Abstract
The quantitative determination of levamisole (LMS), mebendazole (MBZ), and the two metabolites of MBZ, 5-hydroxymebendazole (HMBZ) and 2-amino-5-benzoylbenzimidazole (AMBZ), in poultry eggs (hen, duck, and goose) was achieved with high-performance liquid chromatography–tandem triple quadrupole mass spectrometry (HPLC–MS/MS). Samples were pretreated by liquid–liquid extraction and solid-phase extraction (LLE–SPE) to extract the target compounds, and an Oasis MCX SPE column was used for purification. Determination was performed on an Xbridge C18 column with 0.1% formic acid aqueous solution and acetonitrile as mobile phases. LMS, MBZ, HMBZ, and AMBZ were detected in a triple-quadrupole mass spectrometer with ESI in positive mode and quantified with an external standard. In blank eggs, the target analyte concentrations were within the limits of quantification (LOQs)—25 μg/kg (LMS) and 150 μg/kg (MBZ, HMBZ, and AMBZ)—and the matrix-matched calibration curves had good linearity (R2 ≥ 0.9990). In the same concentration range, the average recoveries of the target analytes were 85.98–97.38% (n = 6); the relative standard deviation (RSD), intraday RSD, and interday RSD ranged from 2.06 to 4.22%, 1.40 to 5.85%, and 2.34 to 6.32%, respectively. The limits of detection (LODs) ranged from 0.03 to 0.33 µg/kg, and the LOQs ranged from 0.08 to 1.00 µg/kg. Experimental verification showed that the HPLC–MS/MS method exhibited high specificity and sensitivity for quantitative analyses of egg samples. This study provides a rapid, efficient, and sensitive method for the simultaneous detection of LMS, MBZ, HMBZ, and AMBZ residues in foods of animal origin.
1. Introduction
Poultry eggs, mainly hen, duck, and goose eggs, are rich in nutrients and are an important source of nutrition in people’s lives [1]. Amino acids contained in eggs are essential substances for the human body, and the utilization rate is up to 99.6%, making eggs the most ideal form of high-quality protein in natural food [2]. In addition, eggs contain rich unsaturated fatty acids and can be easily digested and absorbed by the human body. Therefore, eggs have become a daily staple in people’s diets. To improve poultry production efficiency and health, antibiotics are widely used to prevent and treat disease.
Levamisole (LMS) and mebendazole (MBZ) are widely used in the livestock and poultry industries as efficient, broad-spectrum antiparasitic drugs. LMS can produce a stable S-S chain by inhibiting the activity of fumarase in parasites, thus blocking the reduction of fumaric acid to succinic acid, affecting sugar metabolism and reducing the ATP content in the parasites, resulting in paralysis and death [3,4]. The main mechanism of action of MBZ is that it inhibits the absorption and utilization of glucose, hinders the production of ATP, prolongs the half-life of cellular hydrolases, and accelerates cell lysis, thus leading to the depletion of glycogen, which prevents parasites from surviving or reproducing [5]. LMS can be used in combination with a variety of antiparasitic drugs, has a synergistic effect when paired with benzimidazole antiparasitic drugs, and can improve the efficacy of intestinal deworming treatment [6]. Bennet et al. used a combination of MBZ and LMS to control larval-stage Taenia solium in mice [7]. The study showed that LMS as an adjuvant enhanced the host immune response, which, combined with the known repellent effects of MBZ, increased the efficacy of benzimidazole derivatives [8]. Albonico et al. reported that combined MBZ and LMS treatment had better antiparasitic efficacy than treatment with either compound alone [6]. Therefore, the combination of LMS and MBZ is commonly used to treat intestinal parasitic diseases and a tablet with this combination has been listed in the Chinese Pharmacopoeia [9]. However, LMS abuse may lead to residues in edible animal tissues and the long-term consumption of foods containing LMS may cause skin necrotizing vasculitis, granulophilic hypoxia, neurological side effects, etc., posing potential risks to consumers’ health [10]. Therefore, the European Union [11], the United States (FDA) [12], and South Korea [13] have banned the use of LMS in poultry eggs and set a limit of 10–1100 μg/kg in animal-derived foods. Improper use of MBZ leads to embryo toxicity and teratogenicity, which has caused great concern. China, the European Union, the United States, and Japan stipulate that MBZ must not be detectable in edible poultry and pig tissues. Therefore, it is of great significance to establish a rapid and efficient method to detect LMS, MBZ, and the MBZ metabolites 5-hydroxymebendazole (HMBZ) and 2-amino-5-benzoylbenzimidazole (AMBZ) in poultry eggs.
Currently, the methods used to determine the contents of LMS, MBZ, HMBZ, and AMBZ in animal-derived foods include immunochromatographic detection [14], enzyme-linked immunosorbent assay (ELISA) [15], gas chromatography–mass spectrometry (GC–MS) [16], gas chromatography–tandem mass spectrometry (GC–MS/MS) [17], high-performance liquid chromatography–ultraviolet detector (HPLC–UVD) analysis [18,19], and high-performance liquid chromatography–diode array detector (HPLC–DAD) analysis [20,21]. Due to the basic properties and low volatility of both MBZ and LMS, GC–MS and GC–MS/MS procedures often require derivatization of compound residues to induce volatility and generate substances suitable for MS confirmation analysis. Therefore, GC has been replaced by liquid chromatography (LC) and liquid chromatography–tandem mass spectrometry (LC–MS/MS). Compared with traditional methods such as HPLC–UVD, MS detection has the characteristics of high recovery, high selectivity, good reproducibility, and low interference. Kinsella et al. developed an LC–MS/MS method to analyze residues of 38 deworming veterinary drugs, including benzimidazole, from milk and liver samples [22]. Ning X et al. established a quantitative LC–MS/MS method to identify residues of MBZ and its metabolites, albendazole and its metabolites, and LMS in the edible tissues of aquatic animals [23]. In recent years, the use of HPLC–MS/MS to detect LMS, MBZ, and the metabolite residues of MBZ in animal food has been reported, but the simultaneous detection of LMS, MBZ, and the metabolite residues of MBZ in poultry eggs has not been reported.
Therefore, this study established a method for the simultaneous determination of LMS, MBZ, HMBZ, and AMBZ residues in poultry eggs (hen, duck, and goose eggs) by HPLC–MS/MS, compared the effects of different extractants and solid-phase extraction (SPE) columns on recovery, and optimized the HPLC–MS/MS analysis conditions. Appropriate sample pretreatment not only promoted the accuracy of the quantitative results but also contributed to the maintenance and lengthening of the instrument life. The purpose of this study was to provide a new research method for the simultaneous detection of deworming agents in livestock and poultry, improve the HPLC–MS/MS detection technique for LMS, MBZ, HMBZ, and AMBZ residues in poultry eggs at home and overseas, and provide technical support and a scientific basis for monitoring veterinary drug residues.
2. Materials and Methods
2.1. Sample Collection
Thirty-five Jinghai yellow chickens were randomly selected at 31 weeks of age and collected from the farm of Jiangsu Jinghai Poultry Group Co., Ltd. (Nantong, Jiangsu, China). Thirty-five Gaoyou laying ducks collected from the farm of Jiangsu Gaoyou Duck Group Co., Ltd. (Yangzhou, Jiangsu, China), were randomly selected at 28 weeks of age. Fifty Yangzhou geese collected from the farm of Jiangsu Tiange Goose Industry Development Co., Ltd. (Yangzhou, Jiangsu, China), were randomly selected at 32 weeks of age. Before the experiment, the chickens were free-fed full-price feed without any added drugs (provided by the Feed Factory of Jiangsu Jinghai Group Co., Ltd. and Feed Factory of Yangzhou University) in a single cage for 15 days. Starting on the 16th day, eggs were collected every day between 16:00 and 17:00 for two consecutive weeks. The poultry egg samples were stored at low temperatures for later use.
After poultry egg collection was complete, the blank hen, duck, and goose eggs were collected as whole egg, albumen, and yolk samples or homogenized to make a blank sample and stored in a freezer at −70 °C for later use.
2.2. Chemicals and Reagents
The LMS potassium salt standard (purity 98.0%) was purchased from Sigma–Aldrich (St. Louis, MO, USA). The MBZ standard (purity 98.0%) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Hydroxybenzimidazole (96.28% purity) and aminophenyl benzimidazole (97.70% purity) were purchased from Labour Dr. Ehrenstorfer-Schafers (Augsburg, Germany). HPLC-grade acetonitrile and methanol were both supplied by Merck Co., Ltd. (Huntertown County, NJ, USA). HPLC-grade ethanol was obtained from Fisher Scientific International Inc. (Pittsburgh, PA, USA). HPLC-grade methanol (MeOH) and analytical-grade formic acid, ammonium acetate, hexane, anhydrous sodium sulfate, ethyl acetate (EtOAc), methylene chloride (DCM), potassium hydroxide (KOH), and N-hexane were supplied by Sinopharm Chemical Reagent Co. (Shanghai, China). A nylon syringe filter (13 mm × 0.45 µm) was purchased from ANPEL Laboratory Technologies Inc. (Shanghai, China). Experimental ultrapure water was prepared by a Thermo Scientific Smart2-Pure ultrapure water preparation system (Thermo Fisher Corp., Waltham, MA, USA), and the resistivity of the ultrapure water at room temperature was 18.2 MΩ/cm (25 °C), which met the national laboratory water requirement (GB6682-1992). Filter membranes (0.22 µm) were provided by Anpu Technology Co., Ltd. (Shanghai, China). Oasis HLB cartridges (60 mg/3 mL) and Oasis MCX cartridges (60 mg/3 mL) were supplied by Waters Corporation (Milford, MA, USA), and HyperSep SCX cartridges (60 mg/3 mL) were supplied by Thermo Fisher Corporation (Waltham, MA, USA).
2.3. Solution Preparation
Preparation of standard reserve liquid: Appropriate amounts of LMS, MBZ, HMBZ, and AMBZ were weighed into brown volumetric flasks, dissolved in 1 mL of methanol, and adjusted to 10 mL with methanol. The standard stock solutions were prepared at a concentration of 1.00 mg/mL and were stable for 5 months at −70 °C.
Preparation of standard working fluid: Standard working solutions were prepared by diluting the stock solutions of LMS, MBZ, HMBZ, and AMBZ with an acetonitrile/0.1% formic acid aqueous solution (10:90, v/v) to prepare standard working solutions of different. The standard working solutions (100 μg/mL, 10 μg/mL, and 1 μg/mL) were stable for 1 month at −20 °C. The working solutions were prepared daily and were stored at 4 °C.
MS tuning solution preparation: Standard working solutions (5 µg/mL) of LMS, MBZ, HMBZ, and AMBZ were diluted stepwise with 50% methanol aqueous solution containing 0.1% formic acid to prepare a 50 ng/mL mass spectral tuning solution.
Mobile phase preparation: A total of 1 mL of formic acid solution was diluted with 999 mL of ultrapure water to prepare a 0.1% formic acid aqueous solution, which was placed into a spiral flask as a mobile phase.
2.4. HPLC–MS/MS Conditions
HPLC analysis was carried out on a Waters Alliance e2695 system (Waters Corp., Milford, MA, USA) equipped with an AB SCIEX Triple Quad™ 5500 triple quadrupole mass spectrometer (AB SCIEX Corp., Framingham, MA, USA) and an automatic sampler (AB SCIEX Corp., Massachusetts, USA) and controlled by Analyst software (AB SCIEX Corp., Framingham, MA, USA). A Waters Xbridge MC18 column (150 mm × 4.6 mm; i.d. 5 μm) was used for HPLC; the mobile phase was (A) acetonitrile and (B) 0.1 vol% formic acid solution in water; gradient elution mode was used; the flow rate was 0.6 mL/min; and the column temperature was 35 °C. The elution procedure is detailed in Table 1.

Table 1.
Gradient elution program.
The four prepared spectral tuning solutions (50 ng/mL) were injected by a mass spectrometer needle pump in constant flow mode. The mass spectrometer was operated in ESI+ scanning and MRM modes in positive mode to optimize the ion source parameters by monitoring the MS/MS spectra of the analytes. The fog collision gas (CAD) was high-purity nitrogen; to simultaneously monitor the corresponding protonated molecular ions for all analytes, a spray voltage of 5500 eV, a vaporizer, an ion transport tube temperature of 550 °C, a sheath gas (high-purity nitrogen) pressure of 50 psi, an auxiliary gas (high-purity nitrogen) pressure of 50 psi, a collision gas impact chamber outlet voltage pressure (CXP) of 12 V, and an intake voltage (EP) of 10 V were used. The retention time and optimized MS parameters of LMS, MBZ, HMBZ, and AMBZ are shown in Table 2.

Table 2.
HPLC–MS/MS conditions and retention times for LMS, MBZ, HMBZ, and AMBZ analyses.
2.5. Sample Preparation
In this experiment, LLE and SPE were used to extract and purify egg samples, respectively. After extraction and purification, the samples were subjected to nitrogen blowing, concentration, centrifugation, and redissolution.
LLE extraction method: Poultry egg samples (2.0 ± 0.02 g) were accurately weighed and homogenized in a 50 mL centrifuge tube. Then, 0.5 mL of 50% KOH solution and 8 mL of acetonitrile:water (90:10, v/v) were added. Vortex stirring was performed for 5 min and ultrasonic shock extraction was performed for 5 min. Then, the sample was centrifuged at 8000× g for 8 min, the supernatant was transferred into a new 50 mL polypropylene centrifuge tube and 5 mL of 0.1 mol/L hydrochloric acid solution was added for mixed extraction. To remove fat, 5 mL of n-hexane was added and the mixture was centrifuged at 6000× g for 5 min to separate the n-hexane layer. The LLE process was carried out according to national food safety standards (GB 29685-2013) [24].
SPE purification method: After each sample was processed, it was purified with an Oasis®MCX × 3 cc (60 mg) SPE cartridge. A total of 3 mL of methanol and 3 mL of 0.1 mol/L hydrochloric acid solution was used to reach equilibrium, and the sample solution was added. The mixture was passed through the filter cartridge, which was then rinsed with 3 mL of water and 3 mL of methanol in sequence, followed by depressurization and drying for 3 min on a pump at 2.0 kPa. Finally, 3 mL of 5% ammoniated methanol was used to elute the target compound.
The eluent was concentrated by nitrogen blowing, added to 2 mL of the initial mobile phase for redissolution, vortexed and shaken for 2 min, and filtered through an organic-phase syringe filter (13 mm × 0.45 μm) for analysis. The sample pretreatment process is shown in Figure 1.
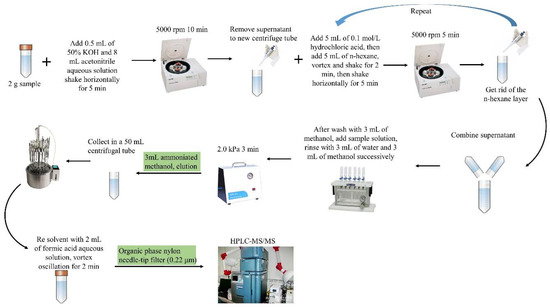
Figure 1.
Schematic diagram of sample pretreatment.
2.6. Method Validation
The linearity of this method was evaluated by establishing calibration curves, which were generated by spiking uncontaminated poultry eggs (whole egg, egg white, and egg yolk) with the target compounds. In this study, the standard working curve was established quantitatively by the external standard method, and the peak areas of LMS, MBZ, HMBZ, and AMBZ were plotted against the concentrations. The correlation coefficients (R2) were determined, and the values were all ≥ 0.999. HPLC–MS/MS method verification was carried out according to the guidelines of European Commission Resolution 2002/657/EC [25]. These guidelines mainly include the assessment of specificity, linearity, accuracy, precision, LOD, and LOQ.
2.6.1. Specificity
The specificity of the sample lies in detection and analysis with HPLC–MS/MS using a blank sample without any target compound. To evaluate the specificity of the detection method, HPLC-ESI/MS/MS was used to analyze blank hen, duck, and goose egg samples (whole egg, albumen, and yolk) after extraction, and 10 replicates were used for each sample. In addition, the pretreatment method and chromatographic analysis conditions were simultaneously optimized, and the specificity of the method was evaluated according to the peak patterns of the analytes [25].
2.6.2. Linearity
The linearity of this method was evaluated by establishing calibration curves, which were generated by spiking uncontaminated poultry eggs (whole egg, egg white, and egg yolk) with the target compounds. Seven standard concentrations of each of the four analytes were used to establish linear regression equations. The poultry egg (whole egg, albumen, and yolk) sample concentration depended on the response parameters of each analyte. The concentrations of LMS were the LOQ and 1, 5, 10, 15, 20 and 25 μg/kg, and the concentrations of MBZ, HMBZ, and AMBZ were the LOQ and 6, 30, 60, 90, 120 and 150 μg/kg. The linear standard curves for the seven target drugs in different sample matrixes were drawn using the spiked concentration as the independent variable (x) and the detected peak area as the dependent variable (y).
2.6.3. Accuracy and Precision
The accuracy and precision of the HPLC–MS/MS method were based on recovery and repeatability. The intraday precision and interday precision of six replicate samples were determined, and for quality control, the spiked concentrations of each sample were set to the LOQ, 0.5 times the maximum residue limit (MRL), 1.0 MRL, and 2.0 MRL to evaluate sample recovery and precision. At different times on the same day, the same instrument and the same standard curve were used to analyze the four concentrations (LOQ, 0.5 MRL, 1.0 MRL, and 2.0 MRL) of spiked samples to calculate the daily RSD. On different days of the week, the daily RSD was determined from the four spiked concentrations.
LMS, MBZ, HMBZ, and AMBZ in the matrix were analyzed by HPLC–MS/MS with six replicates, and the recovery rate was determined at four concentrations. Finally, according to the detected concentration in the calibration curve matching the blank matrix, the peak recovery rate was calculated. The stability of the standard curve, instrument performance, and environmental conditions were evaluated.
2.6.4. LOD and LOQ
Blank matrix extracts of hen, duck, and goose egg samples (whole egg, albumen, and yolk) were prepared, and LMS, MBZ, HMBZ, and AMBZ standard working solutions were added to these blank matrix extracts for HPLC–MS/MS detection. The average S/N was calculated six times for the standard solution at each concentration point. The LOD was defined as the concentration at which the target signal in the blank matrix was three times S/N (S/N ≥ 3). The LOQ refers to the lowest concentration of the target analyte in a sample that can be quantitatively detected and was defined as the concentration at which the target signal in the blank matrix was ten times S/N (S/N ≥ 10). Additionally, the method LOQ was confirmed by a recovery above 70% and accuracy less than 20% [25,26,27].
3. Results and Discussion
3.1. Determination of Precursor Ions and Product Ions
The target compounds in this study showed a high abundance of [M + H]+ parent ions in ESI+ mode. A product ion scan was used to obtain the daughter ion information of each target compound. The two ions with the highest abundance and mutual interference in each group were selected as the analyte monitoring ion pairs of daughter ions. The detection ion pairs of each analyte are shown in Table 3, and the secondary mass spectra of the four analytes are shown in Figure 2.

Table 3.
HPLC–MS/MS conditions and retention times for LMS, MBZ, HMBZ, and AMBZ analysis.
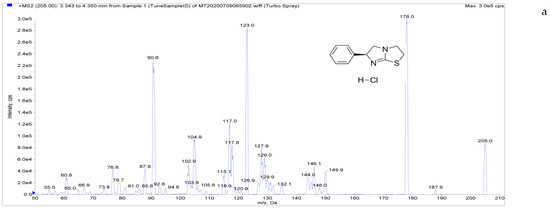
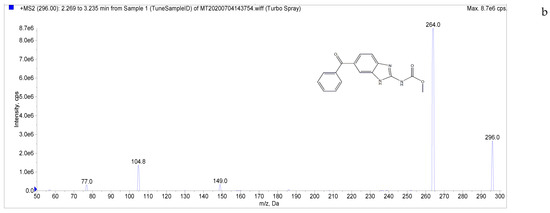
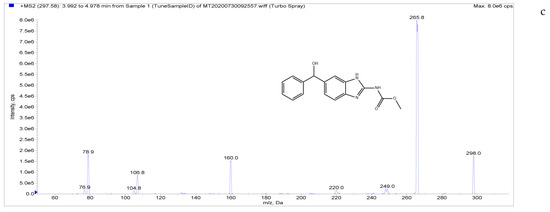
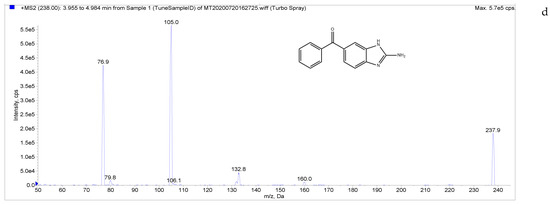
Figure 2.
Chemical structures and MS/MS spectra. ((a–d) correspond to LMS, MBZ, HMBZ, and AMBZ, respectively).
3.2. Selection of Solvents
Poultry eggs are rich in protein, so the organic solvent selected for use in pretreatment typically involves acetonitrile or ethyl acetate. Experimental comparison of the effects of these two organic solvents demonstrated that when using ethyl acetate as the extraction agent, the poultry egg matrix easily emulsified during reverse extraction, so acetonitrile was chosen as the extraction agent. It has been reported that adding water to the extraction solvent improves the recovery rate and peak shape [28,29]. Therefore, different proportions of acetonitrile and water were added, and the peak shapes of the four analytes were evaluated; no tailing was found upon the addition of 10% water. Therefore, acetonitrile:water (90:10, v/v) solution was used in this study. LMS, MBZ, and the two metabolites of MBZ were extracted from eggs, and 0.5 mL of a 50% KOH solution was added as the extraction agent.
3.3. Optimization of the Sample Pretreatment Method
This study used LLE combined with SPE (LLE–SPE) to extract LMS and MBZ and its two metabolites from the samples. Studies have found that LMS, MBZ, HMBZ, and AMBZ tend to be protonated under alkaline conditions [30], which can promote their extraction from tissues. For all analytes, when the extraction solution was alkaline, ionization was inhibited, solubility in the aqueous phase was low, and the solubility in weakly polar organic solvents was high.
SPE is a rapid sample preparation and purification technology used for sample purification, recovery, and enrichment, and it is used for precise quantitative analysis. Compared with the traditional LLE method, SPE improves the recovery of the analyte, separates the analyte and interference elements more efficiently, and reduces the time required for sample pretreatment; furthermore, the operation is simple and saves time and effort. The recoveries realized with different SPE columns were compared according to the method of Sakamoto [20]. Methods for evaluation of chromatographic column and sample processing conditions are described in detail in Section 2.5 Sample Preparation, and the results are shown in Figure 3. The MCX column gave the best recovery rate because its mixed-mode filter cartridge is hydrophobic; the retention performance of cationic exchange after purification leads to perfect separation of drug and impurity peaks. Additionally, the height of the impurity peak decreased substantially, and the peak shape improved. Therefore, MCX was used as the SPE column in this study. Moreover, ammoniated acetonitrile and ammoniated methanol were compared as eluents, and it was found that ammoniated methanol eluted the drugs better and provided higher recovery rates. When the concentration of ammonia in methanol was 5%, the extraction effect was the best, and the peak area obtained was the largest. Therefore, 5% ammoniated methanol was used as the eluent in this experiment.
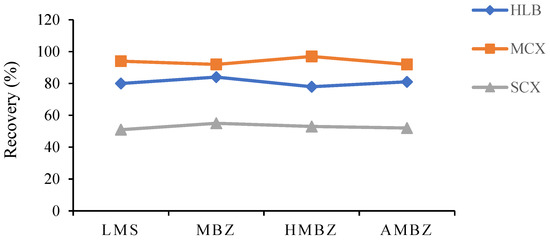
Figure 3.
Comparison of the recovery rates of HLB, MCX, and SCX with different SPE cartridges.
3.4. Optimization of Chromatographic Conditions
When developing an HPLC–MS phase, the chromatographic column and elution method are the key to the correct separation of drugs. The chromatographic column and mobile solvent should be selected according to the sample components and the properties of the compound, solubility, and sample matrix. Methanol and acetonitrile are the most commonly used organic solvents in the mobile phase. It has been reported that gradient elution by LC can shorten the analysis time of multiple drugs, improve efficiency, and increase peak capacity [31]. In this experiment, when 0.1% formic acid water–acetonitrile was used as the mobile phase for gradient elution, it was found that gradient elution was more conducive than isocratic elution to the separation of the drug peak, and the pressure increase in the chromatographic column during isocratic elution was not conducive to long-term use of the chromatographic column.
In chromatographic analyses, the selection of chromatographic columns, mobile phases and gradient elution procedures are key to the optimization of analytical methods. The C18 column is most widely used for the detection of LMS, MBZ, HMBZ, and AMBZ in food of animal origin. Therefore, an XbridgeTM C18 column (4.6 × 150 mm, 5 μm) showed good separation for LMS, MBZ, HMBZ, and AMBZ.
The mobile phase should improve the ionization efficiency and provide appropriate retention times for the analytes to obtain good resolution and high sensitivity. In this experiment, 0.1% formic acid aqueous solution–methanol, 10 mM ammonium formic acid aqueous solution–methanol, 0.1% formic acid aqueous solution–acetonitrile, and 10 mM ammonium formic acid aqueous solution–acetonitrile were used as mobile phases for comparison. When 10 mM ammonium formate was added to the mobile phase, the baseline noise was large and fluctuated greatly, while when 0.1% formate aqueous solution was added, the baseline noise was stable. With methanol as the organic phase, the peaks formed by the methanol and water phases bifurcated and trailed. When acetonitrile was substituted for methanol, the signal-to-noise ratio (S/N) was higher, and the peak shape was improved. Therefore, 0.1% formic acid water and acetonitrile were used as mobile phases. For gradient elution, comparisons between different proportions of the organic phase (acetonitrile) in terms of analyte peak times, recoveries, etc. showed that with proportions under 40%, LMS, MBZ, HMBZ, and AMBZ exhibited retention times less than 20 min. When the ratio of acetonitrile (organic phase) was approximately 50%, the retention times of the four analytes were less than 10 min. Therefore, the final ratio of acetonitrile to 0.1% formic acid in water during gradient elution was 48:52.
3.5. Methodology Validation
3.5.1. Specificity
The specificity of the established method was evaluated by testing different blank poultry egg samples and samples with different drug standard concentrations. The total ion chromatogram and extracted ion chromatogram of a blank hen whole egg sample are shown in Figure 4. Quantitative total ion chromatograms and extraction ion chromatograms after the addition of LMS, MBZ, HMBZ, and AMBZ to blank eggs are shown in Figure 4. The specificity of the analytical method for blank eggs was determined by comparing Figure 4 and Figure 5. Figure 4 shows that LMS, MBZ, HMBZ, and AMBZ were not contained in the blank chicken whole egg samples. This shows that the method has good specificity.
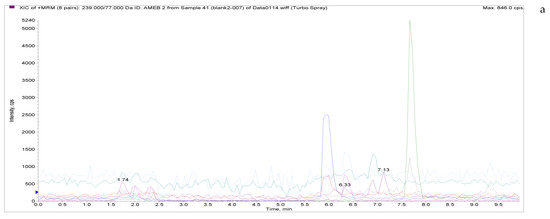
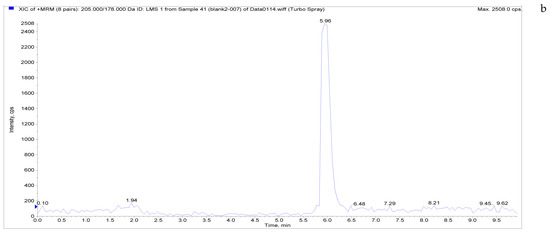
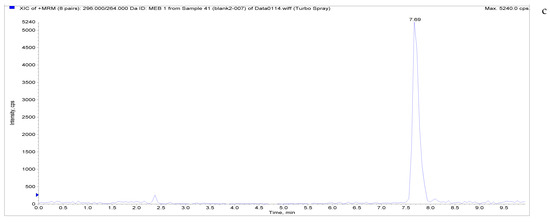
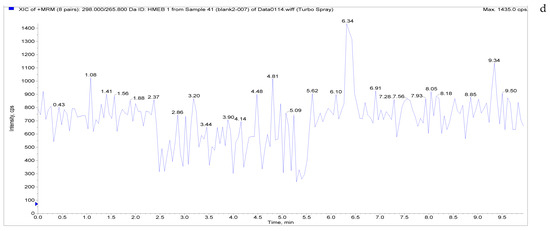
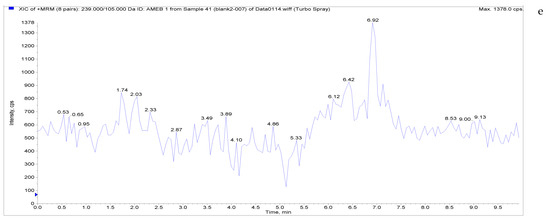
Figure 4.
Total ion chromatograms and extracted ion chromatograms of blank hen whole egg samples. ((a) Total ion chromatogram; (b) extracted ion chromatogram of LMS; (c) extracted ion chromatogram of MBZ; (d) extracted ion chromatogram of HMBZ; (e) extracted ion chromatogram of AMBZ).
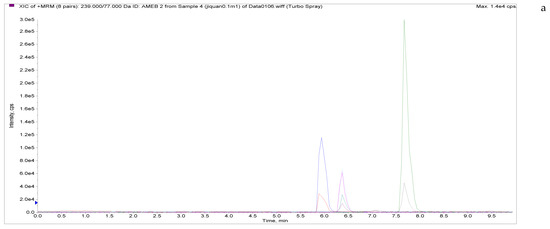
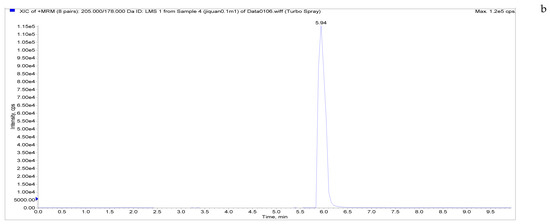
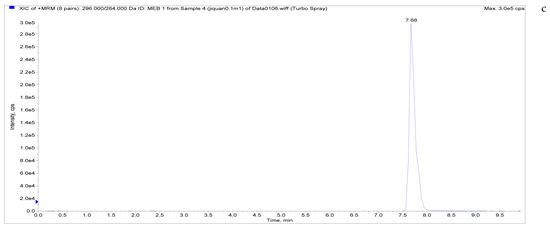
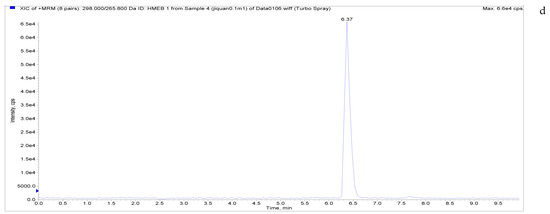
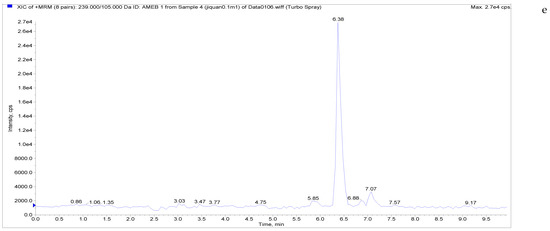
Figure 5.
Total ion chromatograms and extracted ion chromatograms of blank whole egg samples with standard addition. ((a) Total ion chromatogram; (b) extracted ion chromatogram of a sample spiked with 1 µg/kg LMS; (c) extracted ion chromatogram of a sample spiked with 6 µg/kg MBZ; (d) extracted ion chromatogram of a sample spiked with 6 µg/kg HMBZ; (e) extracted ion chromatogram of a sample spiked with 6 µg/kg AMBZ.).
3.5.2. Linearity
The matrix effect (ME) involves enhancement or suppression of the response of the analyte in the sample by components other than the analyte, thus affecting accuracy [32]. To reduce the matrix influence in sample analyses, we optimized the SPE pretreatment method and adopted the blank matrix addition method to establish the matrix matching calibration curve, which fundamentally reduced the influence of the matrix on the accuracy of experimental results. In addition, SPE sample pretreatment purifies and enriches the target compounds in poultry egg samples. The concentration detection ranges of standard analytes in blank poultry egg samples were as follows: LMS: 0.10–25 μg/kg; MBZ: 0.09–150 μg/kg; HMBZ: 0.50–150 μg/kg; and AMBZ: 0.80–150 μg/kg. Peak areas as a function of analyte concentration were used to construct standard working curves. The correlation coefficients (R2 values) were determined, and all were ≥0.9990. The determination coefficients and linear ranges of each of the linear regression equations for each poultry egg sample are shown in Table 4.

Table 4.
Linear regression equations, determination coefficients, and linear ranges for LMS, MBZ, HMBZ, and AMBZ in poultry eggs.
3.5.3. Accuracy and Precision
In this study, four concentrations of each target substance were spiked to the blank samples and then analyzed by HPLC–MS/MS system after the sample pretreatment described in Section 2.5. The recovery is the ratio of the measured concentration to the spiked concentration. The recovery and precision date for the four analytes are shown in Table 5, Table 6 and Table 7. The average recoveries were 86.04–96.64%, 85.98–96.55%, 88.44–96.48%, and 87.89–97.38%; the relative standard deviations (RSDs) were 2.00–4.58%; and the intraday RSDs were 1.40–5.85%. The daily RSD was 2.34–6.32%, and the higher the recovery rate was, the more reliable the test method.

Table 5.
Recovery and precision of LMS, MBZ, HMBZ, and AMBZ added to blank hen eggs (n = 6).

Table 6.
Recovery and precision of LMS, MBZ, HMBZ, and AMBZ added to blank duck eggs (n = 6).

Table 7.
Recovery and precision of LMS, MBZ, HMBZ, and AMBZ added to blank goose eggs (n = 6).
The precision can be used to show the error in the overall test process, and it is divided into the intraday (intrabatch) RSD and interday (interbatch) RSD to determine the accuracy of the method. The smaller the daily RSD is, the more reliable the test method. The above results showed that the method was stable and reliable and fully met the requirements for analysis of LMS, MBZ, HMBZ, and AMBZ residues in poultry eggs.
3.5.4. LOD and LOQ
The LOD and LOQ are very important for the accurate qualitative and quantitative analysis of analytes. Moreover, high sensitivity can be defined by low LODs and LOQs [33]. The LODs and LOQs of LMS, MBZ, HMBZ, and AMBZ in the hen, duck, and goose egg (whole egg, albumen, and yolk) samples are shown in Table 8. The LODs of LMS, MBZ, HMBZ, and AMBZ in poultry eggs were 0.03–0.07 μg/kg, 0.03–0.05 μg/kg, 0.15–0.23 μg/kg, and 0.25–0.33 μg/kg, respectively. The LOQs were 0.10–0.22 μg/kg, 0.08–0.20 μg/kg, 0.50–0.80 μg/kg, and 0.80–1.00 μg/kg.

Table 8.
LODs and LOQs for LMS, MBZ, HMBZ, and AMBZ in poultry eggs.
3.6. Comparison of Different Detection Methods
Currently, there are many methods to detect LMS, MBZ, and the two metabolites of MBZ in foods of animal origin. This study compared sample pretreatment methods and detection methods. A comparison of these data with those from other methods is shown in Table 9. HPLC–MS [29], HPLC–UVD [18], HPLC–DAD [20], MLC–MS [21], LC–MS/MS [34], HPLC–MS/MS [30,35], UHPLC–MS/MS [36], and other methods have been used to detect LMS, MBZ, and the two metabolites of MBZ in meat, poultry eggs, milk, honey, animal tissues, and animal plasma. However, simultaneous detection of these two drugs has only been reported for aquatic products [23]. The results of these studies show that with application of different sample preparation and detection methods, the recovery rates range from 76–118%, and the sensitivity obtained UV and fluorescence detection was low and resulted in high LODs and LOQs. When MS was used, the sensitivity and accuracy of the results are higher, and the LODs and LOQs were lower.

Table 9.
Comparison of the present method with other reported methods.
Zhu et al. established a new method based on a combination of SPE and LC–MS/MS for the simultaneous detection of various veterinary drug residues in milk. The recovery rates were 75.50–104.5%, the LODs were 0.2–2.0 μg/kg, and the LOQs were 0.5–10.0 μg/kg [37]. Kang et al. established an HPLC–UV detection method for screening MBZ, losoluron, diviridin, and toluidine residues in animal foods with a run time of 20 min; the recoveries of MBZ residue in pork, beef, milk, chicken, and eggs were 82.3–104.8%, the LODs were 1–14 μg/kg, and the LOQs were 4–41 μg/kg [18].
This study detected LMS, MBZ, HMBZ, and AMBZ by HPLC–MS/MS with a gradient elution program. The run time was 10 min, a good linear relationship was observed from the standard curve (R2 ≥ 0.9993), and the recovery rates were 85.98–97.38%. The daily RSDs were 2.34–6.32%, the LODs were 0.03–0.33 µg/kg, and the LOQs were 0.08–1.00 µg/kg. Notably, the LODs and LOQs of the samples were low. Therefore, this experiment established a method that is fast and accurate with high recovery rates and sensitivity.
3.7. Real Sample Analysis
To evaluate the feasibility and accuracy of this newly established method, 120 commercial eggs (40 hen, 40 goose, and 40 duck) were collected from a local market and analyzed. Each poultry egg sample was treated and labeled according to the above sample pretreatment method, and then the analyte contents were detected and analyzed by HPLC–MS/MS. The target compounds were not detected in duck or goose eggs, LMS residue was detected in only one hen egg sample (4.2 μg/kg), and AMBZ residue was detected in only one hen egg sample (26.5 μg/kg). Therefore, the established HPLC–MS/MS method can be used for the quantitative analysis of LMS, MBZ, HMBZ, and AMBZ in poultry egg samples.
4. Conclusions
In this study, a fast, accurate, and low-cost HPLC/MS–MS method was established and optimized for the simultaneous determination of LMS, MBZ, HMBZ, and AMBZ residues in poultry eggs. The sample pretreatment method and chromatographic conditions were optimized, the interference effects were reduced, and the extraction efficiency and recovery of analytes were improved. The methodological parameters meet the requirements of China, the United States, and the European Commission for the detection of veterinary drug residues in food of animal origin. This study provides technical support for the simultaneous detection of LMS, MBZ, HMBZ, and AMBZ residues in animal tissues, making market regulation, quality control, and antibiotic contamination assessment possible.
Author Contributions
Conceptualization, K.X.; data management, L.C., P.Z., Y.G. and Y.T.; investigation, Z.H., J.C. and Y.L.; writing—original draft, L.C.; writing—review and editing, L.C. and K.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the China Agriculture Research System of MOF and MARA (CARS-41), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Yangzhou University High-End Talent Support Program.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cotterill, O.J.; Glauert, J.; Froning, G.W. Nutrient Composition of Commercially Spray-Dried Egg Products. Poult. Sci. 1978, 57, 439–442. [Google Scholar] [CrossRef]
- Swendseid, M.E.; Fe Eley, R.J.; Harris, C.L.; Tuttle, S.G. Egg protein as a source of the essential amino acids: Requirement for nitrogen balance in young adults studied at two levels of nitrogen intake. J. Nutr. 1959, 68, 203. [Google Scholar] [CrossRef]
- Feyera, T.; Ruhnke, I.; Sharpe, B.; Elliott, T.; Walkden-Brown, S.W. Comparative therapeutic efficacies of oral and in-water administered levamisole, piperazine and fenbendazole against experimental Ascaridia galli infection in chickens. Vet. Parasitol. 2021, 298, 109514. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Jung, B.-K.; Hong, S.-J. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: An Update. Korean J. Parasitol. 2021, 59, 189–225. [Google Scholar] [CrossRef]
- Friedman, P.A.; Platzer, E.G. Interaction of anthelmintic benzimidazoles with Ascaris suum embryonic tubulin. BBA Gen. Subj. 1980, 630, 271–278. [Google Scholar] [CrossRef]
- Albonico, M.; Bickle, Q.; Ramsan, M.; Montresor, A.; Savioli, L.; Taylor, M. Efficacy of Mebendazole and Levamisole Alone or in Combination against Intestinal Nematode Infections after Repeated Targeted Mebendazole Treatment in Zanzibar. (Research). Bull. World Health Organ. 2003, 81, 343–352. [Google Scholar] [CrossRef]
- Bennet, E.M.; Behm, C.; Bryant, C. Effects of mebendazole and levamisole on tetrathyridia of Mesocestoides corti in the mouse. Int. J. Parasitol. 1978, 8, 463–466. [Google Scholar] [CrossRef]
- Heath, D.D.; Christie, M.J.; Chevis, R. The lethal effect of mebendazole on secondary Echinococcus granulosus, cysticerci of Taenia pisiformis and tetrathyridia of Mesocestoides corti. Parasitology 1975, 70, 273–285. [Google Scholar] [CrossRef]
- Xu, L.; Luan, F.; Wang, L.; Liu, H.; Gao, Y. Development of a Capillary Zone Electrophoresis Method for Determination of Mebendazole and Levamisole Hydrochloride in a Combined Tablet and a Comparison with a LC Method. J. AOAC Int. 2014, 97, 128–132. [Google Scholar] [CrossRef]
- Ejlertsen, B.; Mouridsen, H.T.; Jensen, M.-B.; Andersen, J.; Andersson, M.; Kamby, C.; Knoop, A.S.; Danish Breast Cancer Cooperative Group. Cyclophosphamide, methotrexate, and fluorouracil; oral cyclophosphamide; levamisole; or no adjuvant therapy for patients with high-risk, premenopausal breast cancer. Cancer 2010, 116, 2081–2089. [Google Scholar] [CrossRef]
- Commission, E. Commission Decision (EU) No 37/2010 of December 2009 on Pharmacologically Active Substances and their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. 2010. Available online: https://eur-lex.europa.eu/eli/reg/2010/37(1)/oj (accessed on 15 February 2022).
- FDA. CFR-Code of Federal Regulations Title 21. 2019. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=556&showFR=1 (accessed on 15 June 2020).
- Korea Food & Drug Administration. Notice No. 2012-59 of the Korea Food & Drug Administration. 2012. Available online: https://www.mfds.go.kr/eng/brd/m_60/view.do?seq=67277 (accessed on 15 June 2020).
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2017, 14, 1701782. [Google Scholar] [CrossRef]
- Jinxin, H.; Yan, Z.; Xiaorong, C.; Na, L.; Fang, T.; Nairui, H.; Jianzhong, W.; Shaopeng, G. Development of an enzyme-linked immunosorbent assay for the detection of mebendazole in chicken and mutton. Anal. Methods Adv. Methods Appl. 2021, 13, 1740–1746. [Google Scholar] [CrossRef]
- Woestenborghs, R.; Michielsen, L.; Heykants, J. Determination of levamisole in plasma and animal tissues by gas chromatography with thermionic specific detection. J. Chromatogr. 1981, 224, 25–32. [Google Scholar] [CrossRef]
- Provatas, A.A.; Yeudakimau, A.V.; Stuart, J.D.; Perkins, C.R. Rapid QuEChERS Approach Using Novel Solid Phase Extraction for Insecticides in Lobster and Shellfish Tissue with Gas Chromatography–Tandem Mass Spectrometry. Anal. Lett. 2014, 47, 2461–2474. [Google Scholar] [CrossRef]
- Kang, Y.P.; Yu, J.; Huh, Y.; Oh, J.H.; Kwon, C.H.; Lee, S.J.; Ee, J.W.; Kim, G.T.; Lee, J.G.; Lee, J.; et al. Development of high performance liquid chromatography-ultraviolet detection method for screening mebendazole, clorsulon, diaveridine, and tolfenamic acid in animal-based food samples. Drug Test. Anal. 2014, 6, 246–256. [Google Scholar] [CrossRef]
- Canton, C.; Ceballos, L.; Domínguez, M.P.; Moreno, L.; Fiel, C.; Bernat, G.; Farías, C.; Lanusse, C.; Alvarez, L. Pharmaco-parasitological evaluation of the ricobendazole plus levamisole nematodicidal combination in cattle. J. Vet. Pharmacol. Ther. 2018, 41, 83–91. [Google Scholar] [CrossRef]
- Sakamoto, M.; Take Ba, K.; Fujinuma, K.; Jimbo, K.; Miyazaki, T. Determination of levamisole in livestock products using high performance liquid chromatography. Shokuhinseigaku Zasshi J. Food Hyg. Soc. Jpn. 2002, 43, 6–9. [Google Scholar] [CrossRef][Green Version]
- Pawar, R.P.; Mishra, P.; Durgbanshi, A.; Bose, D.; Albiol-Chiva, J.; Peris-Vicente, J.; García-Ferrer, D.; Esteve-Romero, J. Use of Micellar Liquid Chromatography to Determine Mebendazole in Dairy Products and Breeding Waste from Bovine Animals. Antibiotics 2020, 9, 86. [Google Scholar] [CrossRef]
- Kinsella, B.; Whelan, M.; Cantwell, H.; Mccormack, M.; Furey, A.; Lehotay, S.J.; Danaher, M. A dual validation approach to detect anthelmintic residues in bovine liver over an extended concentration range. Talanta 2011, 83, 14–24. [Google Scholar] [CrossRef]
- Xu, N.; Dong, J.; Yang, Y.; Liu, Y.; Yang, Q.; Ai, X. Development of a liquid chromatography-tandem mass spectrometry method with modified QuEChERS extraction for the quantification of mebendazole and its metabolites, albendazole and its metabolites, and levamisole in edible tissues of aquatic animals. Food Chem. 2018, 269, 442–449. [Google Scholar] [CrossRef]
- GB 29685-2013; National Food Safety Standard: Determination of Lincomycin, Clindamycin and Spectinomycin in Animal Derived Food by Gas Chroma-tography-Mass Spectrometry Method. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2013. Available online: http://down.foodmate.net/standard/yulan.php?itemid=38182 (accessed on 15 February 2022).
- The European Communities. Commission decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, 221, 8–36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002D0657&from=ES (accessed on 15 June 2020).
- Barreca, S.; Busetto, M.; Vitelli, M.; Colzani, L.; Clerici, L.; Dellavedova, P. Online Solid-Phase Extraction LC-MS/MS: A Rapid and Valid Method for the Determination of Perfluorinated Compounds at Sub ng·L 1 Level in Natural Water. J. Chem. 2018, 2018, 3780825. [Google Scholar] [CrossRef]
- Barreca, S.; Orecchio, S.; Pace, A. Photochemical sample treatment: A greener approach to chlorobenzene determination in sediments. Talanta 2014, 129, 263–269. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Vidal, J.; Frenich, A.G. Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life 2012, 895–896, 39–47. [Google Scholar] [CrossRef]
- Yoo, K.H.; Park, D.H.; El-Aty, A.; Kim, S.K.; Jung, H.N.; Jeong, D.H.; Cho, H.J.; Hacimuftuoglu, A.; Shim, J.H.; Ji, H.J. Development of an analytical method for multi-residue quantification of 18 anthelmintics in various animal-based food products using liquid chromatography-tandem mass spectrometry. J. Pharm. Anal. 2021, 11, 68–76. [Google Scholar] [CrossRef]
- Wei, H.; Tao, Y.; Chen, D.; Xie, S.; Pan, Y.; Liu, Z.; Huang, L.; Yuan, Z. Development and validation of a multi-residue screening method for veterinary drugs, their metabolites and pesticides in meat using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 686–701. [Google Scholar] [CrossRef]
- Shea; Jennifer, L. Bioanalytical methods for quantitation of levamisole, a widespread cocaine adulterant. Clin. Chem. Lab. Med. 2013, 51, 205–212. [Google Scholar] [CrossRef]
- Baeza-Baeza, J.J.; García-Alvarez-Coque, M. Peak dispersion in gradient elution: An insight based on the plate model. J. Chromatogr. A 2019, 1613, 460670. [Google Scholar] [CrossRef]
- Soliman, M.; Khorshid, M.A.; Abo-Aly, M.M. Combination of Analyte Protectants and Sandwich Injection to Compensate for Matrix Effect of Pesticides Residue in GC-MS/MS. Microchem. J. 2020, 156, 104852. [Google Scholar] [CrossRef]
- Krupčík, J.; Májek, P.; Gorovenko, R.; Blaško, J.; Kubinec, R.; Sandra, P. Considerations on the determination of the limit of detection and the limit of quantification in one-dimensional and comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2015, 1396, 117–130. [Google Scholar] [CrossRef]
- Zhu, W.X.; Yang, J.Z.; Wang, Z.X.; Wang, C.J.; Liu, Y.F.; Zhang, L. Rapid determination of 88 veterinary drug residues in milk using automated TurborFlow online clean-up mode coupled to liquid chromatography-tandem mass spectrometry. Talanta 2016, 148, 401–411. [Google Scholar] [CrossRef]
- Kolanovic, B.S.; Bilandzic, N.; Kos, B.; Suskovic, J.; Cvetnic, L.; Varenina, I.; Luburic, D.B.; Varga, I.; Pavlicek, D.; Lugomer, M.D. Distribution and elimination of levamisole in eggs and tissues after oral administration to laying hens, determined by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 729–739. [Google Scholar] [CrossRef]
- Whelan, M.; Kinsella, B.; Furey, A.; Moloney, M.; Cantwell, H.; Lehotay, S.J.; Danaher, M. Determination of anthelmintic drug residues in milk using ultra high performance liquid chromatography–tandem mass spectrometry with rapid polarity switching. J. Chromatogr. A 2010, 1217, 4612–4622. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).