Identification of Soluble Degradation Products in Lithium–Sulfur and Lithium-Metal Sulfide Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Model Aging Experiments
2.3. LiS Cells
2.4. Cell Opening Procedure
2.5. Analytical Measurements
3. Results and Discussion
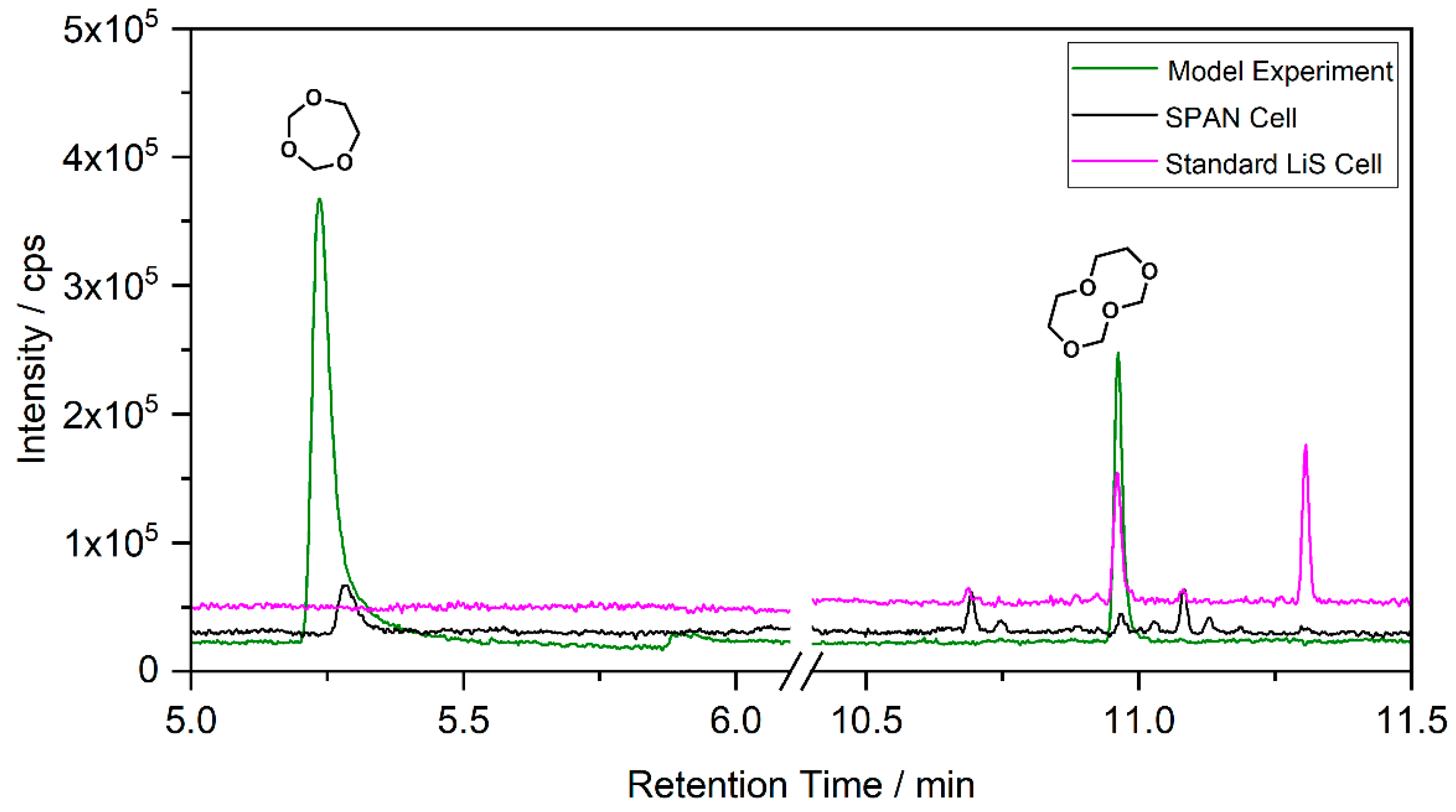
3.1. Ether-Based Systems
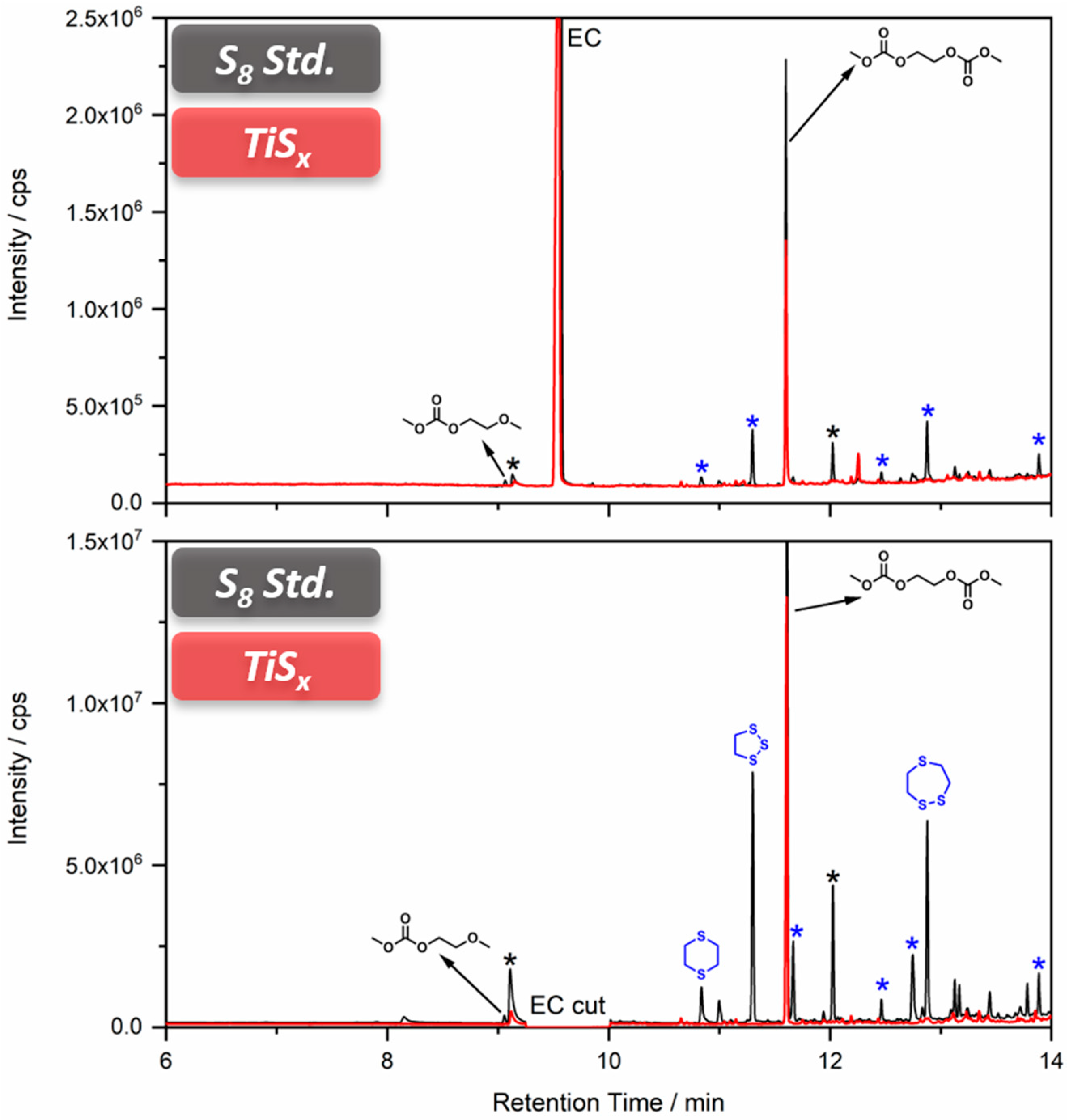
3.2. Carbonate-Based Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Nara, H.; Yokoshima, T.; Mikuriya, H.; Tsuda, S.; Momma, T.; Osaka, T. The Potential for the Creation of a High Areal Capacity Lithium-Sulfur Battery Using a Metal Foam Current Collector. J. Electrochem. Soc. 2017, 164, A5026–A5030. [Google Scholar] [CrossRef] [Green Version]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Kovalev, I.; Schock, R.; Kumaresan, K.; Xu, J.; Affinito, J. High Energy Rechargeable Li-S Cells for EV Application: Status, Remaining Problems and Solutions. ECS Trans. 2010, 25, 23–34. [Google Scholar] [CrossRef]
- Dörfler, S.; Althues, H.; Härtel, P.; Abendroth, T.; Schumm, B.; Kaskel, S. Challenges and Key Parameters of Lithium-Sulfur Batteries on Pouch Cell Level. Joule 2020, 4, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Dühnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Gachot, G.; Ribière, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.-B.; Pilard, S.; Laruelle, S. Gas Chromatography/Mass Spectrometry As a Suitable Tool for the Li-Ion Battery Electrolyte Degradation Mechanisms Study. Anal. Chem. 2011, 83, 478–485. [Google Scholar] [CrossRef]
- Henschel, J.; Peschel, C.; Klein, S.; Horsthemke, F.; Winter, M.; Nowak, S. Clarification of Decomposition Pathways in a State-of-the-Art Lithium Ion Battery Electrolyte through C-13-Labeling of Electrolyte Components. Angew. Chem. Int. Edit. 2020, 59, 6128–6137. [Google Scholar] [CrossRef] [Green Version]
- Barchasz, C.; Molton, F.; Duboc, C.; Leprêtre, J.-C.; Patoux, S.; Alloin, F. Lithium/Sulfur Cell Discharge Mechanism: An Original Approach for Intermediate Species Identification. Anal. Chem. 2012, 84, 3973–3980. [Google Scholar] [CrossRef]
- Diao, Y.; Xie, K.; Xiong, S.Z.; Hong, X.B. Analysis of Polysulfide Dissolved in Electrolyte in Discharge-Charge Process of Li-S Battery. J. Electrochem. Soc. 2012, 159, A421–A425. [Google Scholar] [CrossRef]
- Hagen, M.; Schiffels, P.; Hammer, M.; Dorfler, S.; Tubke, J.; Hoffmann, M.J.; Althues, H.; Kaskel, S. In-Situ Raman Investigation of Polysulfide Formation in Li-S Cells. J. Electrochem. Soc. 2013, 160, A1205–A1214. [Google Scholar] [CrossRef]
- Patel, M.U.M.; Demir-Cakan, R.; Morcrette, M.; Tarascon, J.M.; Gaberscek, M.; Dominko, R. Li-S Battery Analyzed by UV/Vis in Operando Mode. Chemsuschem 2013, 6, 1177–1181. [Google Scholar] [CrossRef]
- Schneider, H.; Weiß, T.; Scordilis-Kelley, C.; Maeyer, J.; Leitner, K.; Peng, H.-J.; Schmidt, R.; Tomforde, J. Electrolyte decomposition and gas evolution in a lithium-sulfur cell upon long-term cycling. Electrochim. Acta 2017, 243, 26–32. [Google Scholar] [CrossRef]
- Kraft, V.; Grutzke, M.; Weber, W.; Winter, M.; Nowak, S. Ion chromatography electrospray ionization mass spectrometry method development and investigation of lithium hexafluorophosphate-based organic electrolytes and their thermal decomposition products. J. Chrom. A 2014, 1354, 92–100. [Google Scholar] [CrossRef]
- Horsthemke, F.; Friesen, A.; Mönnighoff, X.; Stenzel, Y.P.; Grützke, M.; Andersson, J.T.; Winter, M.; Nowak, S. Fast screening method to characterize lithium ion battery electrolytes by means of solid phase microextraction—Gas chromatography—Mass spectrometry. RSC Adv. 2017, 7, 46989–46998. [Google Scholar] [CrossRef] [Green Version]
- Gachot, G.; Grugeon, S.; Eshetu, G.G.; Mathiron, D.; Ribière, P.; Armand, M.; Laruelle, S. Thermal behaviour of the lithiated-graphite/electrolyte interface through GC/MS analysis. Electrochim. Acta 2012, 83, 402–409. [Google Scholar] [CrossRef]
- Hippauf, F.; Nickel, W.; Hao, G.-P.; Schwedtmann, K.; Giebeler, L.; Oswald, S.; Borchardt, L.; Doerfler, S.; Weigand, J.J.; Kaskel, S. The Importance of Pore Size and Surface Polarity for Polysulfide Adsorption in Lithium Sulfur Batteries. Adv. Mater. Interfaces 2016, 3, 1600508. [Google Scholar] [CrossRef]
- Li, S.; Fan, Z. Encapsulation methods of sulfur particles for lithium-sulfur batteries: A review. Energy Storage Mater. 2021, 34, 107–127. [Google Scholar] [CrossRef]
- Li, S.; Leng, D.; Li, W.; Qie, L.; Dong, Z.; Cheng, Z.; Fan, Z. Recent progress in developing Li2S cathodes for Li–S batteries. Energy Storage Mater. 2020, 27, 279–296. [Google Scholar] [CrossRef]
- Sakuda, A.; Takeuchi, T.; Okamura, K.; Kobayashi, H.; Sakaebe, H.; Tatsumi, K.; Ogumi, Z. Rock-salt-type lithium metal sulphides as novel positive-electrode materials. Sci. Rep. 2014, 4, 4883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senoh, H.; Kageyama, H.; Takeuchi, T.; Nakanishi, K.; Ohta, T.; Sakaebe, H.; Yao, M.; Sakai, T.; Yasuda, K. Gallium (III) sulfide as an active material in lithium secondary batteries. J. Power Sources 2011, 196, 5631–5636. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Giebeler, L. Nanosized Li2S-based cathodes derived from MoS2 for high-energy density Li–S cells and Si–Li2S full cells in carbonate-based electrolyte. Energy Storage Mater. 2017, 8, 209–216. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Z.; Elia, G.A.; Wu, Y.; Wahyudi, W.; Abou-Hamad, E.; Emwas, A.-H.; Cavallo, L.; Li, L.-J.; Ming, J. Recognizing the Mechanism of Sulfurized Polyacrylonitrile Cathode Materials for Li–S Batteries and beyond in Al–S Batteries. ACS Energy Lett. 2018, 3, 2899–2907. [Google Scholar] [CrossRef]
- Becking, J.; Gröbmeyer, A.; Kolek, M.; Rodehorst, U.; Schulze, S.; Winter, M.; Bieker, P.; Stan, M.C. Lithium-Metal Foil Surface Modification: An Effective Method to Improve the Cycling Performance of Lithium-Metal Batteries. Adv. Mater. Interfaces 2017, 4, 1700166. [Google Scholar] [CrossRef]
- Weller, C.; Pampel, J.; Dörfler, S.; Althues, H.; Kaskel, S. Polysulfide Shuttle Suppression by Electrolytes with Low-Density for High-Energy Lithium–Sulfur Batteries. Energy Technol. 2019, 7, 1900625. [Google Scholar] [CrossRef]
- Peschel, C.; Horsthemke, F.; Winter, M.; Nowak, S. Implementation of Orbitrap Mass Spectrometry for Improved GC-MS Target Analysis in Lithium Ion Battery Electrolytes. MethodsX 2022, 9, 101621. [Google Scholar] [CrossRef]

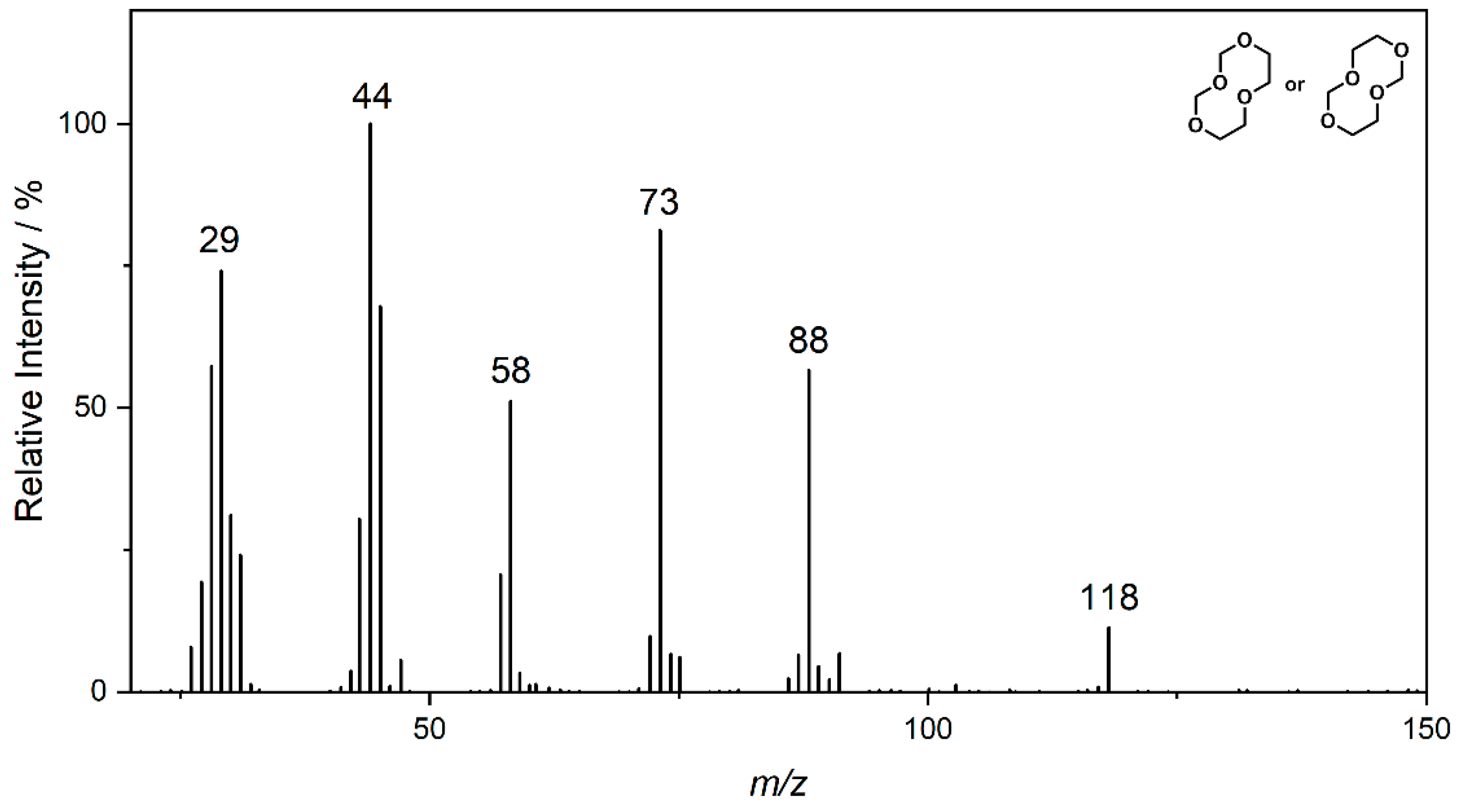

| Compound | 1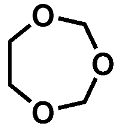 | 2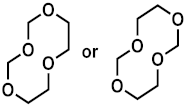 | 3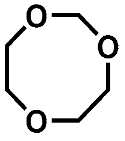 |
|---|---|---|---|
| Ret. Time | 5.25 min | 10.95 min | 7.60 min |
| PCI | 105 [M + H]+ 122 [M + NH4]+ | 149 [M + H]+ 166 [M + NH4]+ | 119 [M + H]+ 136 [M + NH4]+ |
| NCI | 73; 61 | 117 | 87 |
| HRMS | 105.0557 C4H9O3 (5.0 ppm) | 149.0814 C6H13O4 (0.1 ppm) | 119.0718 C5H11O3 (8.2 ppm) |
| (HR)MS2 | - | 75.0446 (C3H7O2) 45.0340 (C2H5O) | 87.0456 (C4H7O2) 71.0503 (C4H7O) 59.0497 (C3H7O) 45.0339 (C2H5O) |
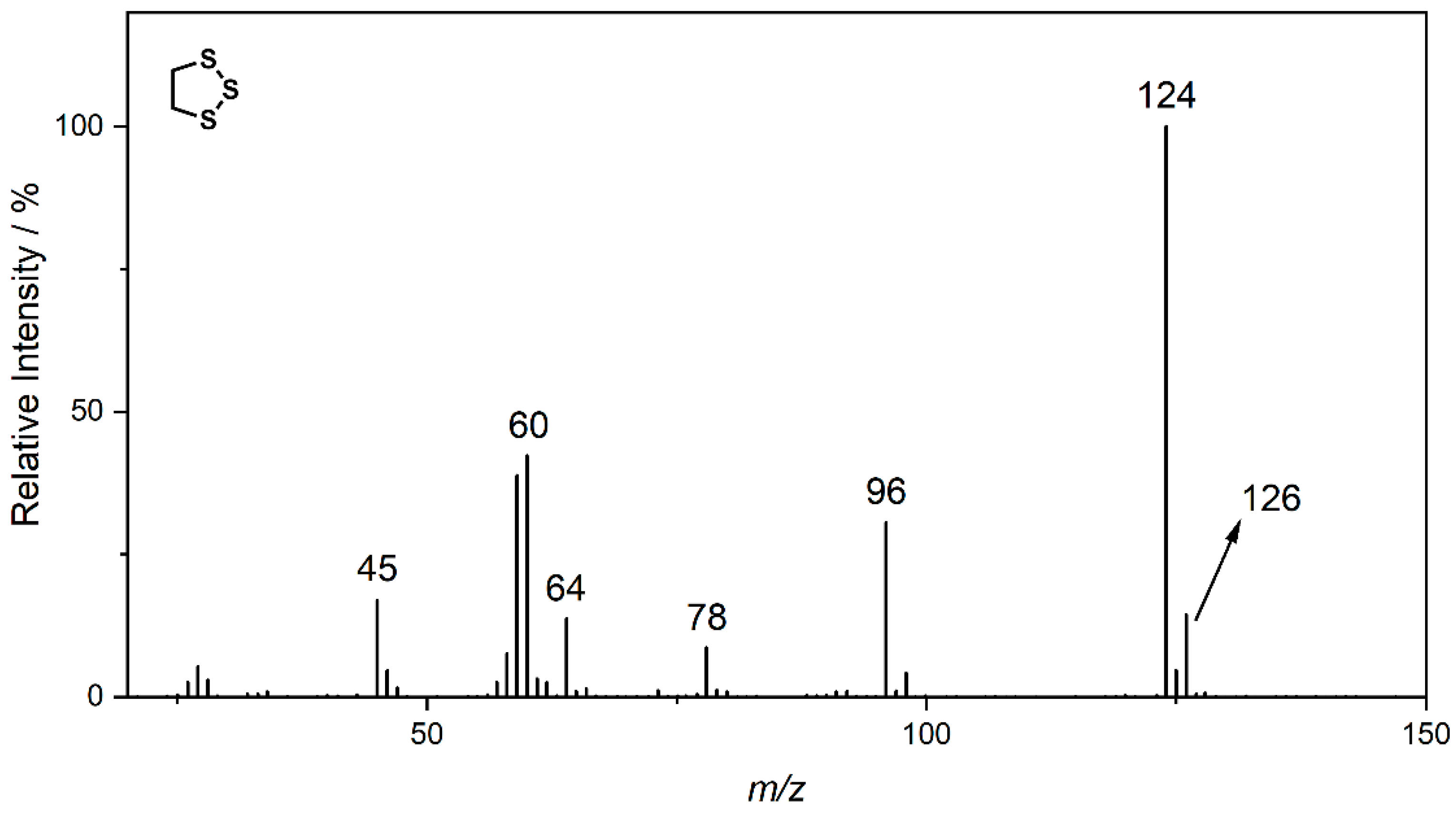
| Compound | A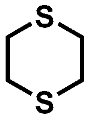 | B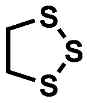 | C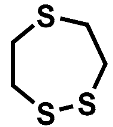 |
|---|---|---|---|
| Ret. Time | 10.84 min | 11.30 min | 12.88 min |
| MS | 120; 60; 61 | 124; 96; 64; 60; 59 | 152; 124; 87; 64; 60; 59 |
| NCI | below limit of detection | 64 | 92 |
| HRMS | 120.0062 C4H8S2 (4.5 ppm) | 123.9470 C2H4S3 (4.1 ppm) | 151.9783 C4H8S3 (3.4 ppm) |
| (HR)MS2 | 61.0106 (C2H5S) 60.0027 (C2H4S) | 95.9157 (S3) 63.9436 (S2) 58.9949 (C2H3S) | 123.9470 (C2H4S3) 87.0263 (C4H7S) 63.9436 (S2) 60.0028 (C2H4S) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horsthemke, F.; Peschel, C.; Kösters, K.; Nowak, S.; Kuratani, K.; Takeuchi, T.; Mikuriya, H.; Schmidt, F.; Sakaebe, H.; Kaskel, S.; et al. Identification of Soluble Degradation Products in Lithium–Sulfur and Lithium-Metal Sulfide Batteries. Separations 2022, 9, 57. https://doi.org/10.3390/separations9030057
Horsthemke F, Peschel C, Kösters K, Nowak S, Kuratani K, Takeuchi T, Mikuriya H, Schmidt F, Sakaebe H, Kaskel S, et al. Identification of Soluble Degradation Products in Lithium–Sulfur and Lithium-Metal Sulfide Batteries. Separations. 2022; 9(3):57. https://doi.org/10.3390/separations9030057
Chicago/Turabian StyleHorsthemke, Fabian, Christoph Peschel, Kristina Kösters, Sascha Nowak, Kentaro Kuratani, Tomonari Takeuchi, Hitoshi Mikuriya, Florian Schmidt, Hikari Sakaebe, Stefan Kaskel, and et al. 2022. "Identification of Soluble Degradation Products in Lithium–Sulfur and Lithium-Metal Sulfide Batteries" Separations 9, no. 3: 57. https://doi.org/10.3390/separations9030057
APA StyleHorsthemke, F., Peschel, C., Kösters, K., Nowak, S., Kuratani, K., Takeuchi, T., Mikuriya, H., Schmidt, F., Sakaebe, H., Kaskel, S., Osaka, T., Winter, M., Nara, H., & Wiemers-Meyer, S. (2022). Identification of Soluble Degradation Products in Lithium–Sulfur and Lithium-Metal Sulfide Batteries. Separations, 9(3), 57. https://doi.org/10.3390/separations9030057







