How Coffee Capsules Affect the Volatilome in Espresso Coffee
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description and Preparation
2.2. GC-MS Analysis Conditions
2.3. GC-MS Data Analysis
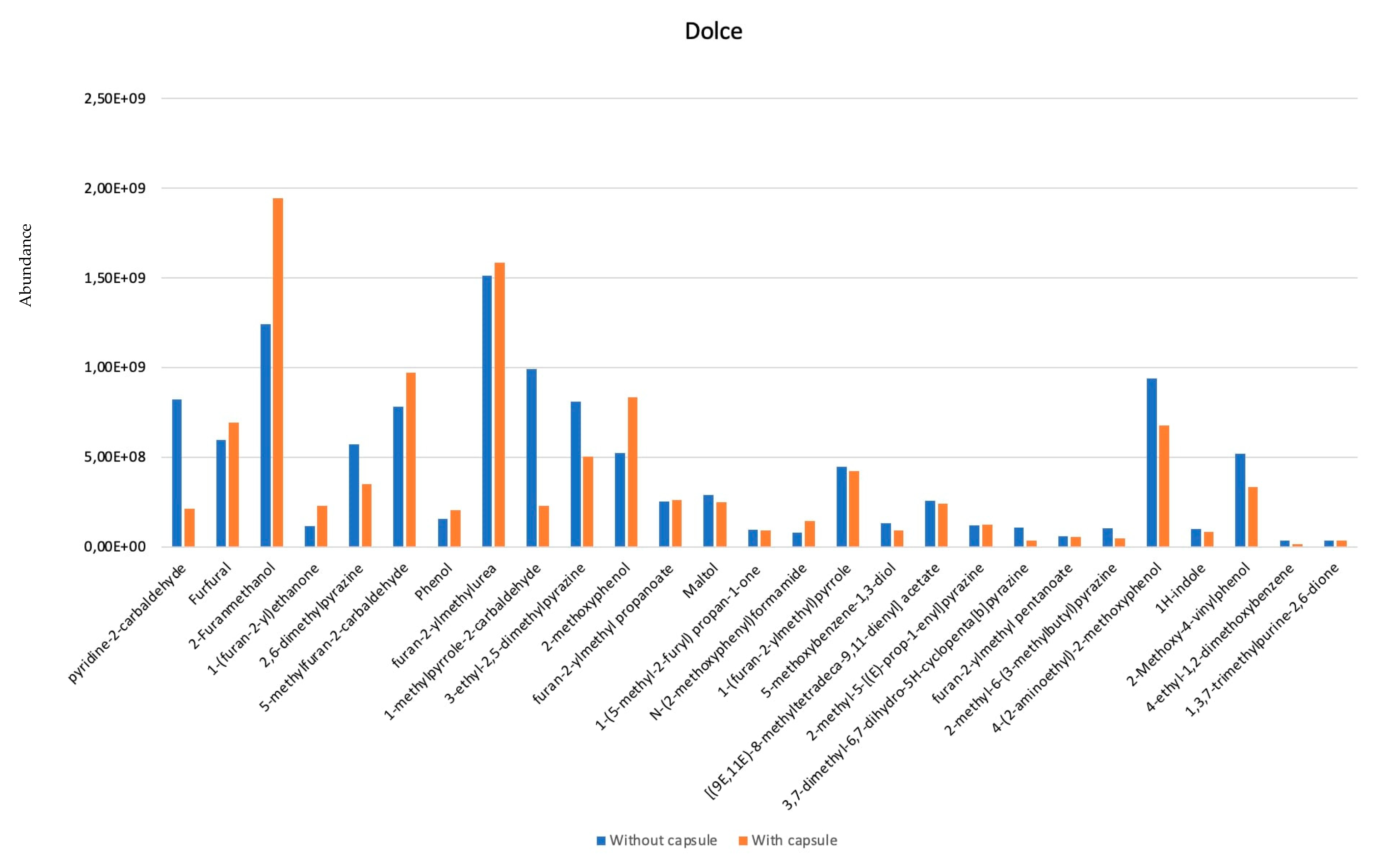
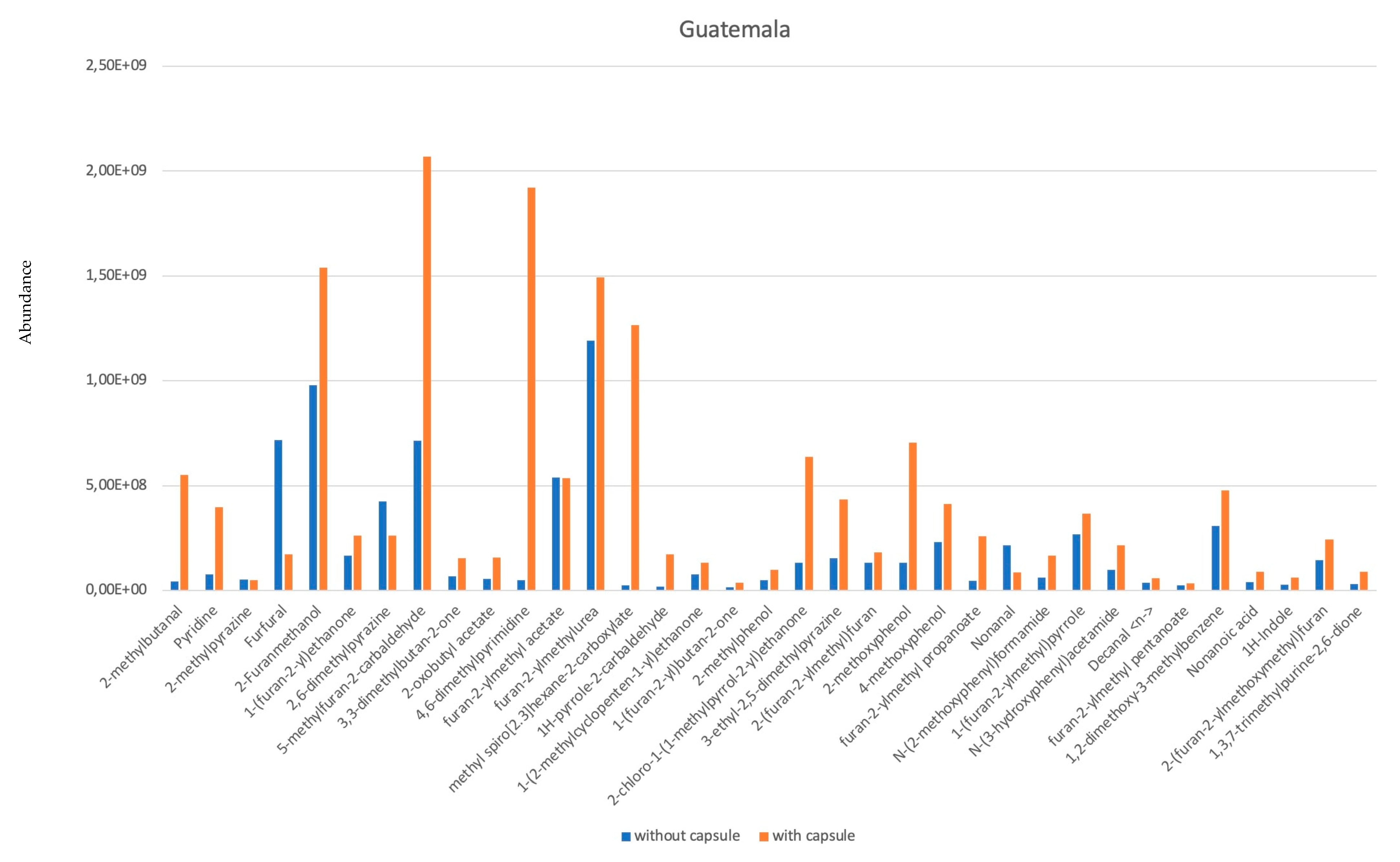
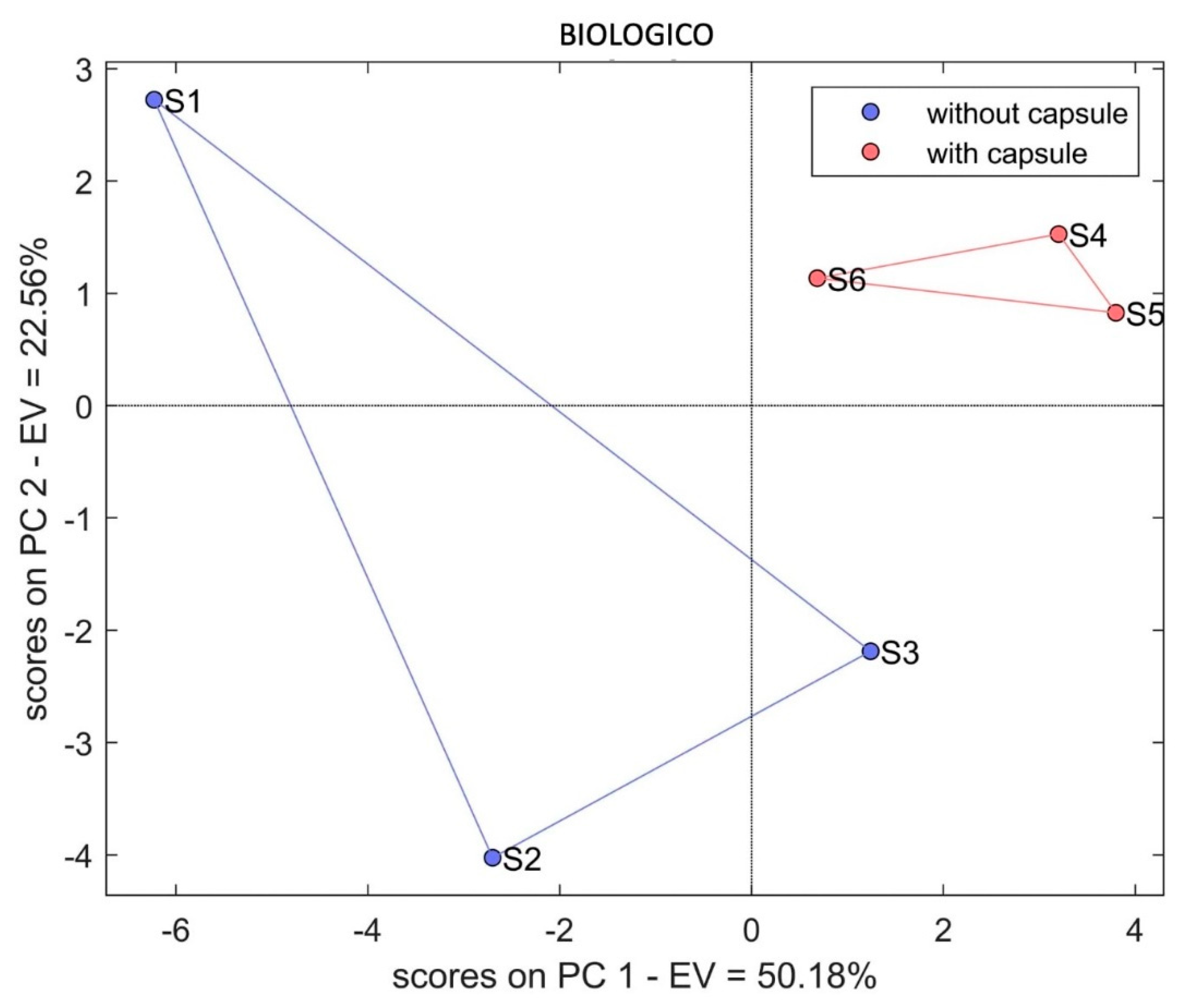
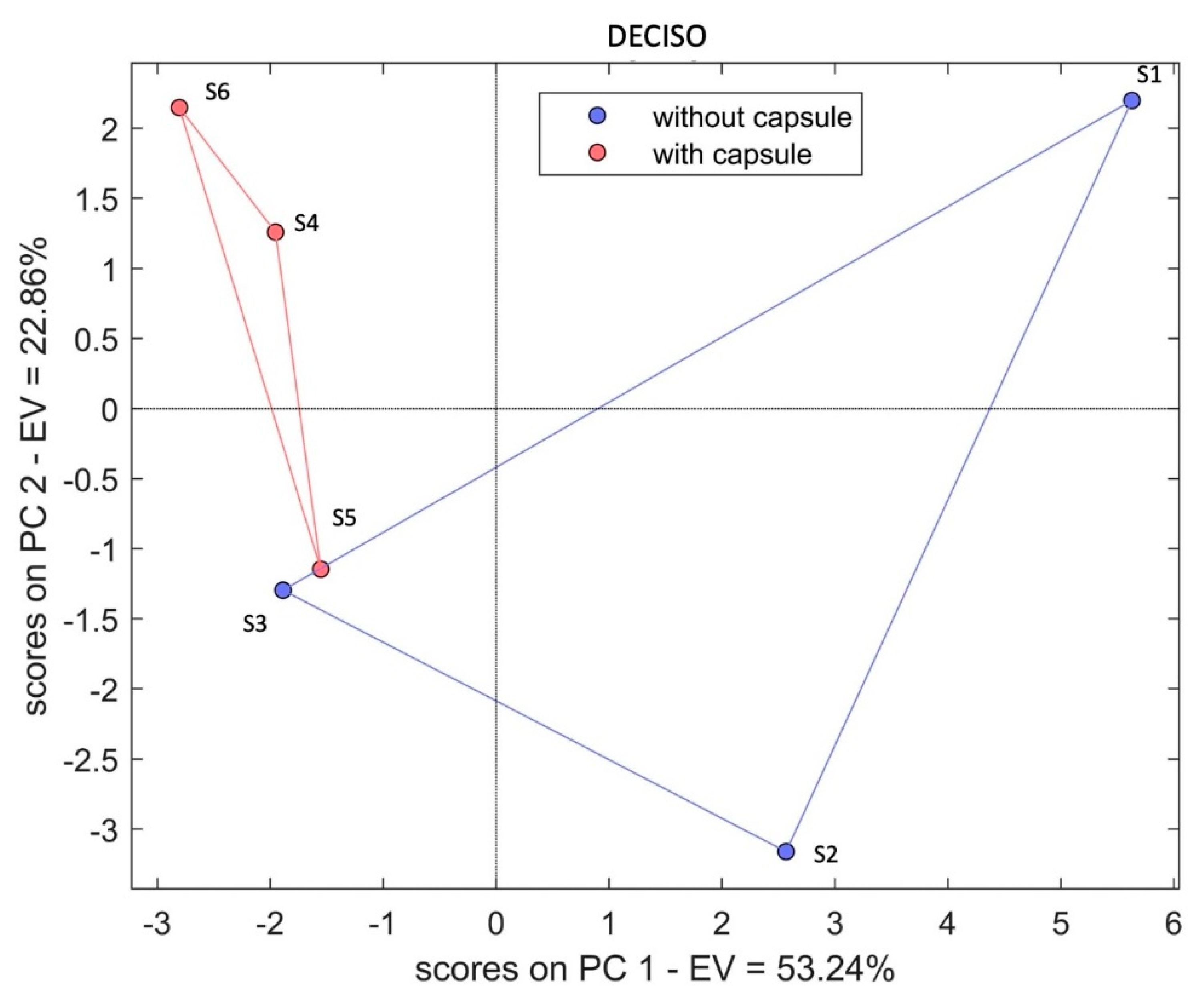
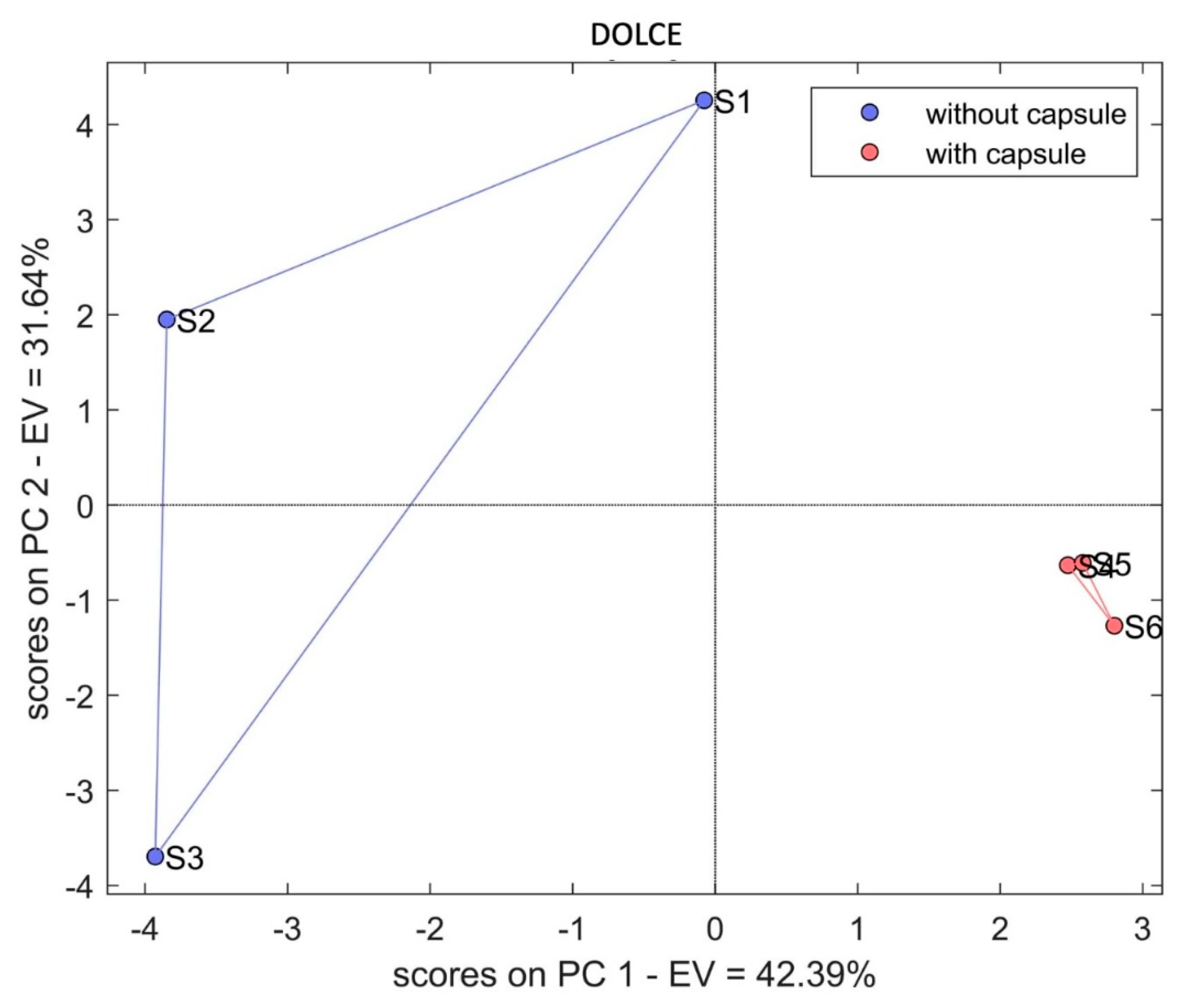
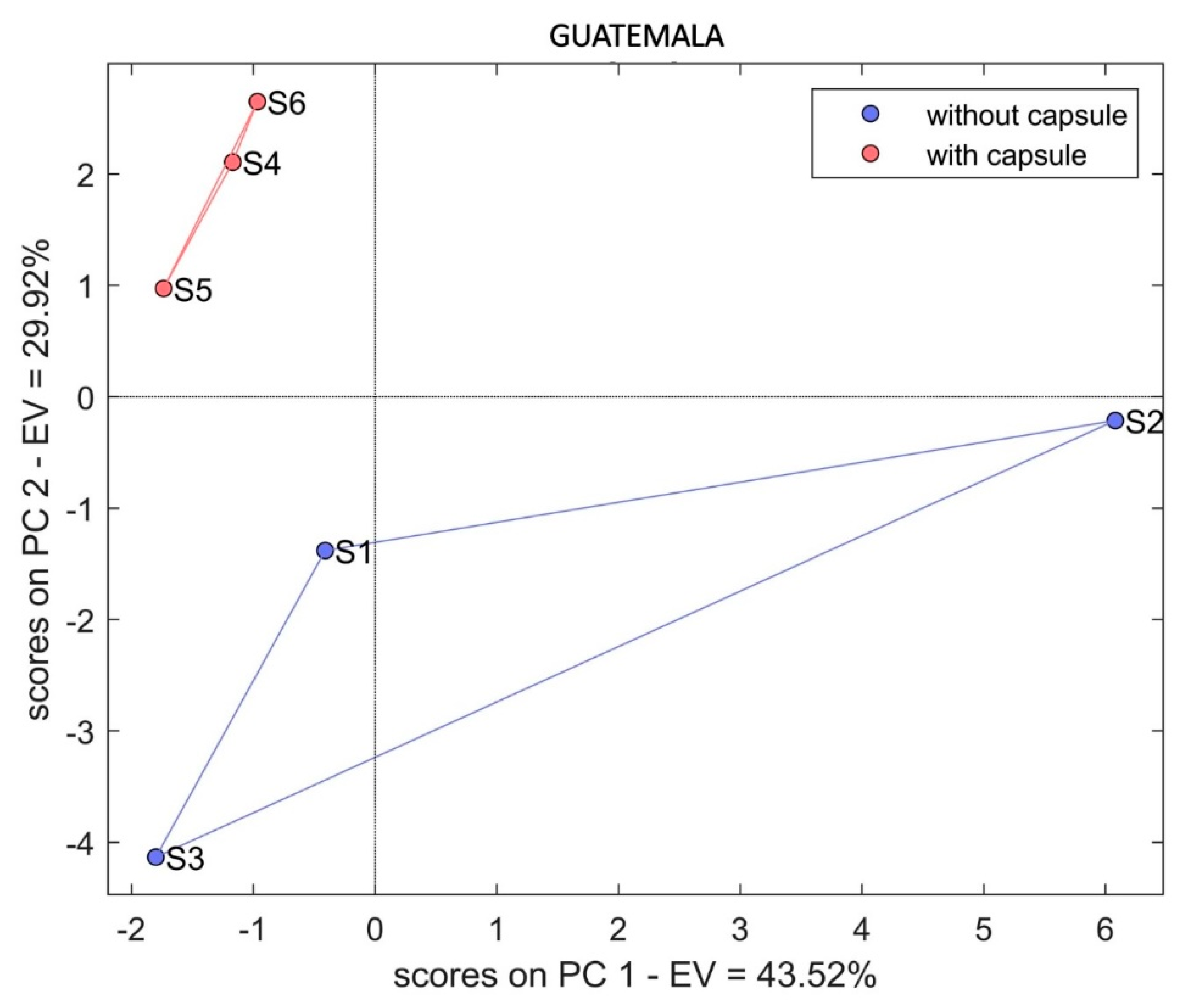
3. Results and Discussion
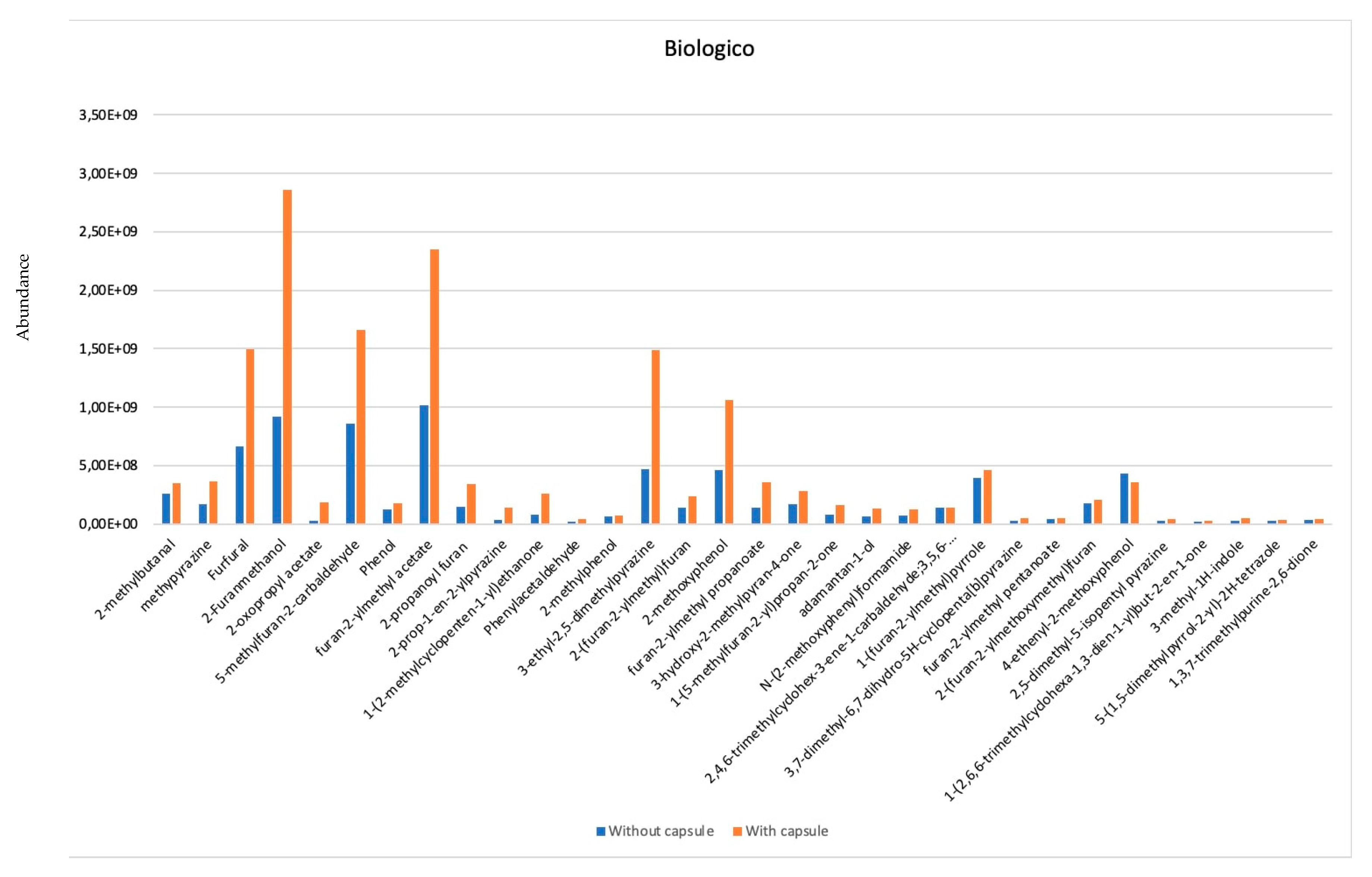
- Five of them are aldehydes: 2-methyl- Butanal; 5-methyl-2-furancarboxaldehyde; 1H-Pyrrole-2-carboxaldehyde; Furfural; and Isocyclocitral. Aldehydes are important molecules because they enrich the volatilome with floral flavors [21].
- Three are pyrazines: methyl-Pyrazine; 2,6-dimethyl-Pyrazine; 2-methyl-Pyrazine; 3-ethyl-2,5-dimethyl Pyrazine. Pyrazines are compounds formed during cooking processes in food matrices consisting of both a sugar fraction and a protein/amino acid fraction, characterizing the typical toasted notes that are perceived from the food.
- Six are of them are alcohols 2-Furanmethanol; 1-1(H-pyrazol-1-yl)-2-Propanone; 2-Furanmethanol acetate; 2-Furanmethanol, propanoate; Maltol; Benzo-2,3-pyrrole. Alcohols can have two functions. They can directly influence the volatilome or indirectly act as precursors of aldehydes and ketones.
- Three are furans: 2-Hexanoylfuran; 2,2′-methylenebis-Furan; 2,2′-[oxybis(methylene)]bis-Furan. Furan is a colorless chemical with a low molecular weight and highly volatile. It forms as a result of food heating and contributes to the roasted taste in some. The coffee and in particular when roasted, represents a source of alimentary exposure to the furan.
- Five are ketones: 1-(2-methyl-1-cyclopenten-1-yl)-Ethanone; 1-(6-Methyl-2-pyrazinyl)-1-ethanone; 1-(5-methyl-2-furanyl)-1-Propanone; 1-(2,5-dihydroxyphenyl)-Ethanone; 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-(E)- 2-Buten-1-one. Ketones are important molecules because they enrich the volatilome with floral flavors.
- Two are phenols: Phenol; 2-methoxy-Phenol. Phenols are molecules possible to find in the raw material. Generally, polyphenols and chlorogenic acid have higher concentration.
- Two acids: Propanoic acid ethenyl ester; Furfuryl pentanoate. Carboxylic acids are a part of the non-volatile component of coffee seeds after roasting, generated as a result of complex reactions that occur during roasting.
- One alkaloid: Caffeine. This compound is responsible for the bitterness.
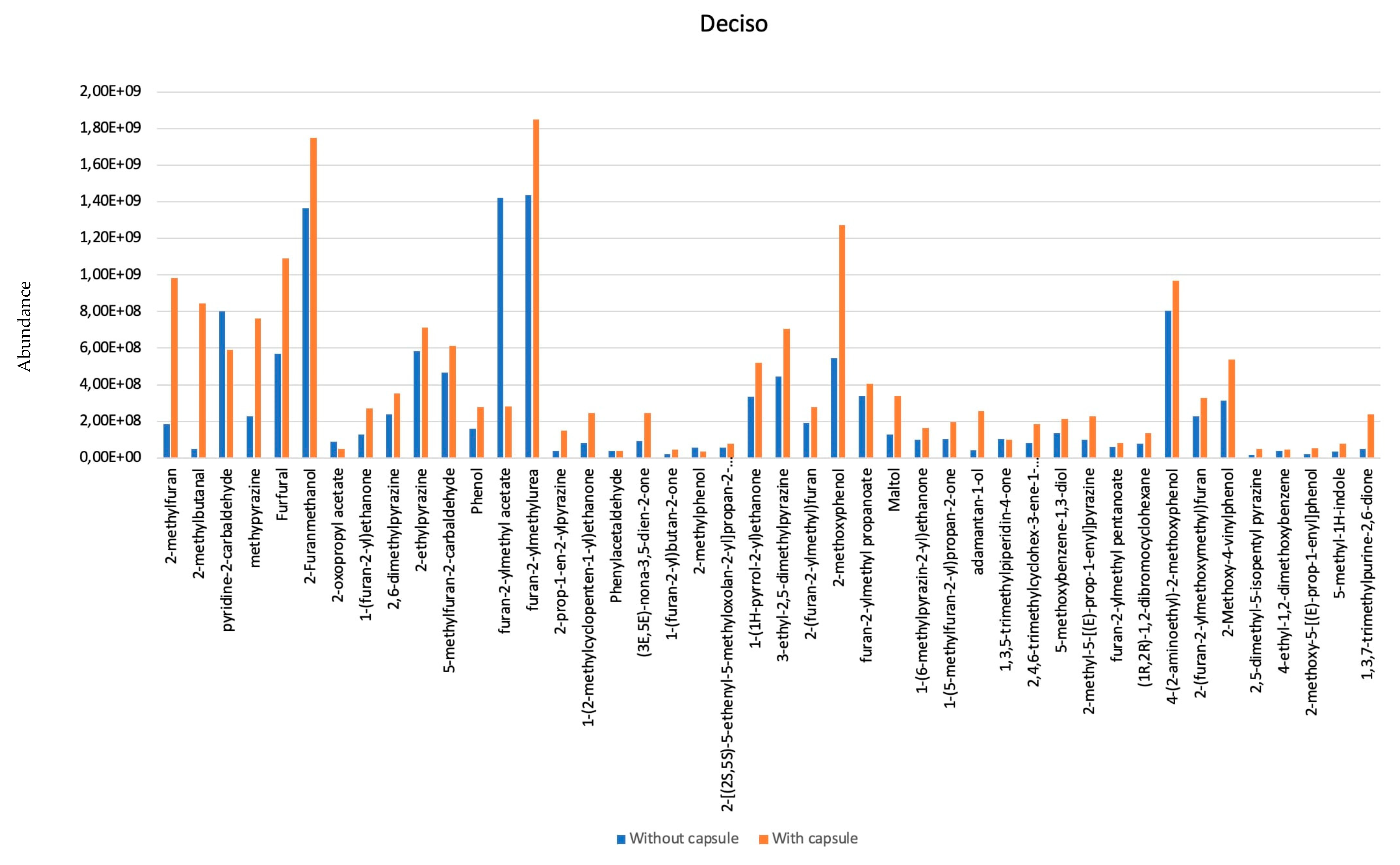
- Six of them are aldehydes: 2-methyl-Butanal; Furfural; Nonanal; Phenylacetaldehyde; 1-methyl-1H-Pyrrole-2-carboxaldehyde.
- Five are pyrazines: methyl-Pyrazine; 2,6-dimethyl-Pyrazine; 3-ethyl-2,5-dimethyl Pyrazine; (1-methylethenyl)-Pyrazine; 3,5-diethyl-2-methyl-) Pyrazine.
- Six of them are alcohols: (2-Furanmethanol; 2-Furanmethanol acetate; Maltol; 1-(2-furanylmethyl)-1H-Pyrrole; Indole; 5-methyl-1H-Indole.
- Three are furans: 2,2′-methylenebis-Furan; 5-methyl-2-furancarboxaldehyde; N-(2-furfuryl)-Urea.
- Five are ketones: 1-(2-methyl-1-cyclopenten-1-yl)-Ethanone; 1-(5-methyl-2-furanyl)-1-Propanone; 1-(acetyloxy)-2-Propanone; 1-(2-furanyl)-Ethanone; 1-(2-furanyl)-2-Butanone; 1-(1H-pyrrol-2-yl)-) Ethanone.
- Five are phenols: Phenol; 2-methoxy- Phenol; 2-methyl-Phenol; 2-methoxy-5-(1-propenyl)-, (E)-Phenol; <4-vinyl-> Guaiacol.
- Two acids: Furfuryl pentanoate; Furfuryl propionate.
- One Benzene: 4-ethyl-1,2-dimethoxy Benzene.
- One Alkaloid: Caffeine.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, G.L. Food Packaging and Shelf Life: A Practical Guide; CRC Press: Boca Raton, FL, USA, 2009; pp. 199–214. [Google Scholar]
- Bates, R.H.; Greif, A.; Levi, M.; Rosenthal, J.L.; Weingast, B.R. Analytic Narratives; Princeton University Press: Princeton, NJ, USA, 2020; 264p. [Google Scholar]
- Arabica e Robusta: Per una Miscela Perfetta Serve Equilibrio. Available online: https://www.dolce-gusto.it/mydolcegusto/tutto-sul-caffe/arabica-robusta-miscela/ (accessed on 21 June 2020).
- Favati, F.; Condelli, N.; Galgano, F.; Caruso, M.C. Extra virgin olive oil bitterness evaluation by sensory and chemical analyses. Food Chem. 2013, 139, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Qualcosa da Sapere Sulle Capsule del Caffè. 2019. Available online: http://www.tbspa.it/qualcosa-da-sapere-sulle-capsule-del-caffe/ (accessed on 15 July 2021).
- Otoukesh, M.; Vera, P.; Wrona, M.; Nerin, C.; Es’Haghi, Z. Migration of dihydroxyalkylamines from polypropylene coffee capsules to Tenax® and coffee by salt-assisted liquid–liquid extraction and liquid chromatography–mass spectrometry. Food Chem. 2020, 321, 126720. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.C.; Calligaris, S.; Manzocco, L. Shelf-Life Testing of Coffee and Related Products: Uncertainties, Pitfalls, and Perspectives. Food Eng. Rev. 2009, 1, 159–168. [Google Scholar] [CrossRef]
- Najar, B.; Nardi, V.; Cervelli, C.; Pistelli, L. Volatiloma analyses of four South African Helichrysum spp. grown in Italy. Nat. Prod. Res. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Hajslova, J. Application of gas chromatography in food analysis. TrAC Trends Anal. Chem. 2002, 21, 686–697. [Google Scholar] [CrossRef]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Internet of Food (IoF), Tailor-Made Metal Oxide Gas Sensors to Sup-port Tea Supply Chain. Sensors 2021, 21, 4266. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Rivetti, D. Water quality for Espresso coffee. Food Chem. 2010, 122, 424–428. [Google Scholar] [CrossRef]
- Chen, P.H.; Richardson, S.D.; Krasner, S.W.; Majetich, G.; Glish, G.L. Hydrogen Abstraction and Decomposition of gmBromopicrin and Other Trihalogenated Disinfection Byproducts by GC/MSPaul H. Glish Environ. Sci. Technol. 2002, 36, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Carmona, E.; Abbatangelo, M.; Sberveglieri, V. Innovative Sensor Approach to Follow Campylobacter jejuni Devel-opment. Biosensors 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbatangelo, M.; Nunez-Carmona, E.; Sberveglieri, V. Novel Equipment for Food Quality Control: An Iot Nanowire Gas Sensors Array. Chem. Eng. Trans. 2019, 75, 25–30. [Google Scholar]
- Zanin, R.C.; Smrke, S.; Kurozawa, L.E.; Yamashita, F.; Yeretzian, C. Modulation of aroma release of instant coffees through microparticles of roasted coffee oil. Food Chem. 2021, 341, 128193. [Google Scholar] [CrossRef]
- Eichelberger, J.W.; Harris, L.E.; Budde, W.L. Reference Compound to Calibrate Ion Abundance Measurements in Gas Chromatography-Mass Spectrometry Systems. Anal. Chem. 1975, 47, 995–1000. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Cristalli, G.; Maggi, F.; Odello, L.; Ricciutelli, M.; Sagratini, G.; Sirocchi, V.; Tomassoni, G.; Vittori, S. Optimization of espresso machine parameters through the analysis of coffee odorants by HS-SPME–GC/MS. Food Chem. 2012, 135, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Chemical Compounds behind the Aroma of Coffee. 2015. Available online: https://www.compoundchem.com/2015/02/17/coffee-aroma/ (accessed on 18 July 2021).
- Wu, C.; Liu, J.; Yan, L.; Chen, H.; Shao, H.; Meng, T. Assessment of odor activity value coefficient and odor contribution based on binary interaction effects in waste disposal plant. Atmos. Environ. 2015, 103, 231–237. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
| RT | Compound Name | Abundance | DEV.ST | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biologico | Deciso | Dolce | Guatemala | ||||||||||||||||
| BI.C. (a) | VOC’s % | BI | VOC’s % | DE | VOC’s % | DE.C. | VOC’s % | DO | VOC’s % | DO.C. | VOC’s % | GU (b) | VOC’s % | GU.C. (a) (b) | VOC’s % | with Capsule | without Capsule | ||
| 0.630 | 2-Methoxyethyl acetate | 6.46 × 107 | 0.37 | ||||||||||||||||
| 0.891 | 2-methylfuran | 1.60 × 108 | 0.97 | 1.30 × 108 | 2.41 | 2.18 × 108 | 1.71 | 6.22 × 107 | |||||||||||
| 0.991 | 2-methylbutanal | 5.22 × 108 | 1.54 | 1.78 × 108 | 1.05 | 8.68 × 107 | 0.52 | 2.24 × 108 | 4.15 | 1.96 × 108 | 1.20 | 5.89 × 108 | 4.61 | 5.61 × 107 | 0.50 | 1.94 × 108 | 6.83 × 107 | ||
| 1.297 | tert-butyl-(2,2-difluoroethoxy)-dimethylsilane | 1.35 × 108 | 1.26 | 3.45 × 108 | 2.70 | 1.48 × 108 | |||||||||||||
| 1.320 | 1-Propionylethyl acetate | 1.83 × 108 | 1.08 | 5.46 × 107 | 0.49 | 9.08 × 107 | |||||||||||||
| 1.581 | 2-Pyridinecarboxaldehyde | 9.32 × 108 | 2.75 | 8.01 × 108 | 4.73 | 7.14 × 108 | 4.04 | 8.23 × 108 | 4.97 | 3.45 × 107 | 0.32 | 2.15 × 108 | 0.85 | 4.19 × 108 | 1.56 × 107 | ||||
| 1.662 | tetrazolo[1,5-b]pyridazin-6-amine | 1.63 × 109 | 4.82 | 5.62 × 107 | 0.52 | 1.60 × 109 | 6.30 | 9.00 × 108 | |||||||||||
| 1.784 | 4-ethenyl-1-methylpyridin-1-ium;bromide | 7.54 × 108 | 6.76 | ||||||||||||||||
| 1.795 | Pyridine | 2.84 × 108 | 1.68 | 4.98 × 108 | 3.01 | 1.50 × 108 | 1.40 | 4.97 × 108 | 3.05 | 4.62 × 108 | 1.82 | 1.74 × 108 | 1.56 | 2.21 × 108 | 1.61 × 108 | ||||
| 2.663 | 2-methyloxolan-3-one | 1.31 × 108 | 0.39 | ||||||||||||||||
| 3.008 | methylpyrazine | 3.49 × 108 | 1.03 | 1.63 × 108 | 0.96 | 2.18 × 108 | 1.23 | 1.88 × 108 | 1.14 | 1.37 × 108 | 1.28 | 1.74 × 108 | 1.07 | 2.67 × 108 | 1.05 | 6.00 × 107 | 0.54 | 8.88 × 107 | 5.84 × 107 |
| 3.188 | Furfural | 1.40 × 109 | 4.14 | 6.60 × 108 | 3.90 | 4.24 × 108 | 2.40 | 4.93 × 108 | 2.98 | 1.33 × 108 | 1.24 | 5.35 × 108 | 3.28 | 6.67 × 108 | 2.63 | 6.40 × 108 | 5.74 | 5.42 × 108 | 8.08 × 107 |
| 3.757 | 2-Furanmethanol | 2.73 × 109 | 8.07 | 1.09 × 109 | 6.44 | 9.83 × 108 | 5.56 | 1.21 × 109 | 7.31 | 7.31 × 107 | 0.68 | 1.09 × 109 | 6.68 | 1.67 × 109 | 6.57 | 9.47 × 108 | 8.49 | 1.12 × 109 | 1.08 × 108 |
| 4.530 | 2-oxopropyl acetate | 1.18 × 108 | 0.35 | 3.36 × 107 | 0.20 | 1.50 × 108 | 0.85 | 7.02 × 107 | 0.42 | 1.16 × 108 | 1.08 | 2.96 × 107 | 0.18 | 2.23 × 108 | 0.88 | 4.33 × 107 | 0.39 | 5.00 × 107 | 1.83 × 107 |
| 5.908 | 1-(furan-2-yl)ethanone | 1.26 × 108 | 0.74 | 1.47 × 108 | 0.83 | 1.13 × 108 | 0.68 | 8.13 × 107 | 0.76 | 1.76 × 108 | 0.69 | 1.54 × 108 | 1.38 | 4.85 × 107 | 2.10 × 107 | ||||
| 6.003 | 2-Hexanoylfuran | 3.00 × 108 | 0.89 | 4.35 × 108 | 4.05 | 2.36 × 108 | 0.93 | 1.02 × 108 | |||||||||||
| 6.048 | 4,6-dimethylpyrimidine | 1.40 × 109 | 4.14 | 1.08 × 109 | 6.10 | 1.15 × 108 | 1.07 | 3.50 × 108 | 1.38 | 6.04 × 108 | |||||||||
| 6.121 | 1-(1H-imidazol-5-yl)ethanone | 1.99 × 108 | 1.12 | ||||||||||||||||
| 6.213 | 2E)-2-pyrrolidin-2-ylideneacetonitrile | 2.32 × 109 | 6.86 | ||||||||||||||||
| 6.244 | 2,6-dimethylpyrazine | 3.84 × 108 | 2.27 | 3.52 × 108 | 1.99 | 3.47 × 108 | 2.10 | 1.27 × 108 | 1.18 | 5.55 × 108 | 3.40 | 1.81 × 108 | 0.71 | 3.99 × 108 | 3.58 | 1.17 × 108 | 9.18 × 107 | ||
| 6.307 | ethylpirazine | 5.03 × 108 | 2.97 | 7.13 × 108 | 4.03 | 5.83 × 108 | 3.52 | 1.33 × 108 | 1.24 | 4.02 × 108 | 2.46 | 4.10 × 108 | 9.07 × 107 | ||||||
| 6.324 | 2-ethenyl-1-methylimidazole | 1.37 × 108 | 1.28 | 1.63 × 109 | 6.42 | 1.06 × 109 | |||||||||||||
| 8.088 | 1-pyrazol-1-ylpropan-2-one | 1.42 × 108 | 0.42 | 5.45 × 107 | 0.31 | 2.53 × 107 | 0.24 | 7.21 × 107 | 0.44 | 1.21 × 108 | 0.48 | 4.77 × 107 | 0.43 | 5.49 × 107 | 1.73 × 107 | ||||
| 8.147 | 1-(2,6,6-trimethylcyclohexen-1-yl)ethanone | 4.40 × 107 | 0.27 | 2.97 × 107 | 0.18 | 1.01 × 107 | |||||||||||||
| 8.436 | 5-methylfuran-2-carbaldehyde | 1.52 × 109 | 4.49 | 8.00 × 108 | 4.72 | 6.11 × 108 | 3.45 | 4.15 × 108 | 2.51 | 2.14 × 107 | 0.20 | 6.57 × 108 | 4.03 | 9.22 × 108 | 3.63 | 7.18 × 108 | 6.44 | 6.25 × 108 | 1.66 × 108 |
| 8.777 | Tripropionin | 9.75 × 107 | 0.91 | 3.05 × 107 | 0.12 | 4.74 × 107 | |||||||||||||
| 8.845 | 2-methyl,3-Pentanone | 7.74 × 108 | 2.29 | ||||||||||||||||
| 8.873 | Ethyl propionate | 3.45 × 108 | 1.02 | 7.13 × 107 | 0.42 | 1.28 × 108 | 1.19 | 3.94 × 108 | 1.55 | 1.43 × 108 | 1.28 | 1.42 × 108 | 5.07 × 107 | ||||||
| 8.883 | 3,3-dimethylbutan-2-one | 2.52 × 107 | 0.15 | 7.17 × 107 | 0.64 | 3.29 × 107 | |||||||||||||
| 9.027 | 2-oxobutyl acetate | 6.76 × 107 | 0.40 | 6.90 × 107 | 0.62 | 9.90 × 105 | |||||||||||||
| 9.437 | Arsenous acid, tris(trimethylsilyl) ester | 5.38 × 107 | 0.32 | 1.01 × 108 | 0.94 | 8.68 × 107 | 0.34 | 1.00 × 107 | |||||||||||
| 9.757 | Phenol | 1.84 × 108 | 0.54 | 1.69 × 108 | 0.96 | 1.75 × 108 | 1.06 | 5.50 × 107 | 0.51 | 1.46 × 108 | 0.89 | 1.89 × 108 | 0.74 | 1.34 × 108 | 1.20 | 6.34 × 107 | 2.11 × 107 | ||
| 9.852 | (4-hydroxyphenyl)phosphonic acid | 8.32 × 107 | 0.49 | ||||||||||||||||
| 9.979 | furan-2-ylmethyl acetate | 2.35 × 109 | 6.95 | 7.53 × 108 | 4.45 | 8.67 × 108 | 4.90 | 1.28 × 109 | 7.73 | 6.27 × 107 | 0.58 | 9.66 × 108 | 5.92 | 1.14 × 109 | 4.49 | 7.95 × 108 | 7.13 | 9.48 × 108 | 2.39 × 108 |
| 10.283 | furan-2-ylmethylurea | 1.77 × 109 | 5.23 | 1.63 × 109 | 9.62 | 1.85 × 109 | 10.46 | 1.43 × 109 | 8.64 | 5.00 × 107 | 0.47 | 1.51 × 109 | 9.25 | 1.42 × 109 | 5.59 | 1.19 × 109 | 10.67 | 8.36 × 108 | 1.86 × 108 |
| 10.479 | 1-methylpyrrole-2-carbaldehyde | 7.66 × 108 | 4.52 | 8.61 × 108 | 4.87 | 7.79 × 108 | 4.71 | 5.60 × 107 | 0.52 | 9.91 × 108 | 6.07 | 9.07 × 108 | 3.57 | 3.71 × 108 | 3.33 | 4.79 × 108 | 2.59 × 108 | ||
| 10.524 | 2-Ethyl-5-methylpyrazine | 6.10 × 108 | 3.60 | 4.28 × 108 | 2.59 | 5.45 × 108 | 3.34 | 9.22 × 107 | |||||||||||
| 10.567 | 2-methoxy-3H-azepine | 1.09 × 109 | 3.22 | 8.13 × 108 | 4.60 | 1.96 × 108 | |||||||||||||
| 10.578 | 4-(2-bromoethyl)-3,5-dimethyl-1H-pyrazole | 7.10 × 108 | 4.29 | 4.02 × 108 | 3.60 | 2.18 × 108 | |||||||||||||
| 10.601 | 2-chloro-1-(1-methylpyrrol-2-yl)ethanone | 1.90 × 107 | 0.18 | 1.48 × 109 | 5.83 | 1.03 × 109 | |||||||||||||
| 10.742 | 2-propanoyl furan | 2.99 × 108 | 0.88 | 1.44 × 108 | 0.85 | 1.02 × 108 | 0.58 | 8.63 × 107 | 0.52 | 8.79 × 107 | 0.79 | 1.39 × 108 | 3.29 × 107 | ||||||
| 10.883 | 2-propylpyrazine | 6.99 × 107 | 0.40 | ||||||||||||||||
| 11.153 | Tetrahydrofurfuryl chloride | 2.80 × 107 | 0.25 | ||||||||||||||||
| 11.163 | 2-ethyl-6-methylpyrazine | 7.40 × 107 | 0.69 | 1.04 × 108 | 0.41 | 2.12 × 107 | |||||||||||||
| 11.211 | 2-prop-1-en-2-ylpyrazine | 1.16 × 108 | 0.34 | 3.65 × 107 | 0.22 | 3.67 × 107 | 0.22 | 3.98 × 107 | 0.24 | 1.85 × 106 | |||||||||
| 11.255 | 1-(1-methoxypropan-2-yloxy)propan-2-ol | 4.62 × 107 | 0.27 | ||||||||||||||||
| 11.439 | 1-(4H-pyridin-1-yl)ethanone | 5.45 × 107 | 0.51 | ||||||||||||||||
| 11.478 | 5-amino-3-methyl-1,2-oxazole-4-carbonitrile | 3.95 × 108 | 1.17 | ||||||||||||||||
| 11.494 | 1-ethyl pyrrole | 1.31 × 108 | 0.77 | 1.13 × 108 | 0.64 | 1.69 × 108 | 1.02 | 1.91 × 108 | 1.17 | 1.05 × 108 | 0.41 | 5.66 × 106 | 3.04 × 107 | ||||||
| 11.522 | 2-methoxybenzenamine | 3.17 × 108 | 0.94 | 1.15 × 108 | 1.07 | 3.27 × 108 | 1.29 | 1.20 × 108 | |||||||||||
| 11.533 | 1-(4H-pyridin-1-yl)ethanone | 2.18 × 108 | 0.86 | ||||||||||||||||
| 11.560 | N-(2-Cyanoethyl)-pyrrole | 2.81 × 108 | 0.83 | ||||||||||||||||
| 11.563 | 1H-pyrrole-2-carbaldehyde | 3.29 × 108 | 0.97 | 8.61 × 108 | 8.02 | 2.43 × 108 | 0.96 | 1.24 × 108 | 1.11 | 3.35 × 108 | |||||||||
| 11.673 | 3-methylcyclopentane-1,2-dione | 2.58 × 107 | 0.23 | ||||||||||||||||
| 11.700 | 2-hydroxy-3-methylcyclopent-2-en-1-one | 1.77 × 107 | 0.10 | 1.72 × 107 | 0.10 | 2.23 × 107 | 0.14 | 2.60 × 107 | 0.23 | 4.16 × 107 | |||||||||
| 12.011 | 2-ethylhexanol | 5.02 × 107 | 0.45 | ||||||||||||||||
| 12.025 | 1-(2-methylcyclopenten-1-yl)ethanone | 2.24 × 108 | 0.66 | 8.38 × 107 | 0.49 | 8.77 × 107 | 0.50 | 8.30 × 107 | 0.50 | 8.56 × 107 | 0.80 | 5.59 × 107 | 0.34 | 1.34 × 108 | 0.53 | 7.61 × 107 | 0.68 | 6.48 × 107 | 1.30 × 107 |
| 12.083 | (4E)-2,6-dimethylhepta-2,4-diene | 6.03 × 107 | 0.34 | 1.37 × 108 | 0.54 | 5.42 × 107 | |||||||||||||
| 12.093 | 3,3,5-trimethylcyclohexene | 5.93 × 107 | 0.36 | 4.76 × 107 | 0.29 | 7.67 × 107 | 0.69 | 1.46 × 107 | |||||||||||
| 12.133 | 6,6-Dimethylhepta-2,4-diene | 4.03 × 107 | 0.36 | ||||||||||||||||
| 12.603 | Benzeneacetaldehyde | 2.56 × 107 | 0.16 | 2.32 × 107 | 0.21 | 1.70 × 106 | |||||||||||||
| 12.623 | Phenylacetaldehyde | 4.28 × 107 | 0.13 | 2.23 × 107 | 0.13 | 3.46 × 107 | 0.20 | 3.94 × 107 | 0.24 | 2.19 × 108 | 2.04 | 1.83 × 107 | 0.11 | 4.21 × 107 | 0.17 | 2.65 × 107 | 0.24 | 8.97 × 107 | 9.15 × 106 |
| 12.675 | N,N-dimethyl-1-(5-methylfuran-2-yl)methanamine | 1.17 × 108 | 0.35 | 3.64 × 108 | 3.39 | 8.90 × 107 | 0.35 | 4.27 × 107 | 0.38 | 1.51 × 108 | |||||||||
| 12.715 | 1,4-dimethyl-5-propan-2-ylcyclopentene | 3.62 × 108 | 1.07 | ||||||||||||||||
| 12.728 | (3E,5E)-nona-3,5-dien-2-one | 9.79 × 107 | 0.58 | 7.23 × 107 | 0.44 | 2.37 × 107 | 0.21 | 3.77 × 107 | |||||||||||
| 12.737 | 1-(1-ethylpyrazol-4-yl)ethanone | 3.34 × 107 | 0.10 | 4.20 × 107 | 0.25 | ||||||||||||||
| 12.771 | 5-methyl-2-Furanmethanethiol | 4.24 × 107 | 0.38 | ||||||||||||||||
| 13.018 | 1-(furan-2-yl)butan-2-one | 2.03 × 107 | 0.12 | 2.15 × 107 | 0.13 | 1.96 × 107 | 0.12 | 1.77 × 107 | 0.16 | 1.59 × 106 | |||||||||
| 13.266 | 2,3,4-trimethylcyclopent-2-en-1-one | 4.80 × 107 | 0.27 | ||||||||||||||||
| 13.290 | 5-ethylfuran-2-carbaldehyde | 9.11 × 107 | 0.27 | 4.65 × 107 | 0.43 | 4.98 × 107 | 0.20 | 2.49 × 107 | |||||||||||
| 13.334 | 2-methylphenol | 8.35 × 107 | 0.25 | 5.66 × 107 | 0.33 | 4.39 × 107 | 0.25 | 5.32 × 107 | 0.32 | 2.84 × 107 | 0.26 | 5.46 × 107 | 0.33 | 5.37 × 107 | 0.21 | 5.35 × 107 | 0.48 | 2.32 × 107 | 1.54 × 106 |
| 13.597 | 1-phenylethanone | 4.93 × 107 | 0.28 | ||||||||||||||||
| 13.690 | [phenyl(propanoyloxy)methyl] propanoate | 2.55 × 107 | 0.15 | 2.31 × 107 | 0.22 | 3.77 × 107 | 0.15 | 1.03 × 107 | |||||||||||
| 13.760 | [(1S,2R,5R)-5-methyl-2-propan-2-ylcyclohexyl] acetate | 1.66 × 108 | 0.49 | ||||||||||||||||
| 13.823 | Linalool oxide <cis-> | 5.93 × 107 | 0.35 | 7.74 × 107 | 0.44 | 5.49 × 107 | 0.33 | 2.46 × 107 | 0.23 | 7.07 × 107 | 0.28 | 2.87 × 107 | 3.11 × 106 | ||||||
| 14.045 | 2-methoxybenzenamine | 3.76 × 108 | 2.22 | 3.03 × 108 | 1.83 | 5.16 × 107 | |||||||||||||
| 14.054 | 1-(1H-pyrrol-2-yl)ethanone | 6.30 × 108 | 1.86 | 1.97 × 108 | 1.16 | 4.45 × 108 | 2.52 | 3.34 × 108 | 2.02 | 3.39 × 107 | 0.32 | 1.35 × 108 | 0.83 | 5.01 × 108 | 1.97 | 8.05 × 107 | 0.72 | 2.58 × 108 | 1.09 × 108 |
| 14.159 | 3-ethyl-2,5-dimethylpyrazine | 1.12 × 109 | 3.31 | 4.09 × 108 | 2.42 | 6.62 × 108 | 3.74 | 3.97 × 108 | 2.40 | 1.37 × 108 | 1.28 | 6.97 × 108 | 4.27 | 7.88 × 108 | 3.10 | 1.91 × 108 | 1.71 | 4.08 × 108 | 2.08 × 108 |
| 14.185 | 3,5-Dimethyl-4-allylpyrazole | 1.59 × 109 | 4.70 | ||||||||||||||||
| 14.373 | 2-(furan-2-ylmethyl)furan | 2.38 × 108 | 0.70 | 1.38 × 108 | 0.81 | 2.69 × 108 | 1.52 | 1.67 × 108 | 1.01 | 2.50 × 107 | 0.23 | 1.04 × 108 | 0.64 | 1.52 × 108 | 0.60 | 1.13 × 108 | 1.01 | 1.09 × 108 | 2.83 × 107 |
| 14.566 | 2-methoxyphenol | 1.03 × 109 | 3.04 | 4.30 × 108 | 2.54 | 8.89 × 108 | 5.02 | 5.01 × 108 | 3.03 | 4.22 × 107 | 0.39 | 5.24 × 108 | 3.21 | 7.99 × 108 | 3.15 | 1.46 × 108 | 1.31 | 4.42 × 108 | 1.74 × 108 |
| 14.612 | 4-methoxyphenol | 3.72 × 108 | 2.28 | 2.30 × 108 | 2.06 | 1.00 × 108 | |||||||||||||
| 14.737 | (2,6-dimethylcyclohexen-1-yl) acetate | 1.00 × 108 | 0.59 | 1.08 × 108 | 0.65 | 4.06 × 107 | 0.38 | 1.27 × 108 | 0.50 | 6.11 × 107 | 5.66 × 106 | ||||||||
| 14.751 | 3-methylcyclohexane-1,2-dione | 8.34 × 107 | 0.75 | ||||||||||||||||
| 14.867 | furan-2-ylmethyl propanoate | 2.03 × 108 | 1.20 | 3.21 × 108 | 1.81 | 2.31 × 108 | 1.40 | 2.76 × 107 | 0.26 | 2.05 × 108 | 1.26 | 2.60 × 108 | 1.02 | 1.20 × 108 | 1.08 | 1.55 × 108 | 4.82 × 107 | ||
| 15.000 | 4,5-dimethylhex-4-en-3-one | 4.29 × 107 | 0.38 | ||||||||||||||||
| 15.246 | 2-methylpropyl [(Z)-non-3-enyl] carbonate | 2.22 × 108 | 1.25 | 8.30 × 107 | 0.51 | 1.47 × 108 | 1.32 | 4.53 × 107 | |||||||||||
| 15.246 | Glutraic acid, di(cis-non-3-enyl) ester | 2.43 × 108 | 0.72 | 1.64 × 108 | 1.01 | ||||||||||||||
| 15.255 | (4Z)-cyclooct-4-en-1-one | 7.30 × 107 | 0.43 | ||||||||||||||||
| 15.268 | 1,1-Dimethyl-4-methylenecyclohexane | 5.59 × 107 | 0.50 | ||||||||||||||||
| 15.291 | 4-O-[(Z)-non-3-enyl] 1-O-propyl (E)-but-2-enedioate | 2.58 × 108 | 1.46 | ||||||||||||||||
| 15.293 | 3-methylidene-3a,4,5,6,7,7a-hexahydro-1-benzofuran-2-one | 7.13 × 107 | 0.44 | ||||||||||||||||
| 15.318 | 3,7-dimethylocta-1,6-dien-3-ol | 2.05 × 108 | 0.61 | ||||||||||||||||
| 15.449 | 3,7-dimethyloctan-3-ol | 6.34 × 107 | 0.37 | ||||||||||||||||
| 15.459 | 2-prop-1-en-2-ylpyrazine | 4.31 × 107 | 0.39 | ||||||||||||||||
| 15.702 | Nonanal | 1.76 × 108 | 0.52 | ||||||||||||||||
| 15.723 | 2-(1-methylpyrrol-2-yl)acetonitrile | 2.47 × 108 | 1.46 | 3.66 × 108 | 2.21 | 3.31 × 107 | 0.31 | 3.20 × 108 | 1.96 | 1.74 × 108 | 0.69 | 1.91 × 108 | 1.71 | 9.96 × 107 | 7.75 × 107 | ||||
| 15.759 | 1-cyclopentylethyl 5-chloropentanoate | 1.21 × 108 | 0.68 | ||||||||||||||||
| 15.794 | 3-hydroxy-2-methylpyran-4-one | 1.31 × 108 | 0.39 | ||||||||||||||||
| 15.849 | 2-phenylethanol | 2.69 × 108 | 0.80 | 1.45 × 108 | 0.86 | 1.69 × 108 | 0.96 | 1.05 × 108 | 0.63 | 2.91 × 108 | 1.78 | 5.78 × 107 | 0.52 | 7.07 × 107 | 1.01 × 108 | ||||
| 15.963 | 2,5-Methano-1H-indene, octahydro- | 4.27 × 107 | 0.25 | 4.77 × 107 | 0.43 | 3.54 × 106 | |||||||||||||
| 16.061 | 1-(3-methylpyrazin-2-yl)ethanone | 2.61 × 108 | 0.77 | ||||||||||||||||
| 16.107 | 3-ethyl-2-hydroxycyclopent-2-en-1-one | 1.35 × 108 | 0.80 | 1.09 × 108 | 0.66 | 1.39 × 108 | 0.85 | 1.63 × 107 | |||||||||||
| 16.131 | ethyl non-2-enoate | 5.95 × 107 | 0.18 | 7.13 × 107 | 0.42 | 1.10 × 108 | 0.66 | 6.62 × 107 | 0.59 | 2.40 × 107 | |||||||||
| 16.138 | 3-butylcyclopentane-1,2,4-trione | 6.40 × 107 | 0.38 | ||||||||||||||||
| 16.167 | 1-(6-methylpyrazin-2-yl)ethanone | 4.41 × 107 | 0.41 | 5.07 × 107 | 0.31 | 1.30 × 108 | 0.51 | 6.07 × 107 | |||||||||||
| 16.381 | prop-2-enyl furan-2-carboxylate | 3.12 × 108 | 0.92 | 7.67 × 107 | 0.45 | 1.60 × 107 | 0.15 | 9.93 × 107 | 0.61 | 2.24 × 108 | 0.88 | 6.53 × 107 | 0.59 | 1.52 × 108 | 1.73 × 107 | ||||
| 16.540 | N-[(4-fluorophenyl)methyl]-2-phenylethanamine | 1.48 × 107 | 0.04 | ||||||||||||||||
| 16.753 | 1-(5-methylfuran-2-yl)propan-2-one | 2.06 × 108 | 0.61 | ||||||||||||||||
| 16.774 | (2E,4E)-2,4-dimethylhepta-2,4-dienal | 1.93 × 108 | 0.57 | 6.77 × 107 | 0.40 | 1.52 × 108 | 0.86 | 8.82 × 107 | 0.53 | 1.86 × 107 | 0.17 | 9.79 × 107 | 0.60 | 1.35 × 108 | 0.53 | 4.83 × 107 | 0.43 | 7.48 × 107 | 2.21 × 107 |
| 16.795 | 2-methyl-3-(2-methylpropyl)pyrazine | 9.70 × 107 | 0.59 | ||||||||||||||||
| 17.097 | 1-(4-methoxyphenyl)-2-(6-methylpyrazin-2-yl)ethanol | 7.89 × 107 | 0.47 | 6.58 × 107 | 0.40 | 5.70 × 107 | 0.35 | 1.10 × 107 | |||||||||||
| 17.120 | 5-methyl-6,7-dihydro-5H-cyclopenta[b]pyrazine | 7.02 × 107 | 0.41 | ||||||||||||||||
| 17.216 | 4-(5-methylfuran-2-yl)butan-2-one | 1.03 × 108 | 0.62 | ||||||||||||||||
| 17.533 | 6-tert-butyl-6-methyloxan-2-one | 1.36 × 108 | 0.40 | 5.75 × 107 | 0.32 | 2.39 × 107 | 0.15 | 2.75 × 107 | 0.25 | 5.55 × 107 | 2.55 × 106 | ||||||||
| 17.550 | adamantan-1-ol | 5.16 × 108 | 4.80 | 3.45 × 107 | 0.14 | 3.40 × 108 | |||||||||||||
| 17.572 | 1,3,5-trimethylpiperidin-4-one | 1.32 × 108 | 0.39 | 6.51 × 107 | 0.38 | 3.89 × 107 | 0.24 | 1.30 × 108 | 1.21 | 7.21 × 107 | 0.44 | 5.62 × 107 | 0.22 | 4.32 × 107 | 1.75 × 107 | ||||
| 17.900 | 3,4-dimethyl-1H-pyrrole-2-carbaldehyde | 1.38 × 108 | 0.81 | 1.39 × 108 | 0.79 | 9.25 × 107 | 0.56 | 1.35 × 108 | 1.26 | 2.05 × 108 | 1.26 | 1.37 × 108 | 0.54 | 5.44 × 107 | 0.49 | 2.00 × 106 | 6.48 × 107 | ||
| 17.946 | 3,5-diethyl-2-methylpyrazine | 1.50 × 108 | 0.59 | ||||||||||||||||
| 17.990 | 2-ethyl-3,5,6-trimethylpyrazine | 6.11 × 108 | 1.81 | 1.07 × 108 | 0.63 | 9.15 × 107 | 0.55 | 3.45 × 107 | 0.32 | 8.16 × 107 | 0.50 | 1.33 × 108 | 0.52 | 4.51 × 107 | 0.40 | 3.08 × 108 | 2.63 × 107 | ||
| 18.123 | N-(2-methoxyphenyl)formamide | 8.68 × 107 | 0.26 | 5.62 × 107 | 0.52 | 3.88 × 107 | 0.24 | 7.31 × 107 | 0.29 | 1.53 × 107 | |||||||||
| 18.291 | 2,3-diethyl-5-methylpyrazine | 1.34 × 108 | 0.40 | 6.23 × 107 | 0.37 | 7.27 × 107 | 0.44 | 1.50 × 108 | 1.40 | 6.89 × 107 | 0.42 | 1.16 × 108 | 0.46 | 5.81 × 107 | 0.52 | 1.70 × 107 | 6.54 × 106 | ||
| 18.511 | 2,5-dimethyl-3-propan-2-ylpyrazine | 1.40 × 108 | 0.41 | 1.37 × 108 | 1.28 | 8.13 × 107 | 0.32 | 3.31 × 107 | |||||||||||
| 18.530 | 1-(3-ethylpyrazin-2-yl)ethanone | 9.88 × 107 | 0.61 | ||||||||||||||||
| 18.531 | 2-hexylfuran | 4.94 × 107 | 0.30 | ||||||||||||||||
| 18.730 | 2,4,6-trimethylcyclohex-3-ene-1-carbaldehyde;3,5,6-trimethylcyclohex-3-ene-1-carbaldehyde | 1.15 × 108 | 0.65 | 3.15 × 107 | 0.19 | ||||||||||||||
| 18.830 | 2-n-Heptylfuran | 1.38 × 108 | 0.41 | 1.02 × 108 | 0.60 | 7.41 × 107 | 0.45 | 1.33 × 108 | 1.24 | 6.96 × 107 | 0.43 | 1.15 × 108 | 0.45 | 1.21 × 107 | 1.76 × 107 | ||||
| 18.841 | (5R)-5-methyl-2-propan-2-ylidenecyclohexan-1-one | 7.22 × 107 | 0.65 | ||||||||||||||||
| 18.850 | 2-(2,6,6-trimethylcyclohexen-1-yl)acetaldehyde | 1.42 × 108 | 0.42 | ||||||||||||||||
| 18.868 | 1-(furan-2-yl)propan-2-one | 1.47 × 108 | 0.43 | ||||||||||||||||
| 19.013 | 1-(furan-2-ylmethyl)pyrrole | 1.07 × 108 | 0.63 | 5.04 × 107 | 0.45 | 4.00 × 107 | |||||||||||||
| 19.192 | 5-methoxybenzene-1,3-diol | 4.53 × 108 | 1.34 | 2.99 × 108 | 1.77 | 3.43 × 108 | 1.94 | 3.79 × 108 | 2.29 | 7.31 × 107 | 0.68 | 4.14 × 108 | 2.54 | 4.35 × 108 | 1.71 | 2.23 × 108 | 2.00 | 1.75 × 108 | 8.54 × 107 |
| 19.456 | 1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene | 1.59 × 108 | 0.94 | 1.36 × 108 | 0.82 | 1.16 × 108 | 1.08 | 9.82 × 107 | 0.60 | 1.33 × 108 | 0.52 | 1.20 × 107 | 3.07 × 107 | ||||||
| 19.468 | 1,3,3a,4,6,6a-hexahydropentalene-2,5-dione | 9.82 × 107 | 0.56 | ||||||||||||||||
| 19.478 | N-(4-hydroxyphenyl)acetamide | 8.44 × 107 | 0.51 | ||||||||||||||||
| 19.569 | N-(3-hydroxyphenyl)acetamide | 7.04 × 107 | 0.63 | ||||||||||||||||
| 19.600 | (Z)-3-(furan-2-yl)-2-methylprop-2-enal | 7.49 × 107 | 0.67 | ||||||||||||||||
| 19.723 | 2-methyl-5-[(E)-prop-1-enyl]pyrazine | 7.42 × 107 | 0.22 | 5.14 × 107 | 0.46 | ||||||||||||||
| 19.896 | 5,6,7,8-tetrahydroquinoxaline | 1.91 × 108 | 0.56 | 7.94 × 107 | 0.47 | 1.57 × 108 | 0.89 | 9.04 × 107 | 0.55 | 8.13 × 107 | 0.76 | 1.21 × 108 | 0.74 | 1.27 × 108 | 0.50 | 4.66 × 107 | 2.16 × 107 | ||
| 19.947 | 2,5-dimethyl-3-propylpyrazine | 1.15 × 108 | 1.07 | 1.37 × 108 | 0.54 | 1.56 × 107 | |||||||||||||
| 20.141 | 1-(8-azabicyclo[3.2.1]oct-2-en-2-yl)ethanone | 4.95 × 107 | 0.15 | 1.70 × 107 | 0.10 | 1.33 × 108 | 1.24 | 1.32 × 107 | 0.08 | 2.14 × 107 | 0.08 | 5.80 × 107 | 2.69 × 107 | ||||||
| 20.150 | 1-(3,6-dimethylpyrazin-2-yl)-3-methylbutan-1-one | 4.35 × 108 | 4.05 | 2.53 × 107 | 0.10 | 2.90 × 108 | |||||||||||||
| 20.163 | benzene-1,2-diol | 4.02 × 107 | 0.23 | ||||||||||||||||
| 20.362 | 2,5-dimethylthiophene | 1.21 × 108 | 0.36 | 6.01 × 107 | 0.36 | 1.27 × 108 | 1.18 | 1.04 × 108 | 0.64 | 9.75 × 107 | 0.38 | 1.56 × 107 | 3.10 × 107 | ||||||
| 20.370 | 2-acetylcyclohexan-1-one | 5.13 × 107 | 0.31 | ||||||||||||||||
| 20.413 | 1-hydroxypyridin-2-imine | 2.97 × 107 | 0.18 | ||||||||||||||||
| 20.419 | (4-hexadecanoyloxy-5-hydroxy-6-methylpyridin-3-yl) hexadecanoate | 1.37 × 108 | 1.28 | 1.28 × 108 | 0.50 | 6.36 × 106 | |||||||||||||
| 20.460 | Decanal | 2.71 × 107 | 0.16 | ||||||||||||||||
| 20.623 | Undecanal | 7.48 × 107 | 0.44 | 6.26 × 107 | 0.38 | 2.53 × 107 | 0.24 | 4.62 × 107 | 0.28 | 1.01 × 108 | 0.40 | 3.49 × 107 | 0.31 | 5.35 × 107 | 1.76 × 107 | ||||
| 20.638 | 3,7-dimethyl-6,7-dihydro-5H-cyclopenta[b]pyrazine | 5.39 × 107 | 0.30 | 2.14 × 107 | 0.20 | 3.71 × 107 | 0.23 | 5.50 × 107 | 0.22 | 1.91 × 107 | |||||||||
| 21.129 | Tridecanedial | 5.78 × 107 | 0.17 | 2.52 × 107 | 0.15 | 2.93 × 107 | 0.17 | 2.27 × 107 | 0.14 | 1.28 × 108 | 1.19 | 5.19 × 107 | 0.32 | 5.00 × 107 | 0.20 | 4.29 × 107 | 1.62 × 107 | ||
| 21.138 | furan-2-ylmethyl pentanoate | 6.27 × 107 | 0.25 | ||||||||||||||||
| 21.300 | 2-butyl-3-methylpyrazine | 4.95 × 107 | 0.15 | 4.06 × 107 | 0.24 | 8.16 × 107 | 0.46 | 5.78 × 107 | 0.35 | 1.01 × 108 | 0.94 | 5.23 × 107 | 0.32 | 5.60 × 107 | 0.22 | 2.09 × 107 | 0.19 | 2.38 × 107 | 1.63 × 107 |
| 22.372 | 2-methoxy-3-propan-2-ylpyrazine | 1.44 × 107 | 0.08 | ||||||||||||||||
| 22.427 | 2-Isoamyl-6-methylpyrazine | 6.27 × 107 | 0.58 | 7.40 × 107 | 0.29 | 7.99 × 106 | |||||||||||||
| 22.427 | 3H-benzimidazole-5-carbaldehyde | 6.53 × 107 | 0.19 | 3.25 × 107 | 0.19 | 3.50 × 107 | 0.20 | 2.56 × 107 | 0.15 | 5.00 × 107 | 0.47 | 2.94 × 107 | 0.18 | 1.90 × 107 | 0.07 | 1.99 × 107 | 3.46 × 106 | ||
| 22.841 | 3H-quinazolin-4-one | 3.99 × 107 | 0.24 | 6.46 × 107 | 0.39 | 1.75 × 107 | |||||||||||||
| 22.879 | heptadecan-9-one | 1.17 × 108 | 0.66 | ||||||||||||||||
| 23.208 | 8-hydroxyneomenthol | 2.60 × 107 | 0.15 | ||||||||||||||||
| 23.236 | 2-[(furan-2-ylmethyldisulfanyl)methyl]furan | 4.15 × 107 | 0.25 | ||||||||||||||||
| 23.239 | 1,2-dibromocyclohexane | 9.36 × 107 | 0.55 | 5.15 × 107 | 0.46 | 2.98 × 107 | |||||||||||||
| 23.279 | 1,4-dimethoxy-2-methyl-5-propylbenzene | 5.50 × 107 | 0.34 | ||||||||||||||||
| 23.339 | 1-(4-propan-2-ylphenyl)ethanone | 3.81 × 107 | 0.34 | ||||||||||||||||
| 23.342 | 1-(2,5-dihydroxyphenyl)ethanone | 5.92 × 107 | 0.17 | 4.62 × 107 | 0.27 | 3.81 × 107 | 0.23 | 5.73 × 106 | |||||||||||
| 23.350 | 1-(2,4-dihydroxyphenyl)-2-phenylethanone | 1.30 × 108 | 0.38 | 6.21 × 107 | 0.37 | 9.45 × 107 | 0.53 | 5.68 × 107 | 0.34 | 5.60 × 107 | 0.52 | 1.15 × 108 | 0.45 | 3.21 × 107 | 3.75 × 106 | ||||
| 23.359 | 2-acetylresorcinol | 8.44 × 107 | 0.50 | 5.09 × 107 | 0.46 | 2.37 × 107 | |||||||||||||
| 23.363 | 4-ethyl-2-methoxyphenol | 4.77 × 107 | 0.19 | ||||||||||||||||
| 23.518 | 1,2-dimethoxy-3-methylbenzene | 2.38 × 108 | 0.94 | ||||||||||||||||
| 23.534 | 4-(2-aminoethyl)-2-methoxyphenol | 2.42 × 108 | 0.95 | ||||||||||||||||
| 23.538 | decan-1-ol | 4.04 × 108 | 2.39 | 5.17 × 108 | 4.81 | ||||||||||||||
| 23.543 | Nonanoic acid | 8.23 × 108 | 2.43 | 7.93 × 108 | 4.68 | 8.62 × 108 | 4.87 | 8.06 × 108 | 4.87 | 1.90 × 107 | 0.18 | 9.42 × 108 | 5.77 | 8.61 × 108 | 3.39 | 4.15 × 108 | 8.25 × 107 | ||
| 23.733 | Benzo-2,3-pyrrole | 2.28 × 107 | 0.13 | ||||||||||||||||
| 24.081 | 1-(5-methylfuran-2-yl)propan-2-one | 3.94 × 107 | 0.35 | ||||||||||||||||
| 24.334 | 1-fluoro-2-[(2-methylpropan-2-yl)oxymethyl]benzene | 1.17 × 108 | 0.35 | ||||||||||||||||
| 24.363 | adamantane-1,2-diamine | 9.38 × 107 | 0.28 | 7.52 × 107 | 0.43 | 8.75 × 107 | 0.53 | 7.40 × 107 | 0.69 | 8.75 × 107 | 0.54 | 8.56 × 107 | 0.34 | 2.58 × 107 | 0.23 | 9.35 × 106 | 3.56 × 107 | ||
| 24.501 | 2-(furan-2-ylmethoxymethyl)furan | 1.76 × 107 | 0.10 | 2.37 × 107 | 0.21 | 4.31 × 106 | |||||||||||||
| 24.503 | 4-ethenyl-2-methoxyphenol | 1.87 × 107 | 0.17 | ||||||||||||||||
| 24.546 | 2,3-dimethyl-5-(3-methylbutyl)pyrazine | 3.14 × 107 | 0.28 | ||||||||||||||||
| 24.762 | 2,5-dimethyl-3-(3-methylbutyl)pyrazine | 1.54 × 108 | 0.91 | 2.91 × 108 | 1.64 | 2.13 × 108 | 1.29 | 5.45 × 107 | 0.51 | 1.43 × 108 | 0.88 | 2.19 × 108 | 0.86 | 1.25 × 108 | 1.12 | 1.21 × 108 | 3.81 × 107 | ||
| 25.204 | 4-ethyl-1,2-dimethoxybenzene | 1.61 × 108 | 0.95 | 1.51 × 108 | 0.85 | 2.03 × 108 | 1.24 | 8.61 × 107 | 0.77 | 5.92 × 107 | |||||||||
| 25.221 | 2,4,4-trimethyl-3-(3-methylbutyl)cyclohex-2-en-1-one | 4.30 × 108 | 2.54 | 2.84 × 108 | 1.72 | 1.15 × 108 | 1.07 | 5.22 × 108 | 3.20 | 3.64 × 108 | 1.43 | 1.76 × 108 | 1.20 × 108 | ||||||
| 25.350 | 2-aminonaphthalen-1-ol | 3.79 × 107 | 0.11 | 8.61 × 108 | 8.02 | 4.65 × 107 | 0.18 | 4.73 × 108 | |||||||||||
| 25.383 | 4-methyl-1H-quinolin-2-one | 4.00 × 107 | 0.12 | 2.70 × 107 | 0.16 | 4.01 × 107 | 0.23 | 1.72 × 107 | 0.10 | 2.28 × 107 | 0.14 | 7.07 × 104 | 4.92 × 106 | ||||||
| 25.870 | 2-methylchromen-4-one | 2.54 × 107 | 0.08 | 1.17 × 107 | 0.07 | 6.85 × 107 | 0.39 | 3.74 × 107 | 0.23 | 8.56 × 107 | 0.80 | 2.62 × 107 | 0.16 | 2.84 × 107 | 0.11 | 1.42 × 107 | 0.13 | 2.98 × 107 | 1.18 × 107 |
| 26.529 | 4-ethenyl-1,2-dimethoxybenzene | 3.54 × 107 | 0.10 | ||||||||||||||||
| 26.737 | 2-methoxy-5-[(E)-prop-1-enyl]phenol | 2.57 × 107 | 0.15 | 1.63 × 107 | 0.10 | 2.19 × 108 | 2.04 | 2.31 × 107 | 0.09 | 1.12 × 108 | |||||||||
| 26.761 | 4-ethoxy-3-hydroxybenzaldehyde | 1.19 × 108 | 0.35 | 2.92 × 107 | 0.17 | 3.64 × 108 | 3.39 | 2.46 × 107 | 0.10 | 1.59 × 108 | |||||||||
| 26.904 | 2-methoxy-4-prop-2-enylphenol | 3.67 × 107 | 0.22 | 7.34 × 107 | 0.41 | 1.85 × 107 | 0.17 | 1.29 × 107 | |||||||||||
| 27.813 | 7-methyl-4-oxo-1H-1,8-naphthyridine-3-carboxylic acid | 3.39 × 107 | 0.20 | ||||||||||||||||
| 27.817 | 1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one | 2.07 × 107 | 0.12 | 1.83 × 107 | 0.11 | 4.65 × 107 | 0.43 | 1.50 × 107 | 0.09 | 3.39 × 107 | 0.13 | 8.91 × 106 | 2.86 × 106 | ||||||
| 27.825 | 3-methyl-1H-indole | 5.83 × 107 | 0.33 | ||||||||||||||||
| 27.842 | dodecan-2-ol | 3.97 × 107 | 0.12 | ||||||||||||||||
| 28.016 | Tetradecane | 2.84 × 107 | 0.26 | 1.37 × 108 | 0.54 | 7.68 × 107 | |||||||||||||
| 28.057 | 5-(1,5-dimethylpyrrol-2-yl)-2H-tetrazole | 2.31 × 107 | 0.22 | 2.50 × 107 | 0.10 | 1.34 × 106 | |||||||||||||
| 28.321 | (5E)-6,10-dimethylundeca-5,9-dien-2-one | 2.30 × 107 | 0.14 | 4.88 × 107 | 0.28 | 3.09 × 107 | 0.19 | 2.46 × 107 | 0.23 | 3.58 × 107 | 0.22 | 4.22 × 107 | 0.17 | 1.01 × 107 | 0.09 | 1.25 × 107 | 1.12 × 107 | ||
| 29.385 | 1-(furan-2-ylmethyl)pyrrole | 5.54 × 107 | 0.33 | ||||||||||||||||
| 30.200 | dodecan-1-ol | 3.39 × 107 | 0.32 | 4.06 × 107 | 0.16 | 4.74 × 106 | |||||||||||||
| 30.590 | Nonatriacontane <n-> | 2.50 × 107 | 0.15 | 1.24 × 107 | 0.08 | 8.91 × 106 | |||||||||||||
| 31.182 | Pentadecane <n-> | 1.37 × 108 | 1.28 | 2.76 × 107 | 0.11 | 7.74 × 107 | |||||||||||||
| 32.049 | 2,3,5-trimethylbenzene-1,4-diol | 9.42 × 106 | 0.06 | 1.17 × 107 | 0.07 | 1.61 × 106 | |||||||||||||
| 32.316 | 1-iodohexadecane | 5.58 × 107 | 0.50 | ||||||||||||||||
| 33.663 | 1,3,7-trimethylpurine-2,6-dione | 2.50 × 107 | 0.23 | 3.31 × 107 | 0.13 | 5.73 × 106 | |||||||||||||
| 34.323 | Pentadecane <n-> | 4.22 × 107 | 0.39 | 4.41 × 107 | 0.17 | 1.43 × 107 | 0.13 | 1.34 × 106 | |||||||||||
| 34.795 | 2,3,5-trimethylbenzene-1,4-diol | 4.06 × 107 | 0.38 | 1.60 × 107 | 0.06 | 1.74 × 107 | |||||||||||||
| 36.303 | 1-iodohexadecane | 2.76 × 107 | 0.26 | 1.86 × 107 | 0.07 | 1.21 × 107 | 0.11 | 6.36 × 106 | |||||||||||
| 45.432 | 1,3,7-trimethylpurine-2,6-dione | 4.34 × 107 | 0.13 | 2.83 × 107 | 0.17 | 1.94 × 108 | 1.10 | 3.89 × 107 | 0.24 | 3.31 × 107 | 0.31 | 1.92 × 108 | 1.18 | 6.62 × 107 | 0.26 | 3.94 × 107 | 0.35 | 7.45 × 107 | 7.84 × 107 |
| TOT | 3.38 × 1010 | 100 | 1.69 × 107 | 100 | 1.77 × 107 | 100 | 1.66 × 107 | 100 | 1.07 × 107 | 100 | 1.63 × 107 | 100 | 2.54 × 107 | 100 | 1.12 × 107 | 100 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, G.; Núñez-Carmona, E.; Abbatangelo, M.; Fava, P.; Sberveglieri, V. How Coffee Capsules Affect the Volatilome in Espresso Coffee. Separations 2021, 8, 248. https://doi.org/10.3390/separations8120248
Greco G, Núñez-Carmona E, Abbatangelo M, Fava P, Sberveglieri V. How Coffee Capsules Affect the Volatilome in Espresso Coffee. Separations. 2021; 8(12):248. https://doi.org/10.3390/separations8120248
Chicago/Turabian StyleGreco, Giuseppe, Estefanía Núñez-Carmona, Marco Abbatangelo, Patrizia Fava, and Veronica Sberveglieri. 2021. "How Coffee Capsules Affect the Volatilome in Espresso Coffee" Separations 8, no. 12: 248. https://doi.org/10.3390/separations8120248
APA StyleGreco, G., Núñez-Carmona, E., Abbatangelo, M., Fava, P., & Sberveglieri, V. (2021). How Coffee Capsules Affect the Volatilome in Espresso Coffee. Separations, 8(12), 248. https://doi.org/10.3390/separations8120248