or AOAC salt forms a buffer system in the solution state, the results showed that the recovery using an MgSO4 + NaCl combination best met the requirements, accounting for 78%. The reason for this result was that the volume of the extract from the QuEChERS method was relatively small. If the amount of extraction salt was too large, the heat emitted during water absorption destroys the structure of thermally unstable pesticides and affects their recovery. Therefore, 4 g MgSO4 and 1 g NaCl with less salt consumption were finally selected as the salting-out agents.
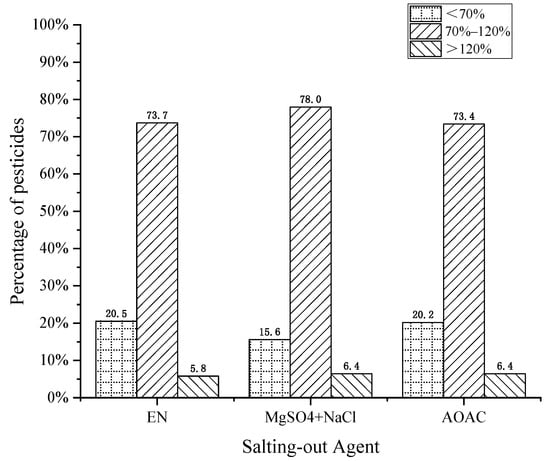
Figure 3.
Effect of salting-out agents on pesticide recovery.
3.4. Optimization of Types and Amounts of Clean-Up Sorbents
A clean-up procedure was a key step in the pretreatment of the oily matrix. Its purpose was to effectively purify the analyzed matrix, and most of target pesticides had acceptable recovery, precision, and matrix effect [14]. Although acetonitrile had low liposolubility, which can slightly reduce the interference of a fat-soluble matrix on target compounds [15], in order to effectively reduce the influence of high-fat matrix co-extraction on the detection sensitivity of pesticides, as well as instrument loss, the clean-up procedure was necessary. Theurillat established a d-SPE clean-up method containing 150 mg C18 and 150 mg PSA to determine 176 pesticide residues in fatty foods [12]. Therefore, this study was optimized on this basis.
In this work, the ability of MgSO4 + PSA + C18 + Z-sep and MgSO4 + PSA + C18 sorbents were compared. The structure of PSA had -NH2, which can form a strong hydrogen bond with -COOH, so it was often used to adsorb polar compounds, such as fatty acids, lipids, and carbohydrates. C18 was often used to adsorb non-polar compounds, such as long-chain aliphatic compounds and sterols [8,25]. Z-sep was a new adsorbent, based on zirconia, which can be used for the adsorption of hydrophobic compounds in the fat matrix [28]. It was seen that the bottom of the purification tube after Z-sep purification was dark yellow, while the sample without Z-sep purification was light yellow, indicating that Z-sep had an obvious effect on degreasing.
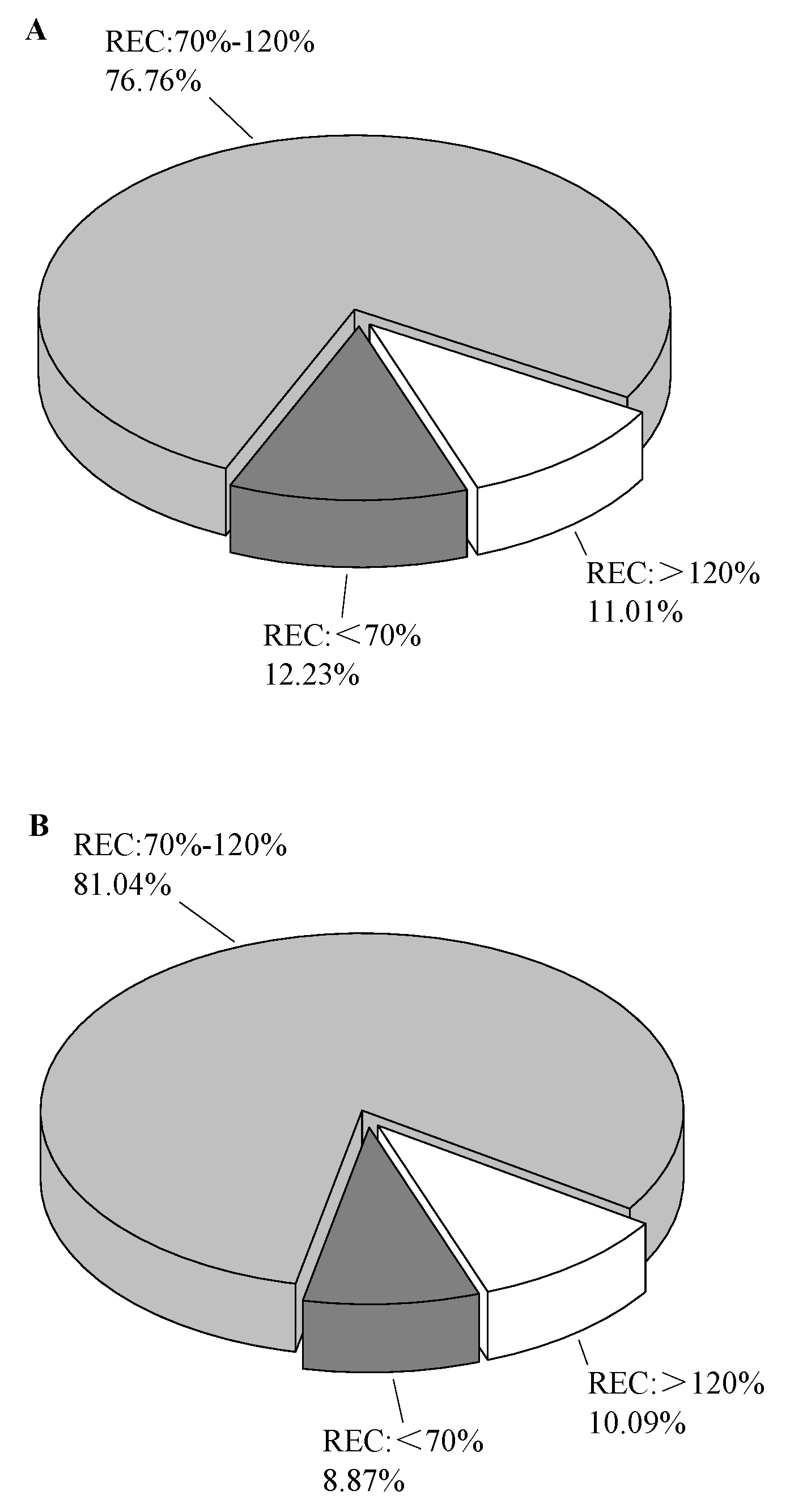
In order to further verify the ability of sorbents, the spiked experiments were carried out. As shown in Figure 4, A was the sorbent combination of MgSO4 + PSA + C18 + Z-sep, and B was the sorbent combination of MgSO4 + PSA + C18. As a result, the sorbent combination without Z-sep accounted for more pesticides that meet the requirements, reaching 81.04%. The reason for this result was that Z-sep adsorbs some target pesticides while removing lipids. According to the Lewis theory, the affinities of Z-sep on the analyte containing different substituent characteristics can be sorted in the following order: chloride < formate < acetate < sulphate< citrate < fluoride < phosphate < hydroxide [25]. In this work, a variety of pesticides, such as trinexapac-ethyl, abamectin containing -OH, fenamiphos sulfoxide containing phosphate, and sulfoxaflor containing sulphate, had substituents with a strong affinity to Z-sep. Therefore, the recovery of sorbent combinations with Z-sep was significantly lower than that without Z-sep. Although Z-sep was more efficient in removing lipid compounds, the sorbent combination of MgSO4 + PSA + C18 was finally selected as the purification filler in this work, from the perspective of method versatility.
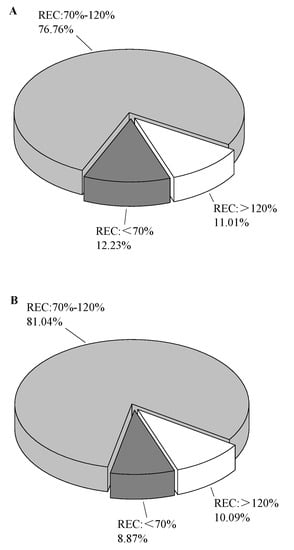
Figure 4.
Effect of clean-up sorbents on pesticide recovery. (A) MgSO4 + PSA + C18 + Z-sep; (B) MgSO4 + PSA + C18.
The amount of PSA and C18 was also optimized. The effects of PSA (50–150 mg) and C18 (100–300 mg) on the recovery of various pesticides were optimized by controlling other variables. The results showed that when the amount of PSA was 100 mg, the greatest number of pesticides with satisfactory recovery was obtained, accounting for 73.7%. With the increase in PSA amount, the recovery of organic nitrogen pesticides, such as propanil and fenbuconazole, and carbamate pesticides, such as aldicarb-sulfone and thiophanate-methyl, gradually decreased. When the amount of C18 was 100 mg, the proportion of pesticides that met satisfactory recovery was 82.0%. With an increase in the C18 amount, the recovery of various organic nitrogen pesticides obviously decreased, especially the chlorides with a benzene ring structure, such as monolinuron, novaluron, propanil, and pretilachlor. Therefore, 100 mg PSA and 100 mg C18 were finally selected as the optimal amounts of clean-up sorbents.
3.5. Evaluation of Matrix Effect
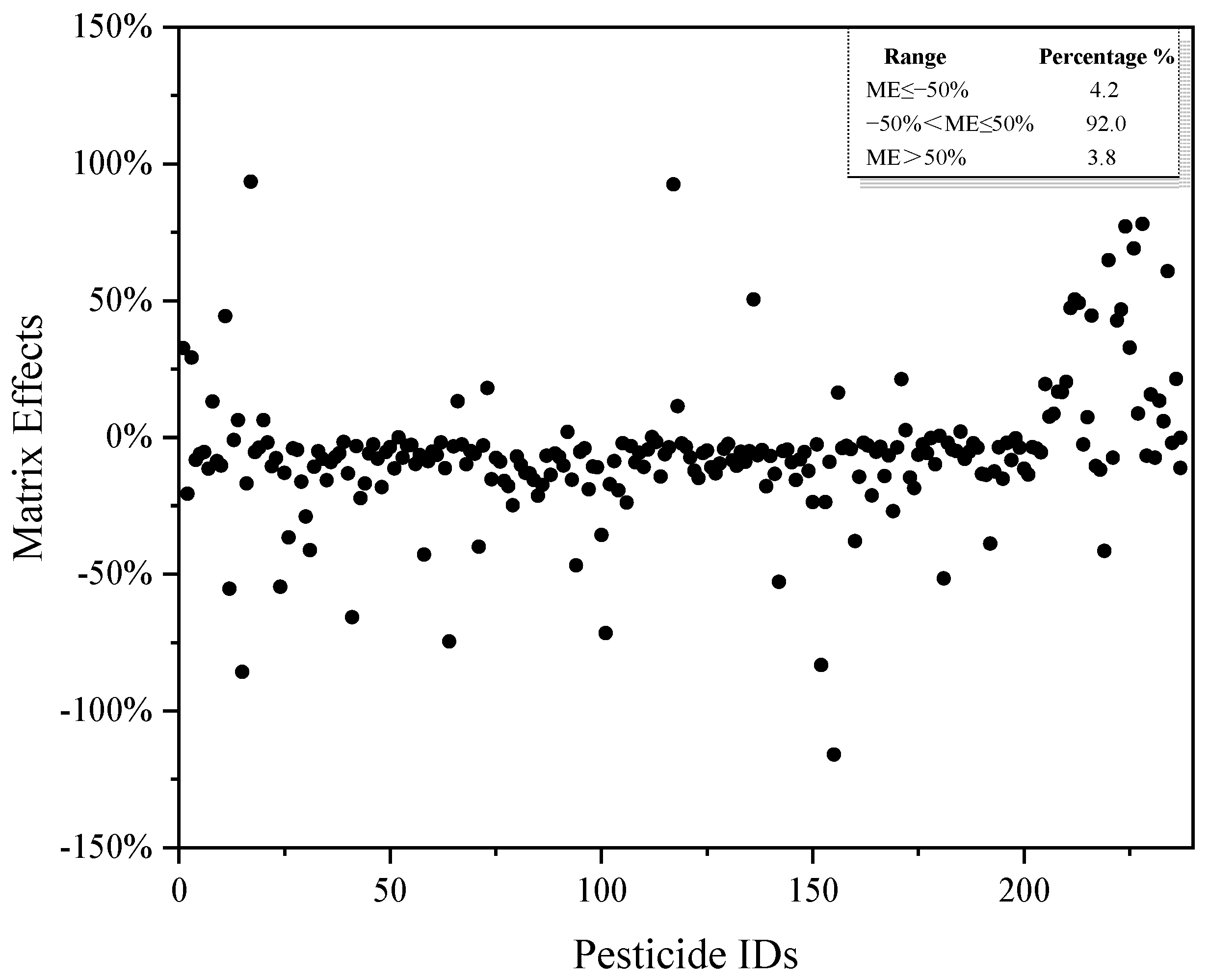
Analysis of pesticide residues in the oil matrix may be adversely affected by the matrix effect. The main result of the matrix effect is to increase or decrease the analyte signal when the same analyte exists in the solvent [29]. The methods for eliminating or reducing the matrix effect include: (1) optimizing the sample preparation method and reducing co-extraction; (2) changing the chromatographic mass spectrometry conditions; (3) diluting the samples; and (4) using matrix-matched standards or an additional standard method [30]. In this work, the purifying agent was optimized, and the matrix-matched standard was used to reduce the interference of the matrix effect on target compounds. The matrix effect distribution of 237 pesticides is shown in Figure 5. Among the 237 pesticides investigated in cottonseed hull samples, the proportion of pesticides with a negative matrix effect accounted for 81.4%, indicating that the substrate had a suppression effect on the tested pesticides as a whole. The matrix effect can be divided into three categories: no matrix effect (|ME| ≤ 20%); a weak matrix effect (20% < |ME| < 50%); and a strong matrix effect (|ME| ≥ 50%). In this work, only 8% of the pesticides in the cottonseed hull matrix showed a strong matrix effect; the weak matrix effect and no matrix effect accounted for 13.1% and 78.9%, respectively, indicating that this research method had a strong anti-matrix interference ability.
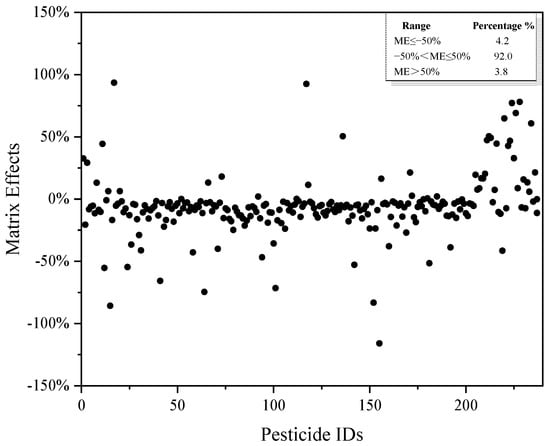
Figure 5.
Matrix effect distribution of 237 pesticides.
3.6. Method Validation and Method Performance
3.6.1. SDL, LOQ, and Standard Curve
The method validation was carried out under the optimal sample preparation procedure, and the results are shown in Table 1. The typical extraction ion chromatograms of GC-Q TOF/MS and LC-Q TOF/MS are shown in Figure 6 and Figure 7, respectively. The SDLs were in the range of 0.2–20 μg/kg, of which 224 pesticides (accounting for 94.5%) were in the range of 0.2–5 μg/kg. The LOQs were in the range of 0.2–20 μg/kg; 215 pesticides (accounting for 90.7%) had an LOQ range of 0.2–5 μg/kg. Shinde developed and verified 222 and 220 multi-pesticides residue analysis methods in sesame seeds, using LC-MS/MS and GC-MS/MS, respectively, and most pesticides offered an LOQ of 10 μg/kg for most compounds [16]. Kuzukiran et al. developed an SPE sample preparation method, combined with GC-MS, GC-MS/MS and LC-MS/MS, to analyze the residues of 322 organic pollutants in bats [31]. The LOQ of the method was in the range of 0.27–19.26 μg/kg, which was similar to that in our work; however, they paid more attention to environmental pollutants. This indicated that this method had high sensitivity in the detection of pesticide residues in cottonseed hull matrix. It is noteworthy that due to the large number of pesticides spiked, the retention time of some pesticides may overlap or be very close; for example, the RTs of Chloridazon and Mevinphos were 3.62 min. However, the excellent resolution of high-resolution mass spectrometry was sufficient to separate compounds that had a similar RT but a different mass (the quantitative ion mass of Chloridazon and that of Mevinphos were 222.04287 and 225.05230, respectively).

Table 1.
Compound information, screening detection limits (SDLs), limit of quantification (LOQ), linear range, R2, recovery, and RSD of 237 pesticides (n = 6).
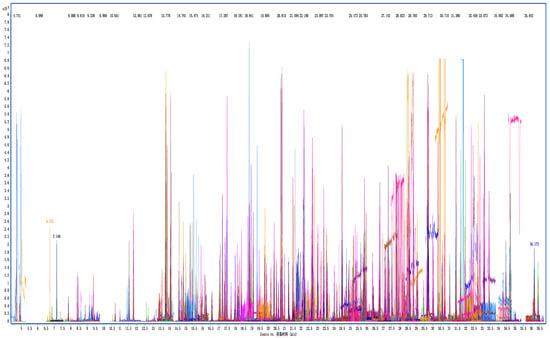
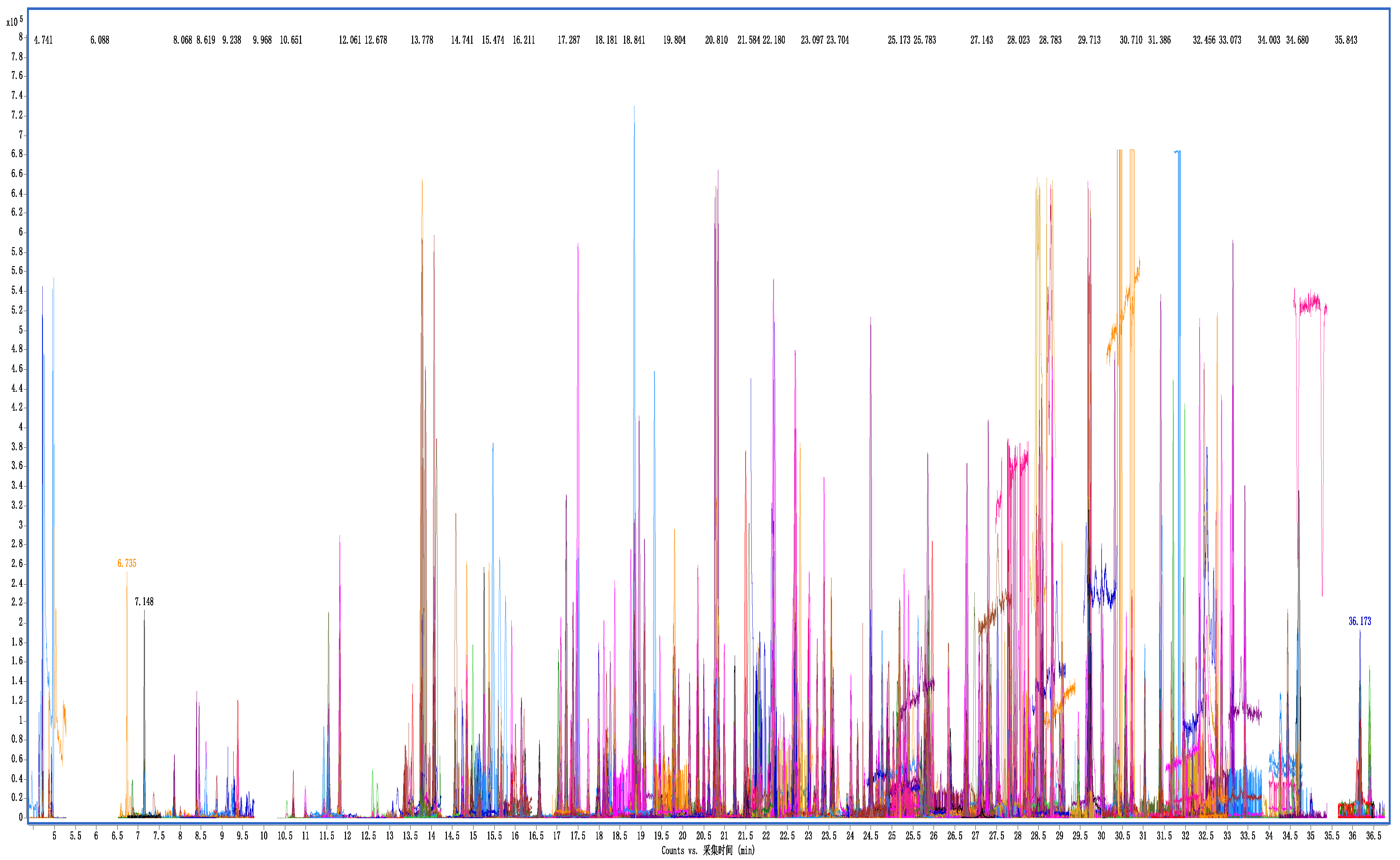
Figure 6.
Overlay extraction ion chromatograms of GC-Q TOF/MS of cottonseed hull sample at spiking level of 200 μg/kg.
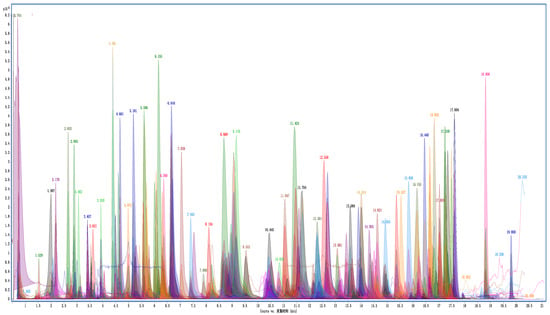
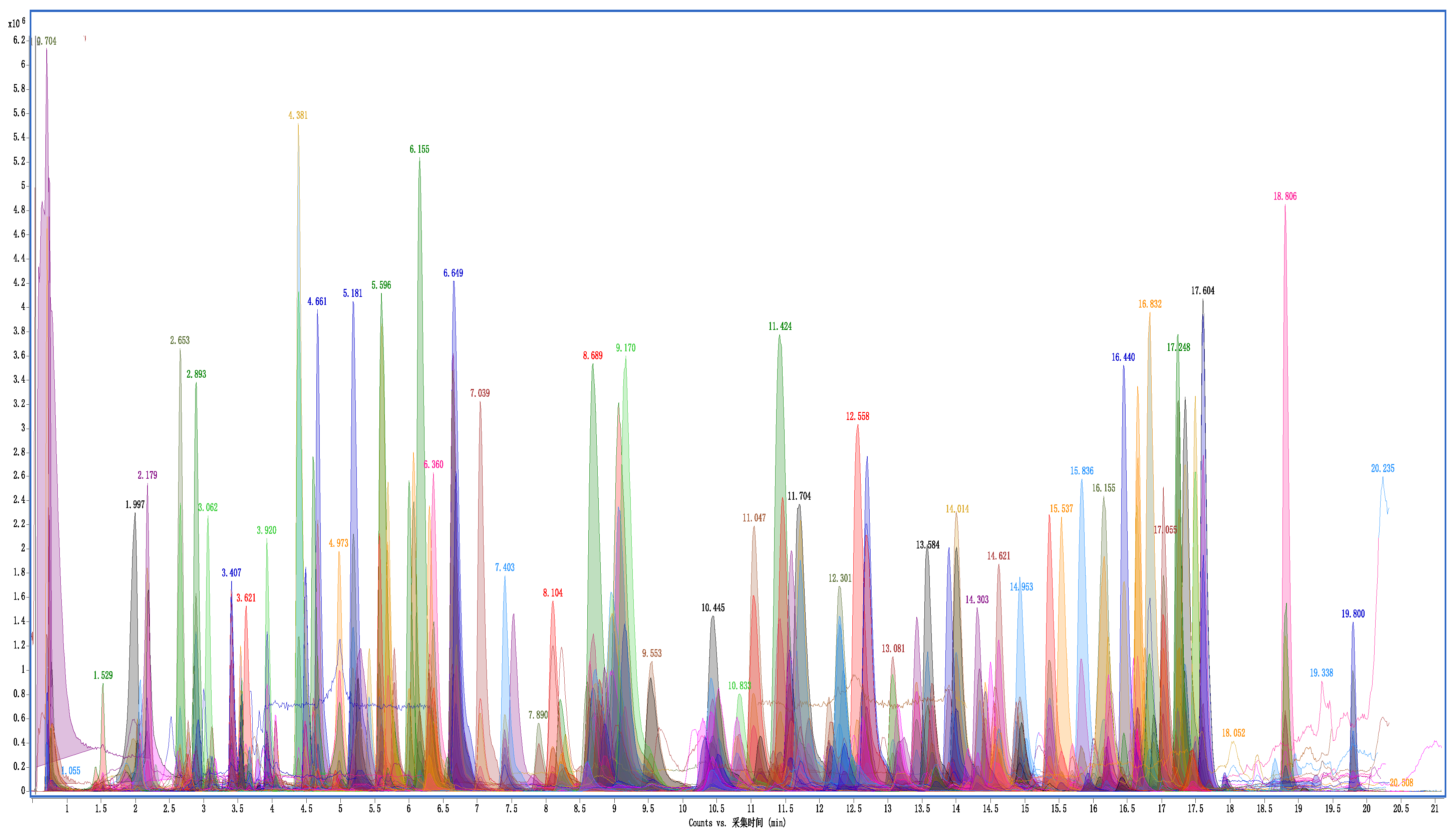
Figure 7.
Overlay extraction ion chromatograms of LC-Q TOF/MS of cottonseed hull sample at spiking level of 200 μg/kg.
The calibration curve was plotted using the matrix matching calibration method and the target analytes at 10 spiked levels (0.2, 0.5, 1, 2, 5, 10, 20, 50, 100, and 200 μg/kg) were spiked to the blank cottonseed hull sample. The linear ranges of 237 pesticide analytes were 1–200 μg/L. All target pesticides showed good linearity in the concentration range, and R2 was greater than 0.99, indicating that this method could meet the requirements of quantitative analysis.
3.6.2. Recovery and Precision
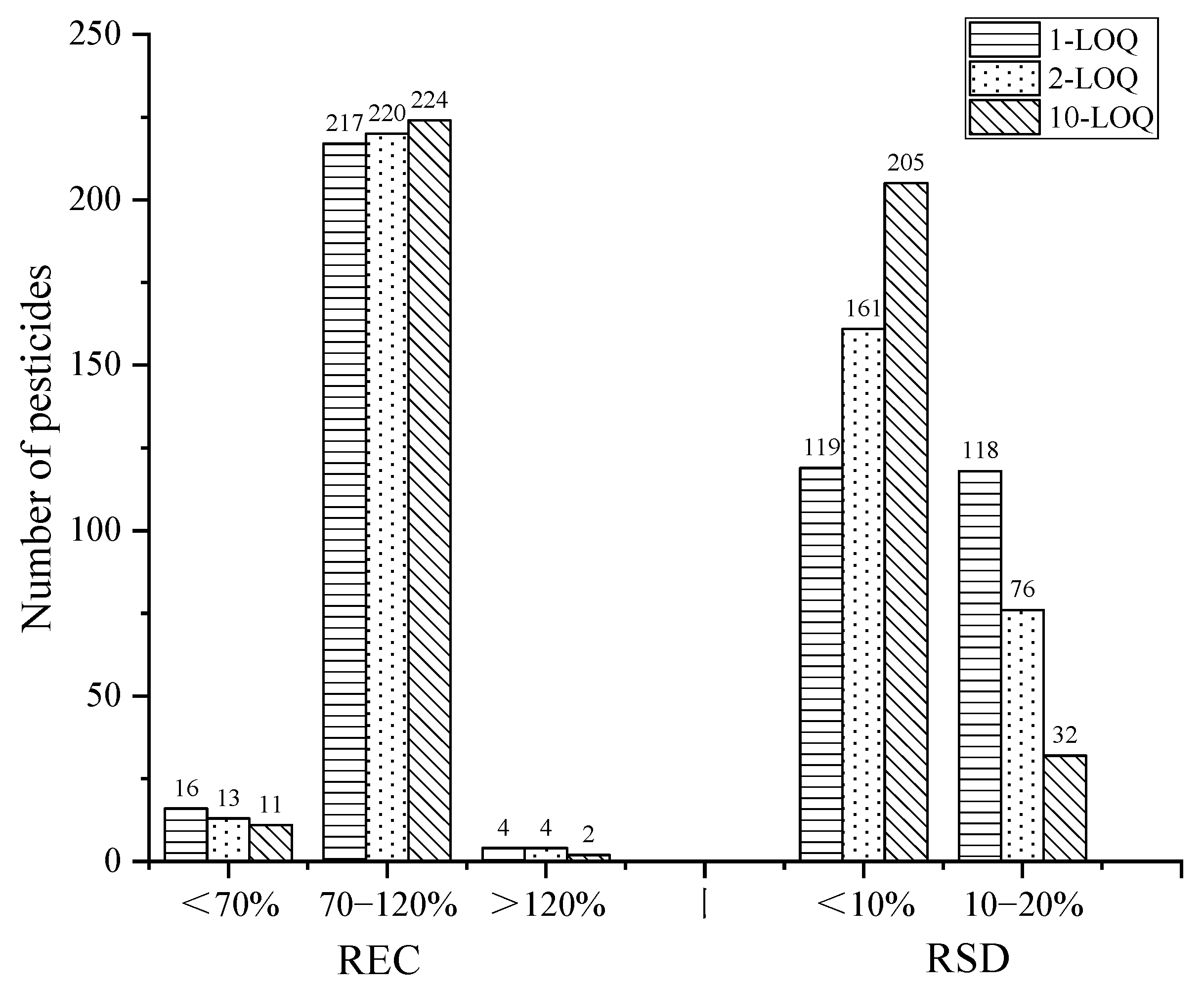
The recovery and precision of the method was evaluated by spiked standard solutions at the levels of 1-, 2-, and 10-times LOQ for the cottonseed hull samples with six parallels at each spiked level. The results are shown in Figure 8. At the levels of 1-, 2-, and 10-times LOQ, the recoveries of the 237 pesticides in the range of 70–120% were 91.6%, 92.8%, and 94.5%, respectively, and the RSD of all the pesticides was less than 20%, indicating that the method had satisfactory recovery and precision.
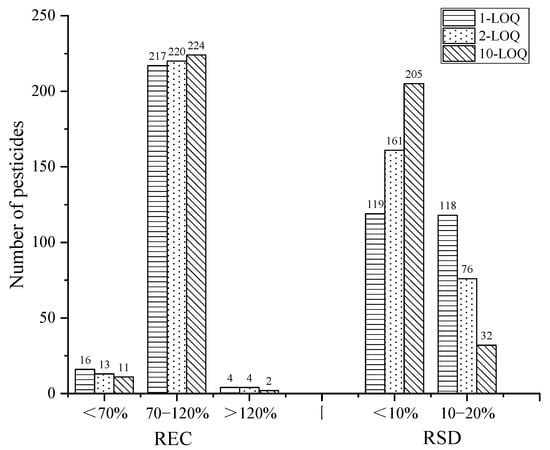
Figure 8.
The recovery and RSD of the target pesticides at three spiked levels.
Among the 237 pesticides, 60 pesticides were detected by two detection techniques, and most of them showed similar performance; however, individual pesticides were different in the two techniques. For example, the average recovery (81.2%) of clodinafop-propargyl detected by GC-QTOF/MS was lower than that (95.7%) detected by LC-QTOF/MS. In terms of precision, the RSD (10.8%) of the compound detected by GC-QTOF/MS was higher than that (4.8%) detected by LC-QTOF/MS. For Propiconazole, the average recovery and RSD of GC-QTOF/MS (89.0%, 5.5%) were better than those of LC-QTOF/MS (80.0%, 6.4%). Therefore, appropriate detection techniques should be selected in pesticide residue analysis, especially when compounds are suitable for these two detection techniques.
3.7. Analysis of Real Samples
The established method was applied to the analysis of 11 real cottonseed hull samples collected from several domestic pastures. The results showed that three pesticide residues were found in 11 cottonseed hull samples (butylate (three times), fenbuconazole (three times), and Diuron (two times)), with concentrations ranging from 10 to 28 μg/kg and above the LOQ. The determined three pesticides were slightly hazardous, according to WHO [32]. This method can be used for high-throughput trace detection of pesticide residues in cottonseed hull samples and improve the ability of risk-screening.
4. Conclusions
In this work, GC-QTOF/MS and LC-QTOF/MS were used to develop a high throughput method for qualitative screening and quantitative analysis of 237 pesticides in the cottonseed hull matrix. The modified QuEChERS extraction process seems to effectively eliminate the interference caused by the oily matrix, and the SDL, LOQ, recovery, and precision of the analysis method were verified under optimal conditions. In addition, compared with other methods for the oily matrix, this method has the advantages of being fast and simple, with high throughput and low solvent consumption. The results showed that the developed method could be applied to the screening of pesticide residues in the cottonseed hull matrix, effectively and generally.
Author Contributions
Conceptualization, H.C. and C.F.; methodology, H.C.; validation, K.T., Y.X. and X.W.; investigation, S.H. and Y.L.; resources, K.T.; data curation, Y.X.; writing—original draft preparation, K.T.; writing—review and editing, H.C., X.W. and C.F.; supervision, M.L. and W.W.; project administration, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Science and Technology Project of the State Administration for Market Regulation (2021MK165).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moretti, D.B.; Jimenez, C.R.; Trinca, H.M.; Machado-Neto, R.; Louvandini, H. Cottonseed feeding changes oxidative stress markers in ewes during the peripartum period and increases the quality of colostrum. Vet. J. 2019, 247, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Eiras, C.E.; Guerrero, A.; Valero, M.V.; Pardo, J.A.; Ornaghi, M.G.; Rivaroli, D.C.; Sanudo, C.; Prado, I.N. Effects of cottonseed hull levels in the diet and ageing time on visual and sensory meat acceptability from young bulls finished in feedlot. Animal 2017, 11, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.A.S.; Valadares, S.D.; Valadares, R.F.D.; Paulino, M.F.; Pina, D.D.; Paixao, M.L. Nutritional and productive parameters of beef cattle on pasture fed different amounts of supplement Parâmetros nutricionais e produtivos em bovinos de corte a pasto alimentados com diferentes quantidades de suplemento. Rev. Bras. Zootec. 2011, 40, 1303–1312. [Google Scholar] [CrossRef]
- Buah-Kwofie, A.; Humphries, M.S. Validation of a modified QuEChERS method for the analysis of organochlorine pesticides in fatty biological tissues using two-dimensional gas chromatography. J. Chromatogr. B 2018, 1105, 85–92. [Google Scholar] [CrossRef]
- Shi, Z.H.; Zhang, S.L.; Huai, Q.R.; Xu, D.; Zhang, H.Y. Methylamine-modified graphene-based solid phase extraction combined with UPLC-MS/MS for the analysis of neonicotinoid insecticides in sunflower seeds. Talanta 2017, 162, 300–308. [Google Scholar] [CrossRef]
- Walorczyk, S.; Drozdzynski, D. Improvement and extension to new analytes of a multi-residue method for the determination of pesticides in cereals and dry animal feed using gas chromatography–tandem quadrupole mass spectrometry revisited. J. Chromatogr. A 2012, 1251, 219–231. [Google Scholar] [CrossRef]
- David, F.; Devos, C.; Dumont, E.; Yang, Z.; Sandra, P.; Huertas-Perez, J.F. Determination of pesticides in fatty matrices using gel permeation clean-up followed by GC-MS/MS and LC-MS/MS analysis: A comparison of low- and high-pressure gel permeation columns. Talanta 2017, 165, 201–210. [Google Scholar] [CrossRef]
- Xue, J.Y.; Li, H.C.; Liu, F.M.; Jiang, W.Q.; Chen, X.C. Determination of strobilurin fungicides in cotton seed by combination of acetonitrile extraction and dispersive liquidliquid microextraction coupled with gas chromatography. J. Sep. Sci. 2014, 37, 845–852. [Google Scholar] [CrossRef]
- Zhan, J.; Li, J.D.; Liu, D.H.; Liu, C.; Yang, G.G.; Zhou, Z.Q.; Wang, P. A simple method for the determination of organochlorine pollutants and the enantiomers in oil seeds based on matrix solid-phase dispersion. Food Chem. 2016, 194, 319–324. [Google Scholar] [CrossRef]
- Piao, H.L.; Jiang, Y.X.; Li, X.P.; Ma, P.Y.; Wang, X.H.; Song, D.Q.; Sun, Y. Matrix solid-phase dispersion coupled with hollow fiber liquid phase microextraction for determination of triazine herbicides in peanuts. J. Sep. Sci. 2019, 42, 2123–2130. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Li, Y.J.; Jiang, Y.T.; Li, J.G.; Pan, C.P. Determination of multiresidues in rapeseed, rapeseed oil, and rapeseed meal by acetonitrile extraction, low-temperature cleanup, and detection by liquid chromatography with tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 5089–5098. [Google Scholar] [CrossRef] [PubMed]
- Theurillat, X.; Dubois, M.; Huertas-Pérez, J.F. A multi-residue pesticide determination in fatty food commodities by modified QuEChERS approach and gas chromatography-tandem mass spectrometry. Food Chem. 2021, 353, 129039. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.B.; Li, Z.N.; Zhang, H.Y.; Hong, H.J.; Rebeyev, N.; Ye, Y.; Ma, Y.Q. Amine modified graphene as reversed-dispersive solid phase extraction materials combined with liquid chromatography–tandem mass spectrometry for pesticide multi-residue analysis in oil crops. J. Chromatogr. A 2013, 1286, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, E.; Ozowicka, B.; Kaczyński, P. Compensation of matrix effects in seed matrices followed by gas chromatography-tandem mass spectrometry analysis of pesticide residues. J. Chromatogr. A 2020, 1614, 460738. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Mastovska, K.; Dorweiler, K.J.; Leepipatpiboon, N. Extension of the QuEChERS Method for Pesticide Residues in Cereals to Flaxseeds, Peanuts, and Doughs. J. Agric. Food Chem. 2010, 58, 5950–5958. [Google Scholar] [CrossRef]
- Shinde, R.; Pardeshi, A.; Dhanshetty, M.; Anastassiades, M.; Banerjee, K. Development and validation of an analytical method for the multiresidue analysis of pesticides in sesame seeds using liquid- and gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2021, 1652, 462346. [Google Scholar] [CrossRef]
- Gonzalez-Curbelo, M.A.; Socas-Rodriguez, B.; Herrera-Herrera, A.V.; Gonzalez-Salamo, J.; Hernandez-Borges, J.; Rodriguez-Delgado, M.A. Evolution and applications of the QuEChERS method. TrAC Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Farre, M.; Kantiani, L.; Petrovic, M.; Perez, S.; Barcelo, D. Achievements and future trends in the analysis of emerging organic contaminants in environmental samples by mass spectrometry and bioanalytical techniques. J. Chromatogr. A 2012, 1259, 86–99. [Google Scholar] [CrossRef]
- Dankyi, E.; Carboo, D.; Gordon, C.; Fomsgaard, I.S. Application of the QuEChERS Procedure and LC-MS/MS for the Assessment of Neonicotinoid Insecticide Residues in Cocoa Beans and Shells. J. Food Compos. Anal. 2016, 44, 149–157. [Google Scholar] [CrossRef]
- Chawla, S.; Patel, H.K.; Vaghela, K.M.; Pathan, F.K.; Gor, H.N.; Patel, A.R.; Shah, P.G. Development and validation of multiresidue analytical method in cotton and groundnut oil for 87 pesticides using low temperature and dispersive cleanup on gas chromatography and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 983–997. [Google Scholar] [CrossRef]
- Naik, R.H.; Pallavi, M.S.; Bheemanna, M.; PavanKumar, K.; Reddy, V.C.S.; Nidoni, R.U.; Paramasivam, M.; Yadav, S. Simultaneous determination of 79 pesticides in pigeonpea grains using GC-MS/MS and LC-MS/MS. Food Chem. 2021, 347, 128986. [Google Scholar] [CrossRef]
- Elbashir, A.A.; Aboul-Enein, H.Y. Application of gas and liquid chromatography coupled to time-of-flight mass spectrometry in pesticides: Multiresidue analysis. Biomed. Chromatogr. 2018, 32, e4038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chow, W.; Leung, D. Applications of LC/ESI-MS/MS and UHPLC QqTOF MS for the determination of 148 pesticides in fruits and vegetables. J. AOAC Int. 2011, 396, 1513–1538. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.; Rajski, L.; Ucles, S. Evaluation of zirconium dioxide-based sorbents to decrease the matrix effect in avocado and almond multiresidue pesticide analysis followed by gas chromatography tandem mass spectrometry. Talanta 2014, 118, 68–83. [Google Scholar] [CrossRef]
- Pang, G.F.; Chang, Q.Y.; Bai, R.B.; Fan, C.L.; Zhang, Z.J.; Yan, H.Y.; Wu, X.Q. Simultaneous Screening of 733 Pesticide Residues in Fruits and Vegetables by a GC/LC-Q-TOFMS Combination Technique. Engineering 2020, 6, 432–441. [Google Scholar] [CrossRef]
- Dos-Reis, R.R.; Sampaio, S.C.; De Melo, E.B. The effect of different log P algorithms on the modeling of the soil sorption coefficient of nonionic pesticides. Water Res. 2013, 47, 5751–5759. [Google Scholar] [CrossRef]
- Mol, H.G.J.; Plaza-BolanOs, P.; Zomer, P.; De Rijk, T.C.; Stolker, A.A.M.; Mulder, P.P.J. Toward a generic extraction method for simultaneous determination of pesticides, mycotoxins, plant toxins, and veterinary drugs in feed and food matrixes. Anal. Chem. 2008, 80, 9450–9459. [Google Scholar] [CrossRef]
- Tuzimski, T.; Rejczak, T. Application of HPLC-DAD after SPE/QuEChERS with ZrO2-based sorbent in d-SPE clean-up step for pesticide analysis in edible oils. Food Chem. 2016, 190, 71–79. [Google Scholar] [CrossRef]
- Lagunas-Allue, L.; Sanz-Asensio, J.; Martínez-Soria, M.T. Comparison of four extraction methods for the determination of fungicide residues in grapes through gas chromatography-mass spectrometry. J. Chromatogr. A 2012, 1270, 62–71. [Google Scholar] [CrossRef]
- Ucles, S.; Lozano, A.; Sosa, A.; Vazquez, P.P.; Valverde, A.; Fernandez-Alba, A.R. Matrix interference evaluation employing GC and LC coupled to triple quadrupole tandem mass spectrometry. Talanta 2017, 174, 72–81. [Google Scholar] [CrossRef]
- Kuzukiran, O.; Simsek, I.; Yorulmaz, T.; Yurdakok-Dikmen, B.; Ozkan, O.; Filazi, A. Multiresidues of environmental contaminants in bats from Turkey. Chemosphere 2021, 282, 131022. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).