Post-Functionalization of Bromo-Substituted Ether-Linked Polymers via Ullman Coupling Reaction: Synthesis, Characterization and Their Role toward Carbon Dioxide Capture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Physical Properties
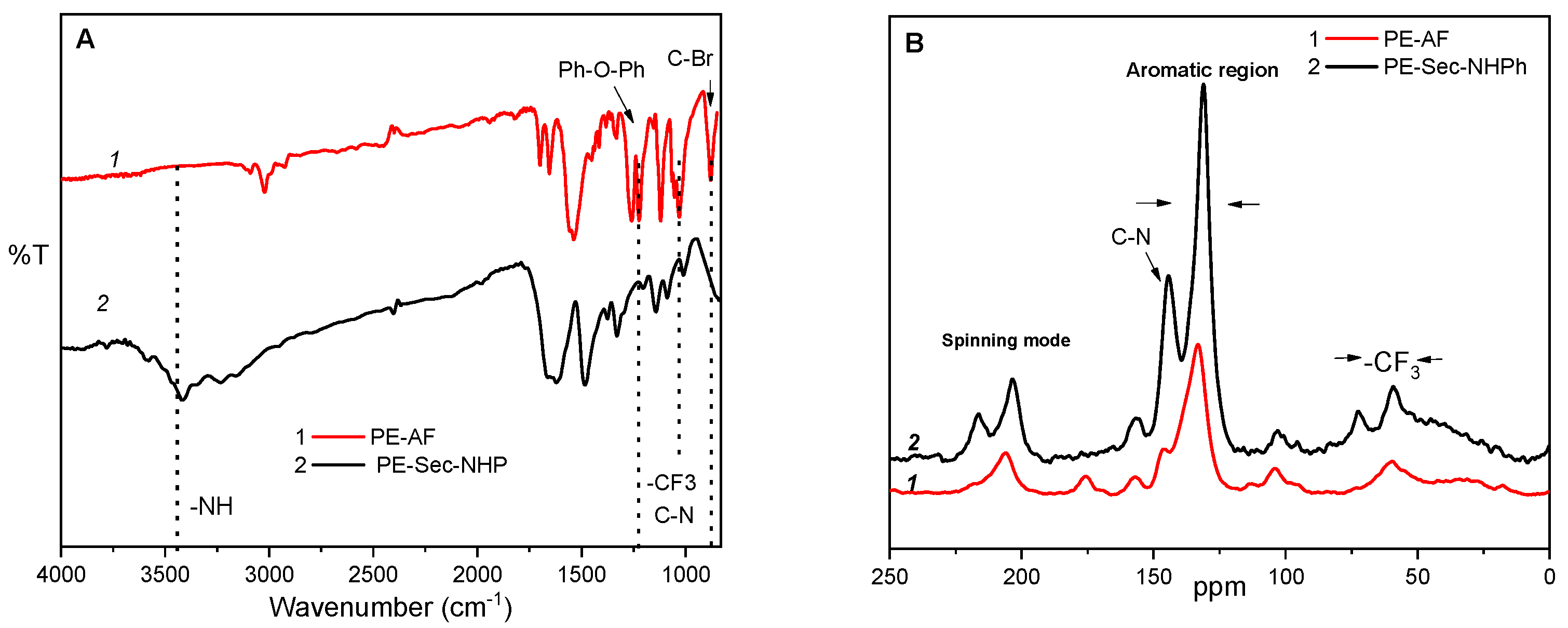
2.2. Infrared Spectroscopy (FTIR), 1H-NMR, 13C-NMR and Solid State 13C-C-MAS
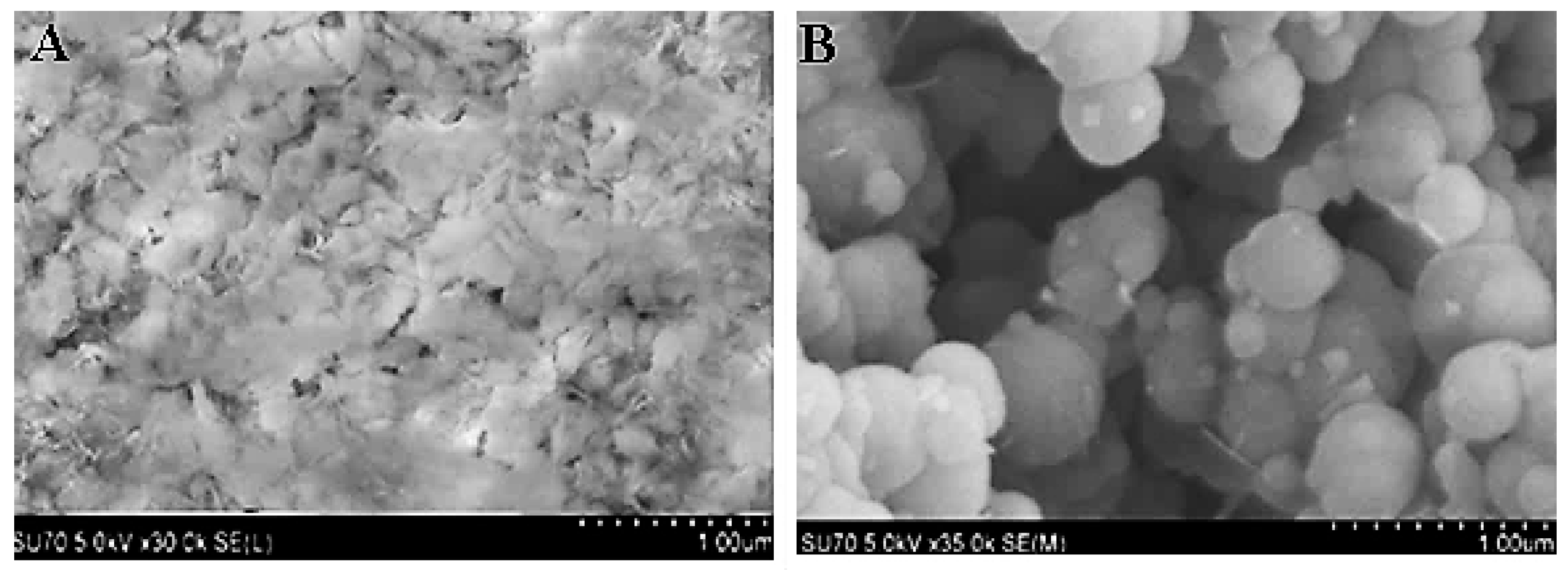
2.3. Scanning Electron Microscopy
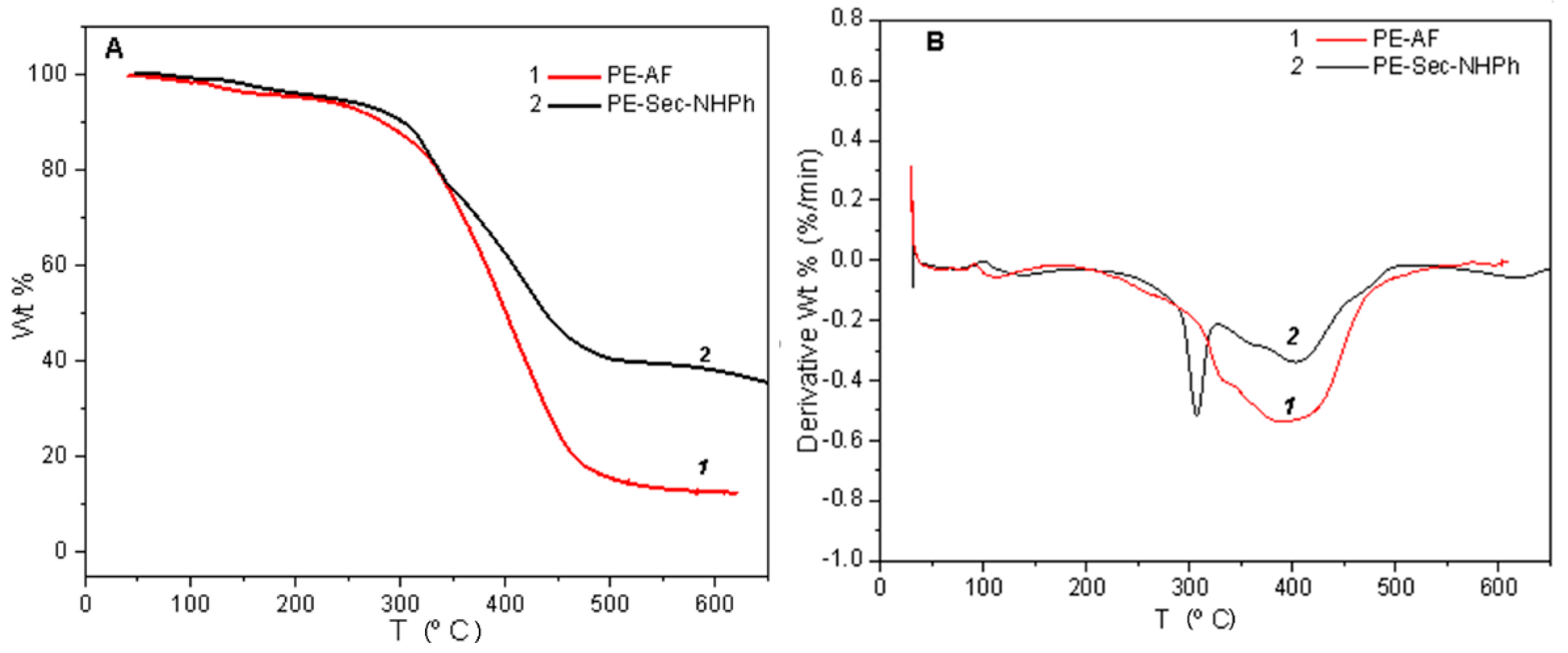
2.4. Thermal Stability
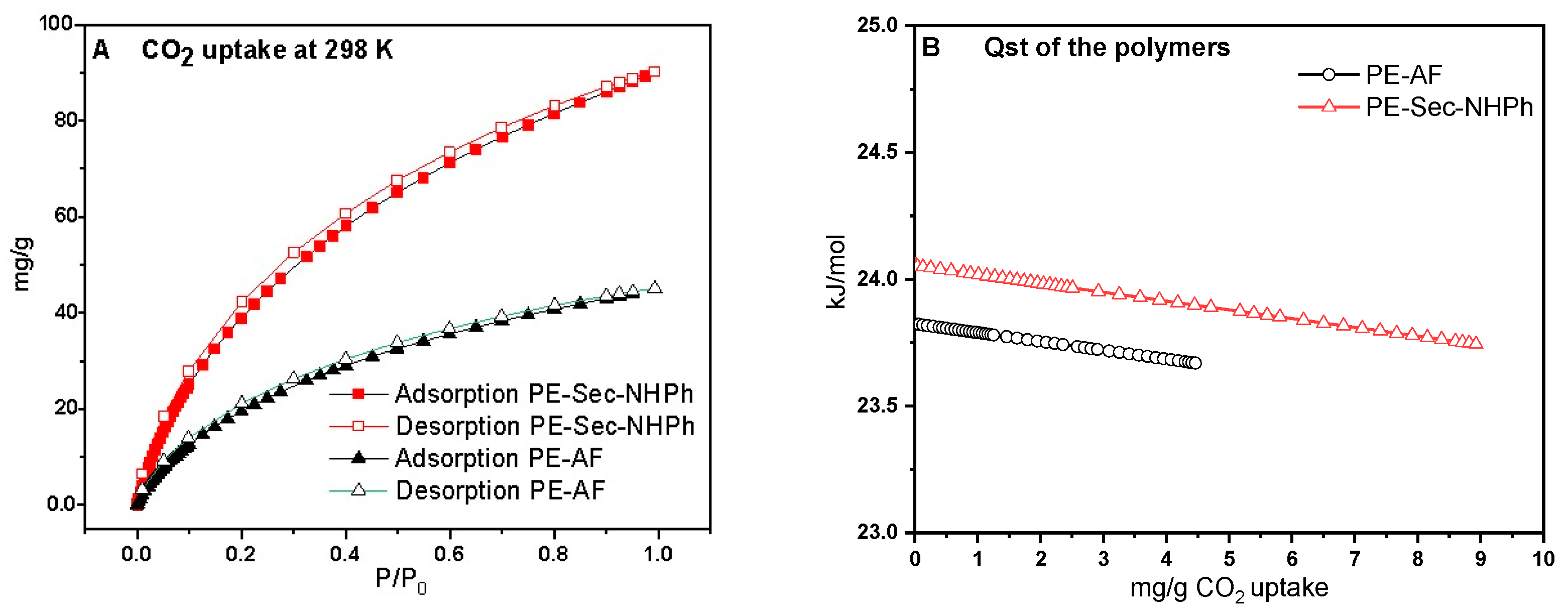
2.5. Carbon Dioxide (CO2) Adsorption
| Polymer | CO2 at 1 Bar, 298 k (mg/g) | Binding Affinity (kJ/mol) | Reference |
|---|---|---|---|
| PE-Sec-NHPh | 90.0 | 24.2 | This work |
| PE-AF | 41.0 | 23.7 | This work |
| BILP-polymers | 80.7 | 32.0 | [37] |
| BOLP-polymer | 79.0 | 32.9 | [38] |
| BTLP-polymer | 87.0 | 29.1 | [38] |
| TPIm-polymer | 75.0 | 24.0 | [34] |
3. Materials and Methods
3.1. Chemicals and Characterization Methods
3.2. Synthesis of Bromo-Substituted Ether-Based Polymer (PE-AF)
3.3. Synthesis of Aniline-Substituted Ether-Based Polymer (PE-Sec-NHPh)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bricaud, Q.; Cravino, A.; Leriche, P.; Roncali, J. Terthiophene-cyanovinylene π-conjugated polymers as donor material for organic solar cells. Synthmet 2009, 159, 2534–2538. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Sum, Y.; Ying, J.; Zhang, Y. A mesoporous poly-melamine-formaldehyde polymer as a solid sorbent for toxic metal removal. Energy Environ. Sci. 2013, 6, 3254–3259. [Google Scholar] [CrossRef]
- Jung, J.; Jo, J.; Chueh, C.; Liu, F.; Jo, W.; Russell, T.; Jen, A. Fluoro-Substituted n-Type Conjugated Polymers for Additive-Free All-Polymer Bulk Heterojunction Solar Cells with High Power Conversion Efficiency of 6.71%. Adv. Mater. 2015, 27, 3310–3317. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, J.; Kou, Y.; Pang, H.; Zhang, S.; Li, N.; Liu, C.; Weng, Z.; Jian, X. Enabling low voltage losses and high photocurrent in fullerene-free organic photovoltaics. Nat. Commun. 2019, 10, 570. [Google Scholar]
- Liu, B.; Hu, W.; Chen, C.; Jiang, Z.; Zhang, W.; Wu, Z.; Matsumoto, T. Soluble aromatic poly(ether ketone)s with a pendant 3,5-ditrifluoromethylphenyl group. Polymer 2004, 45, 3241–3247. [Google Scholar] [CrossRef]
- Chang, G.-J.; Xu, Z.; Lin, R.-X.; Luo, X. Synthesis and Properties of Novel Poly(amine ether)s. Chin. J. Chem. 2007, 25, 1207–1211. [Google Scholar] [CrossRef]
- Hemvichian, K.; Ishida, H. Thermal decomposition processes in aromatic amine-based polybenzoxazines investigated by TGA and GC–MS. Polymer 2002, 43, 4391–4402. [Google Scholar] [CrossRef]
- Vasanthi, B.J.; Ravikumar, L. Synthesis and Characterization of Poly(azomethine ester)s with a Pendent Dimethoxy Benzylidene Group. Open. J. Pol. Chem. 2013, 3, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Shieh, S.; LeGoff, E.; Kanatzidis, M.G. Synthesis and Characterization of a New Conjugated Aromatic Poly(azomethine) Derivative Based on the 3′, 4′-Dibutyl-α-Terthiophene Building Block. Macromolecules 1996, 29, 3147–3156. [Google Scholar] [CrossRef]
- Mihai, I.; Ivanoiu, M.; Stafie, L.V.; Grigoras, M. Suzuki polycondensation: A simple way for synthesis of new polyazines containing thiophene and fluorene moieties. e-Polymers 2011, 31, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.; Gong, C.; Zhang, S.; Shao, Y.; Li, Y. Synthesis of a new pyridine-containing diamine and related polyimide. Chin. Chem. Lett. 2010, 21, 277–278. [Google Scholar] [CrossRef]
- Rai, S.; Bajpai, A.; Lokhandwal, S. Condensation Polymers of Terephthalic Acid and 1,4-Diaminobutane and Their Schiff Base Complexes. J. Polym. 2013, 2013, 278576. [Google Scholar] [CrossRef]
- Tabish, A.; Varghese, A.M.; Wahab, M.A.; Karanikolos, G.N. Perovskites in the energy grid and CO2 conversion: Current context and future directions. Catalysts 2020, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Varghese, A.; Mittal, V. Performance of Various Adsorbents Towards Diverse Gases: A Comparative Study, Functional Nanomaterials and Nanotechnologies: Applications for Energy & Environment; Central West Publishing: Orange, NSW, Australia, 2018; pp. 305–404. [Google Scholar]
- Varghese, A.M.; Reddy, K.S.K.; Singh, S.; Karanikolos, G.N. Performance enhancement of CO2 capture adsorbents by UV treatment: The case of self-supported graphene oxide foam. Chem. Eng. J. 2020, 386, 124022. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Maroto-Valer, M.M. Development of adsorbents for CO2 capture from waste materials: A review. Greenh. Gases Sci. Technol. 2012, 2, 20–35. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Dzubak, A.L.; Lin, L.C.; Kim, J.; Swisher, J.A.; Poloni, R.; Maximoff, S.N.; Smit, B.; Gagliardi, L. Ab initio carbon capture in open-site metal–organic frameworks. Nat. Chem. 2012, 4, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Favvas, E.P.; Heliopoulos, N.S.; Karousos, D.S.; Devlin, E.; Papageorgiou, S.K.; Petridis, D.; Karanikolos, G.N. Mixed matrix polymeric and carbon hollow fiber membranes with magnetic iron-based nanoparticles and their application in gas mixture separation. Mat. Chem. Phys. 2019, 223, 220–229. [Google Scholar] [CrossRef]
- Iqbal, N.; Wang, X.; Yu, J.; Jabeen, N.; Ullah, H.; Ding, B. In situ synthesis of carbon nanotube doped metal–organic frameworks for CO2 capture. RSC Adv. 2016, 6, 4382–4386. [Google Scholar] [CrossRef]
- Labropoulos, A.; Veziri, C.; Kapsi, M.; Pilatos, G.; Likodimos, V.; Tsapatsis, M.; Kanellopoulos, N.K.; Romanos, G.E.; Karanikolos, G.N. Carbon nanotube selective membranes with subnanometer, vertically aligned pores, and enhanced gas transport properties. Chem. Mater. 2015, 27, 8198–8210. [Google Scholar] [CrossRef]
- Perdikaki, A.V.; Labropoulos, A.I.; Siranidi, E.; Karatasios, I.; Kanellopoulos, N.; Boukos, N.; Falaras, P.; Karanikolos, G.N.; Romanos, G.E. Efficient CO oxidation in an ionic liquid-modified, Au nanoparticle-loaded membrane contactor. Chem. Eng. J. 2016, 305, 79–91. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. Identifying cost-effective CO2 control levels for amine-based CO2 capture systems. Ind. Eng. Chem. Res. 2006, 45, 2421–2429. [Google Scholar] [CrossRef]
- Tzialla, O.; Veziri, C.; Papatryfon, X.; Beltsios, K.; Labropoulos, A.; Iliev, B.; Adamova, G.; Schubert, T.; Kroon, M.; Francisco, M. Zeolite imidazolate framework-ionic liquid hybrid membranes for highly selective CO2 separation. J. Phys. Chem. C 2013, 117, 18434–18440. [Google Scholar] [CrossRef]
- Zainab, G.; Iqbal, N.; Babar, A.A.; Huang, C.; Wang, X.; Yu, J.; Ding, B. Freestanding, spider-web-like polyamide/carbon nanotube composite nanofibrous membrane impregnated with polyethyleneimine for CO2 capture. Compos. Commun. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Irvin, J.; Neef, C.; Kane, K.; Cassidy, P.; Tullos, G.; Clair, A.J. Polyethers derived from bisphenols and highly fluorinated aromatics. Polym. Sci. Part A Polym. Chem. 1992, 30, 1675–1679. [Google Scholar] [CrossRef]
- Tkachenko, I.; Kurioz, Y.; Kovalchuk, A.; Kobzar, Y.; Shekera, O.; Tereshchenko, O.; Nazarenko, V.; Shevchenko, V. Optical properties of azo-based poly(azomethine)s with aromatic fluorinated fragments, ether linkages and aliphatic units in the backbone. Mol. Cryst. Liq. Cryst. 2020, 697, 85–96. [Google Scholar] [CrossRef]
- Chen, S.; Ren, D.; Li, B.; Li, K.; Chen, L.; Xu, M.; Liu, X. Benzoxazine Containing Fluorinated Aromatic Ether Nitrile Linkage: Preparation, Curing Kinetics and Dielectric Properties. Polymers 2019, 11, 1036. [Google Scholar] [CrossRef] [Green Version]
- Puthiaraj, P.; Ahn, W. Ullmann coupling of aryl chlorides in water catalyzed by palladium nanoparticles supported on amine-grafted porous aromatic polymer. Mol. Catal. 2017, 437, 73–79. [Google Scholar] [CrossRef]
- Reis, L.; Ligiéro, C.; Andrade, A.; Taylor, J.; Miranda, P. Preparation of Polyaminopyridines Using a CuI/l-Proline-Catalyzed C-N Polycoupling Reaction. Materials 2012, 5, 2176–2189. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Cai, Q. L-Proline Promoted Ullmann-Type Coupling Reactions of Aryl Iodides with Indoles, Pyrroles, Imidazoles or Pyrazoles. Synletter 2004, 1, 128–130. [Google Scholar] [CrossRef]
- Quivelli, A.; Vitale, P.; Perna, F.; Capriati, V. Reshaping Ullmann Amine Synthesis in Deep Eutectic Solvents: A Mild Approach for Cu-Catalyzed C–N Coupling Reactions with No Additional Ligands. Front. Chem. 2019, 7, 723. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, S.; Ababneh, T.; Al-Momani, L.; Aljaafreh, I. New Microporous Thiophene-Pyridine Functionalized Imine-Linked Polymer for Carbon-Dioxide Capture. Polym. Sci. Ser. B 2018, 60, 789–797. [Google Scholar] [CrossRef]
- Alves, W.; Malmonge, J.; Mattoso, L.; De Medeiros, E. Non-isothermal decomposition kinetics of conductive polyaniline and its derivatives. Polimeros 2018, 28, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Gus’Kov, V.; Kudasheva, F.; Bogolyuk, G. Second virial coefficients of adsorption isotherms of organic molecules on porous polymers. Russ. J. Phys. Chem. A 2013, 87, 879–881. [Google Scholar] [CrossRef]
- Altarawneh, S.; İslamoğlu, T.; Sekizkardes, A.; El-Kaderi, H. Effect of Acid-Catalyzed Formation Rates of Benzimidazole-Linked Polymers on Porosity and Selective CO2 Capture from Gas Mixtures. Environ. Sci. Technol. 2015, 49, 4715–4723. [Google Scholar] [CrossRef]
- Rabbani, M.; Islamoglu, T.; El-Kaderi, H. Benzothiazole- and Benzoxazole-Linked Porous Polymers for Carbon Dioxide Storage and Separation. J. Mater. Chem. A 2017, 5, 258–264. [Google Scholar] [CrossRef]
- Varghese, A.; Karanikolos, G. CO2 capture adsorbents functionalized by amine–Bearing polymers: A. review. Int. J. Greenh. Gas Control 2020, 96, 103005. [Google Scholar] [CrossRef]

| Polymer | Stage | Ts (°C) | Wt (%) | T50% (°C) | T100% (°C) | CR% | LOI |
|---|---|---|---|---|---|---|---|
| PE-Sec-NHPh | S1: 286–326 | 300 | 10 | 450 | 470 | 40 | 33.5 |
| S2: 326–483 | 410 | 40 | |||||
| PE-AF | S1: 250–460 | 400 | 70 | 400 | 450 | 10 | 21.5 |
| Polymer */ Formula (Monomer)n | FW ǁ (g/mol) | Appearance | Yield (%) | Elemental Analyses Calculated (Found) (Wt%) | ||||
|---|---|---|---|---|---|---|---|---|
| (PE-AF) | 552.1 | White solid | 77.0 | C | H | Br | O | Fǂ |
| 45.68 (45.62) | 1.83 (2.00) | 28.95 (28.33) | 2.90 (2.78) | 20.65 (20.62) | ||||
| (PE-Sec-NHPh) | 576.54 | Pale brown solid | 85.0 | C | H | N | O | F |
| 68.75 (68.11) | 3.85 (3.55) | 4.86 (4.76) | 2.78 (2.67) | 19.77 20.91 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shboul, T.M.A.; Al-Tarawneh, S.S.; Ababneh, T.S.; Jazzazi, T.M.A. Post-Functionalization of Bromo-Substituted Ether-Linked Polymers via Ullman Coupling Reaction: Synthesis, Characterization and Their Role toward Carbon Dioxide Capture. Separations 2022, 9, 55. https://doi.org/10.3390/separations9030055
Al-Shboul TMA, Al-Tarawneh SS, Ababneh TS, Jazzazi TMA. Post-Functionalization of Bromo-Substituted Ether-Linked Polymers via Ullman Coupling Reaction: Synthesis, Characterization and Their Role toward Carbon Dioxide Capture. Separations. 2022; 9(3):55. https://doi.org/10.3390/separations9030055
Chicago/Turabian StyleAl-Shboul, Tareq M. A., Suha S. Al-Tarawneh, Taher S. Ababneh, and Taghreed M. A. Jazzazi. 2022. "Post-Functionalization of Bromo-Substituted Ether-Linked Polymers via Ullman Coupling Reaction: Synthesis, Characterization and Their Role toward Carbon Dioxide Capture" Separations 9, no. 3: 55. https://doi.org/10.3390/separations9030055
APA StyleAl-Shboul, T. M. A., Al-Tarawneh, S. S., Ababneh, T. S., & Jazzazi, T. M. A. (2022). Post-Functionalization of Bromo-Substituted Ether-Linked Polymers via Ullman Coupling Reaction: Synthesis, Characterization and Their Role toward Carbon Dioxide Capture. Separations, 9(3), 55. https://doi.org/10.3390/separations9030055







