In Situ XRD, Raman Characterization, and Kinetic Study of CO2 Capture by Alkali Carbonate-Doped Na4SiO4
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorbents
2.2. Characterization
2.3. CO2 Sorption
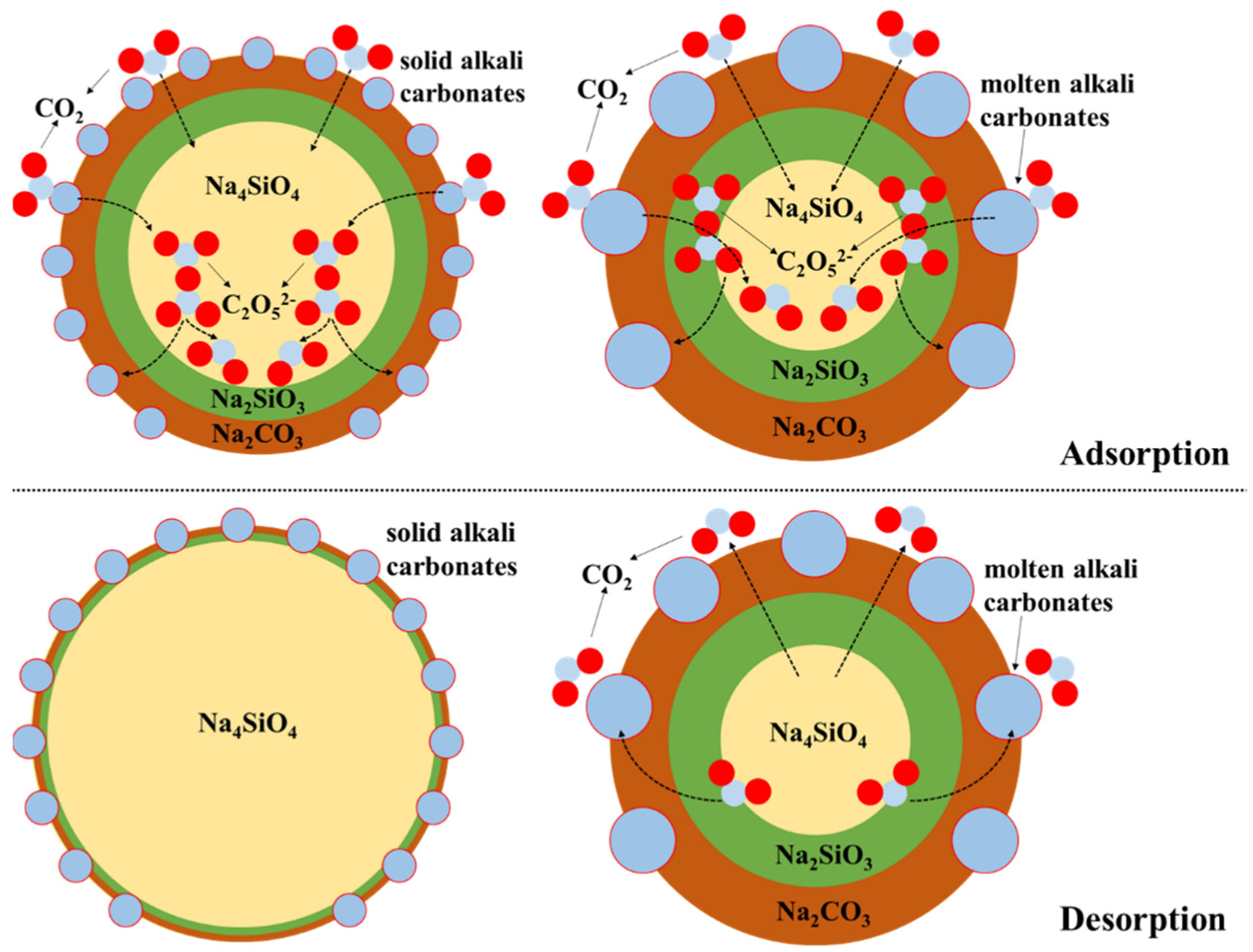
3. Results and Discussions
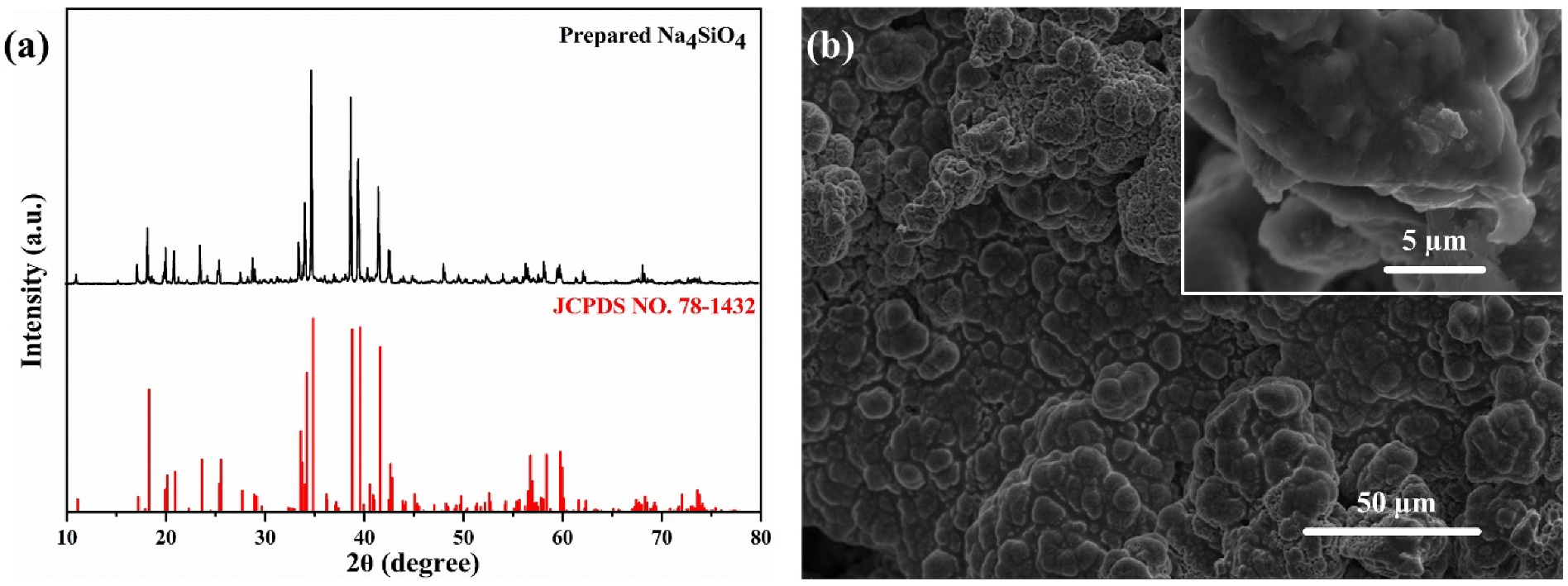
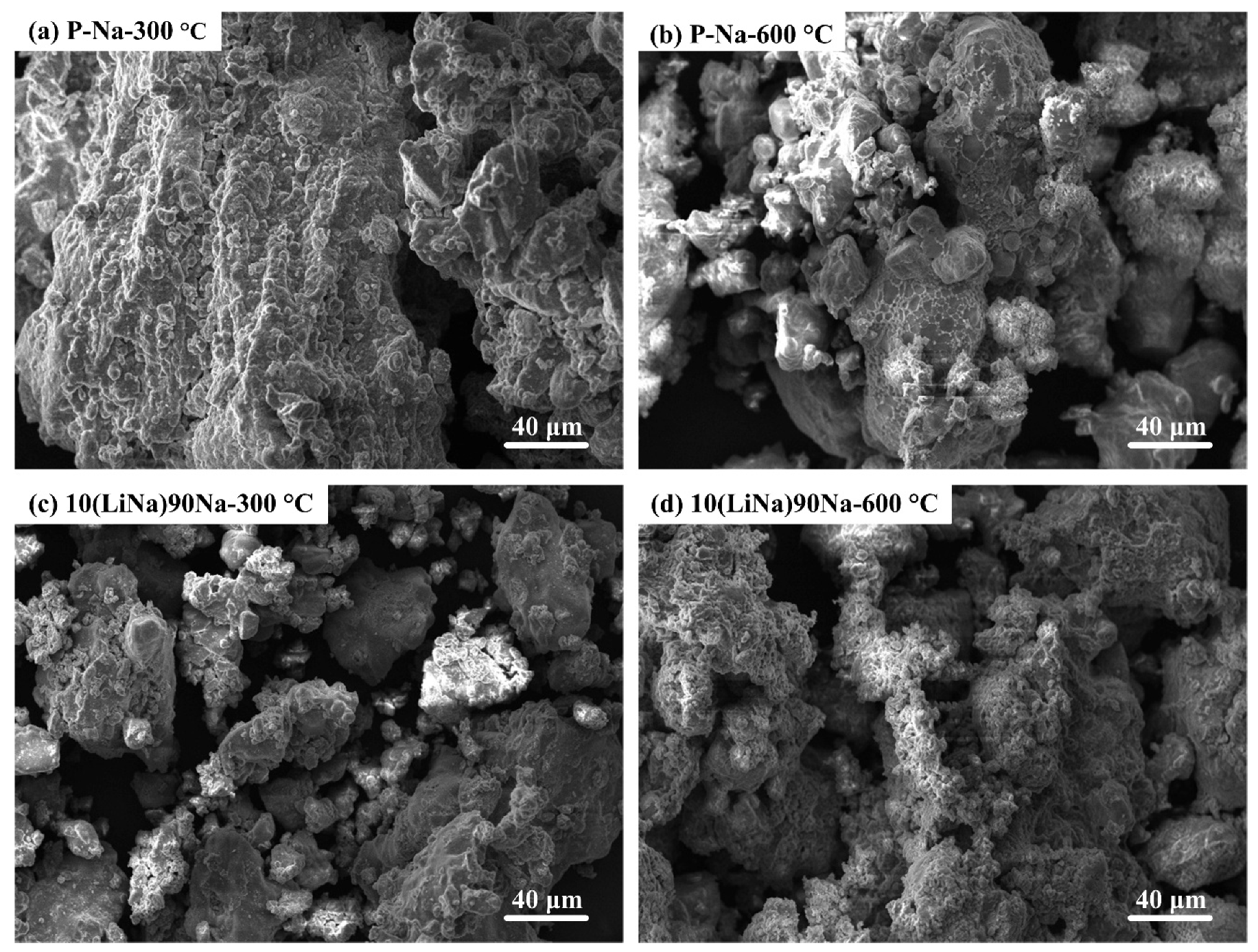
3.1. Characterization of the Prepared Na4SiO4
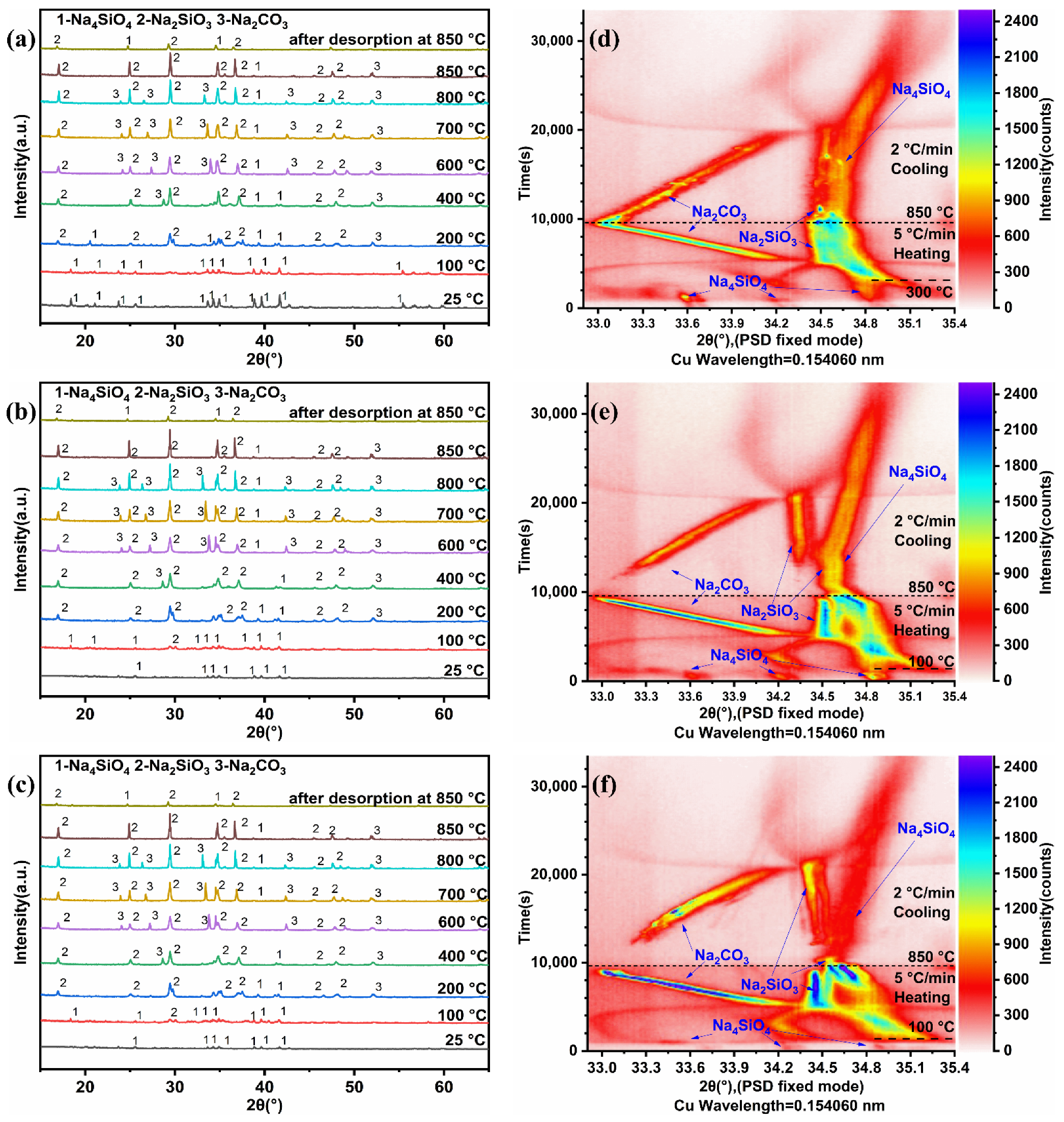
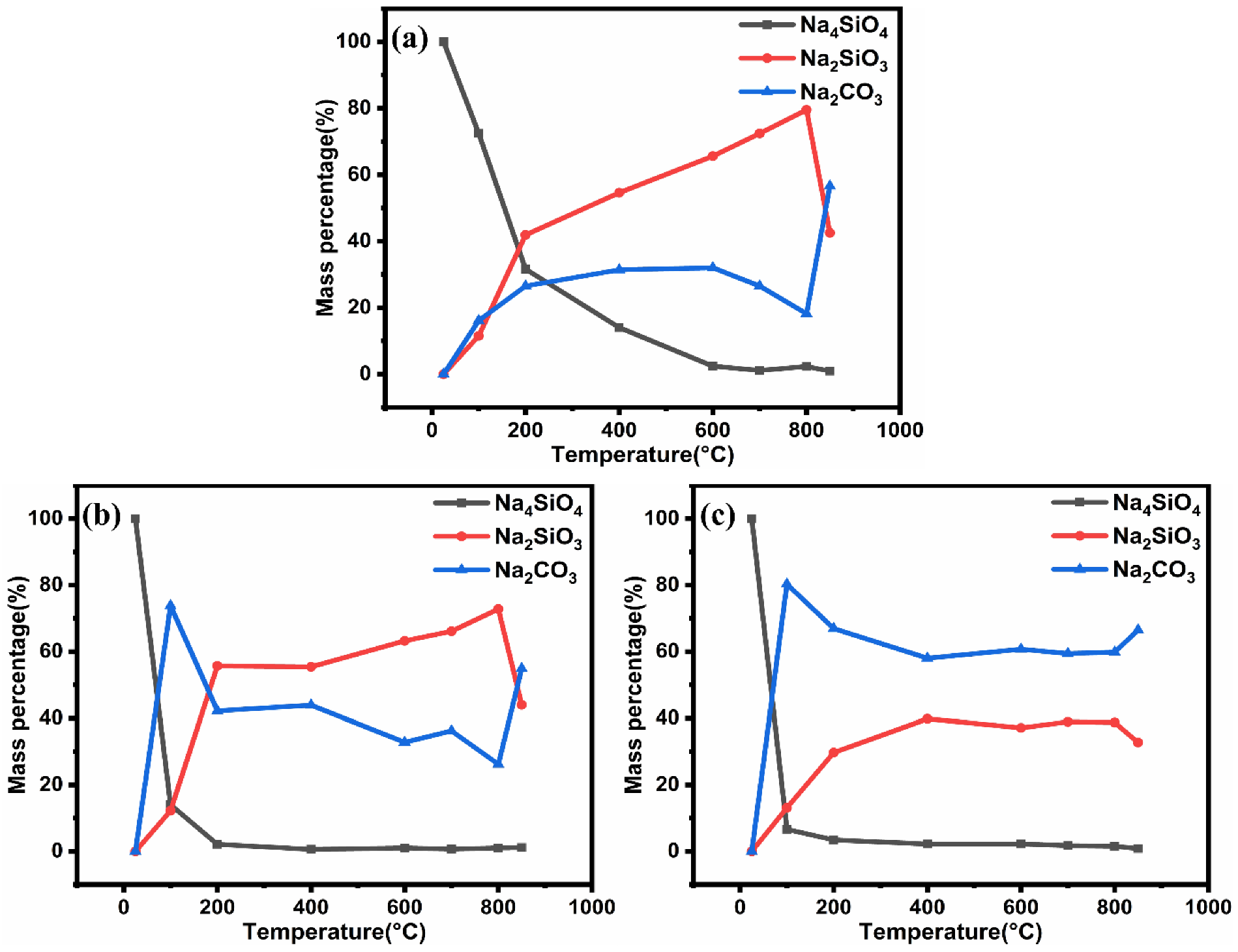
3.2. In Situ XRD
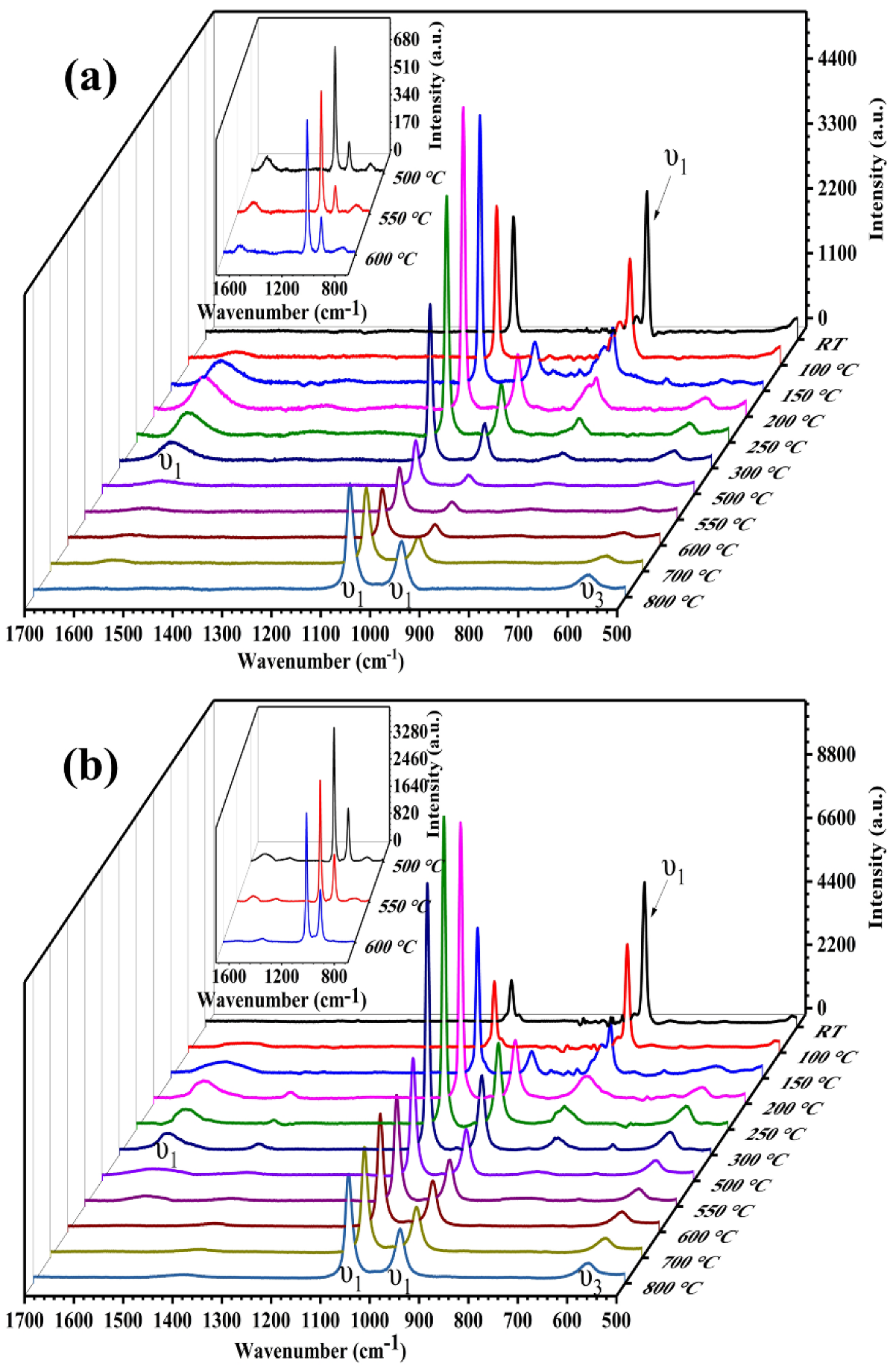
3.3. In Situ Raman
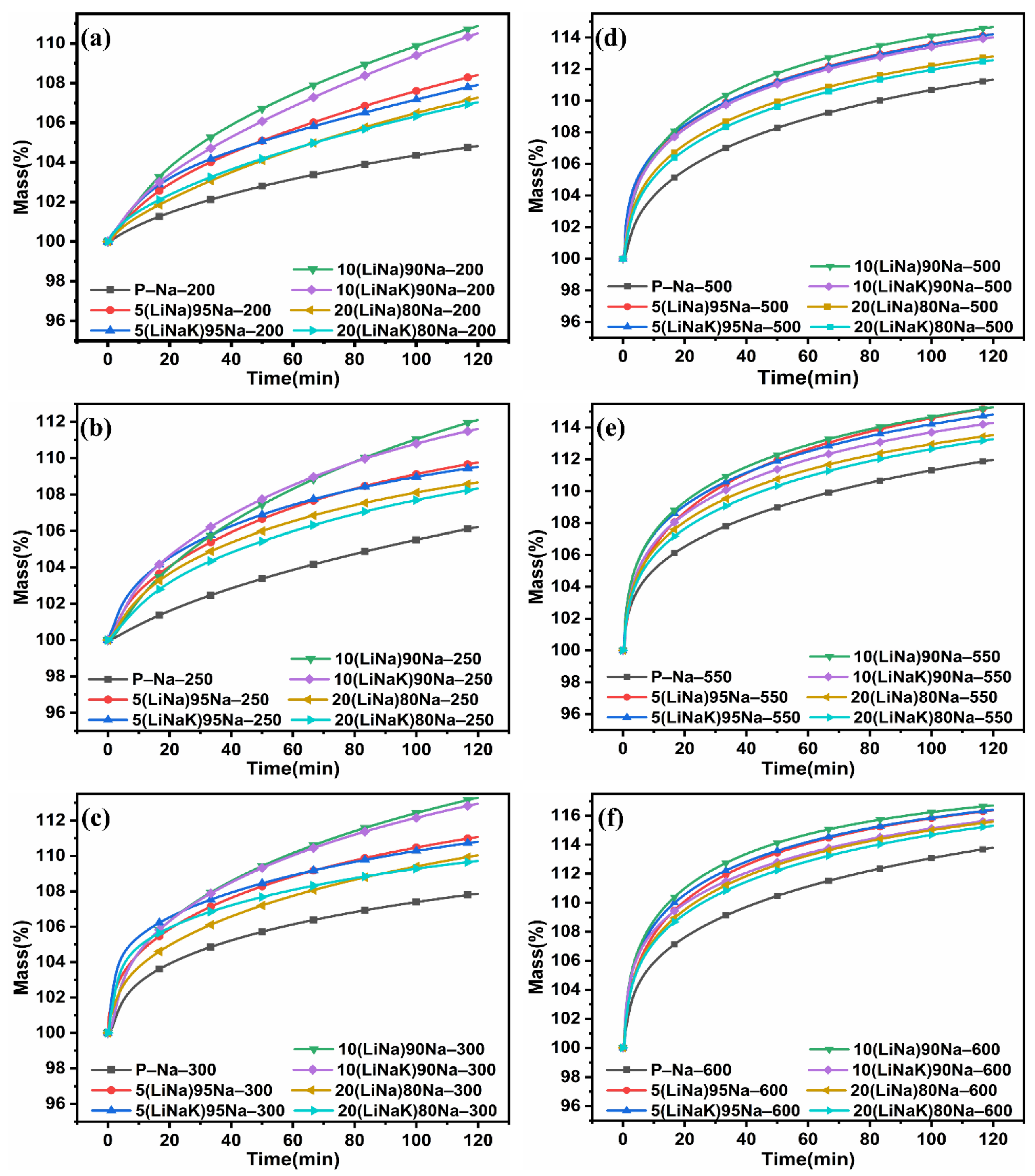
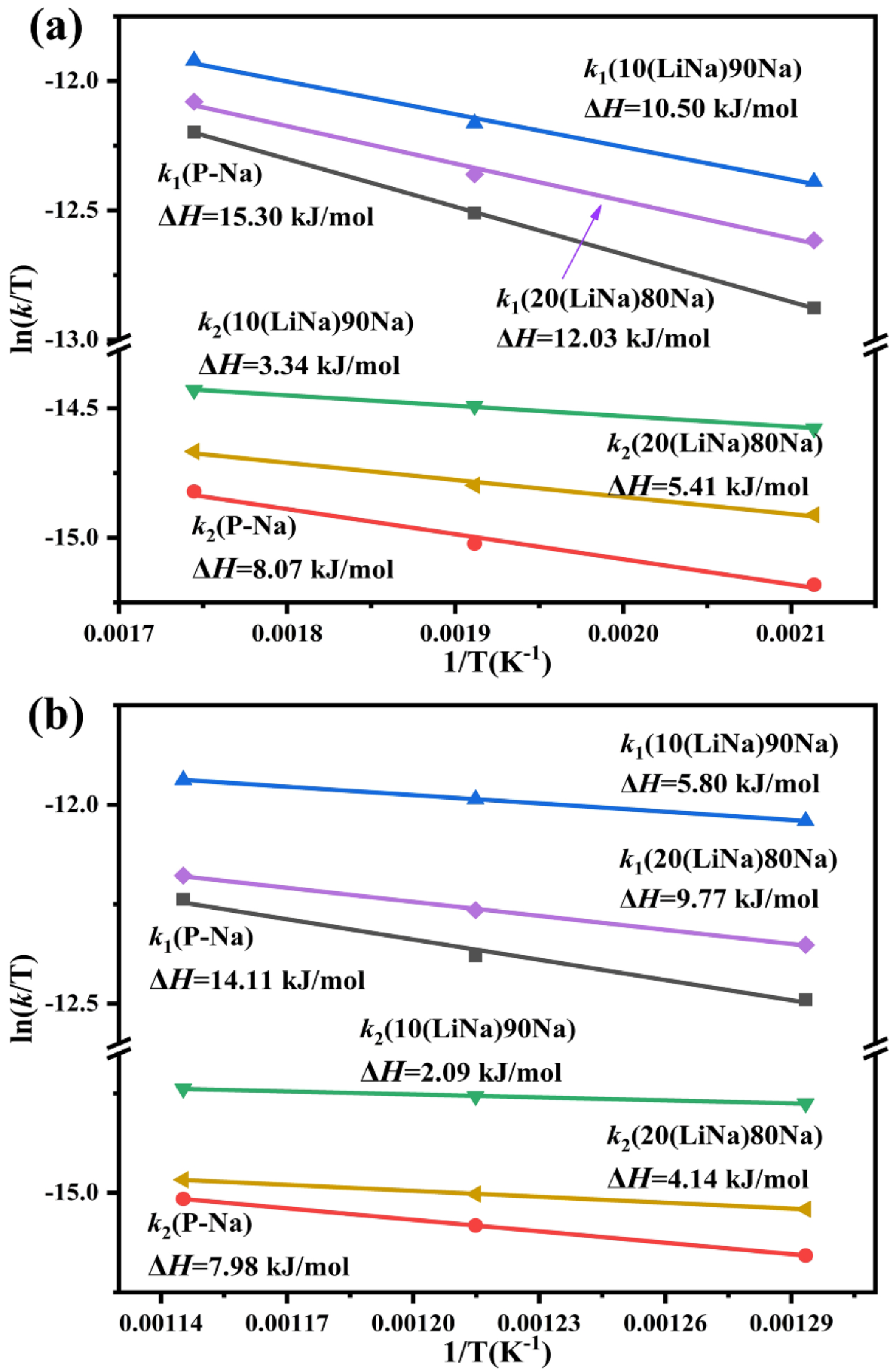
3.4. Isothermal Analyses and Kinetic Evaluation
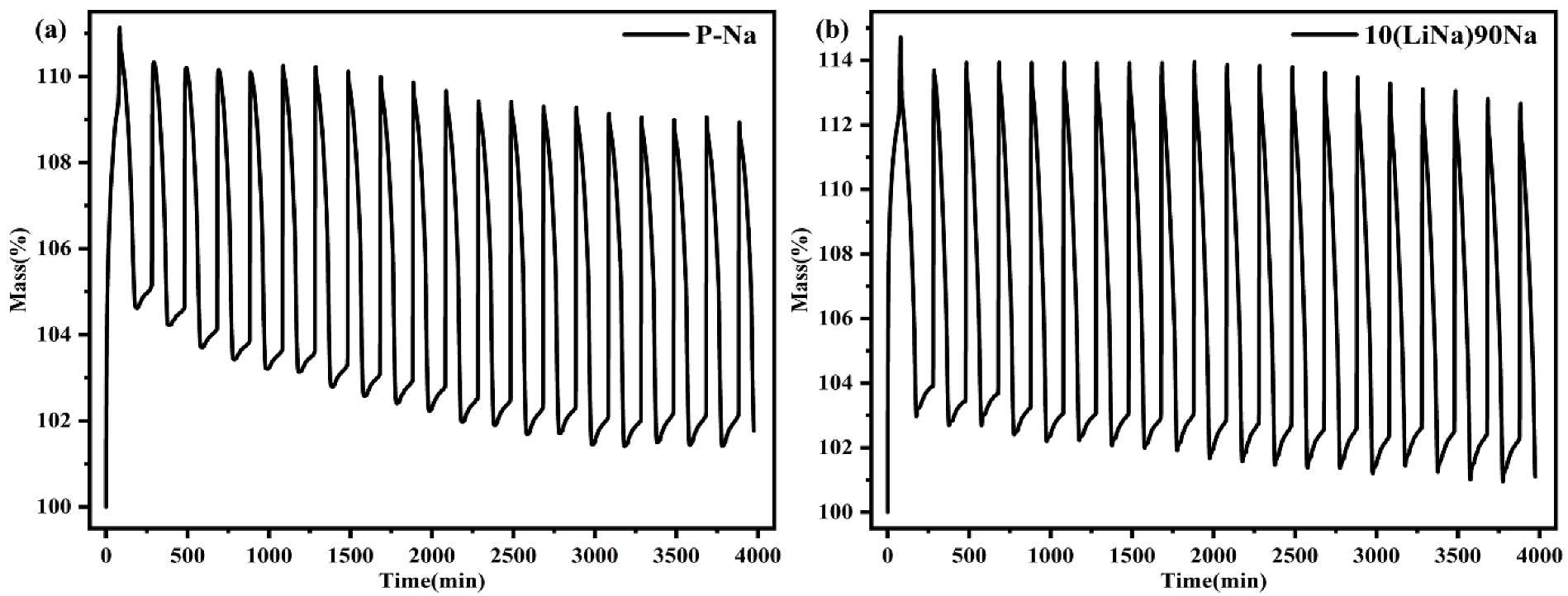
3.5. Cyclic Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crippa, M.; Oreggioni, G.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Lo Vullo, E.; Solazzo, E.; Monforti-Ferrario, F.; Olivier, J.G.; Vignati, E. Fossil CO2 and GHG Emissions of All World Countries; Publication Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Robinson, S.A. Climate change adaptation in SIDS: A systematic review of the literature pre and post the IPCC Fifth Assessment Report. Wiley Interdiscip. Rev. Clim. Change 2020, 11, e653. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Selma, L.; Seigo, O.; Dohle, S.; Siegrist, M. Public perception of carbon capture and storage (CCS): A review. Renew. Sustain. Energy Rev. 2014, 38, 848–863. [Google Scholar]
- Valverde, J.M. Ca-based synthetic materials with enhanced CO 2 capture efficiency. J. Mater. Chem. A 2013, 1, 447–468. [Google Scholar] [CrossRef]
- Tomkute, V.; Solheim, A.; Olsen, E. CO2 capture by CaO in molten CaF2–CaCl2: Optimization of the process and cyclability of CO2 capture. Energy Fuels 2014, 28, 5345–5353. [Google Scholar] [CrossRef]
- Nygård, H.S.; Tomkute, V.; Olsen, E. Kinetics of CO2 absorption by calcium looping in molten halide salts. Energy Procedia 2017, 114, 250–258. [Google Scholar] [CrossRef]
- Jo, S.-I.; An, Y.-I.; Kim, K.-Y.; Choi, S.-Y.; Kwak, J.-S.; Oh, K.-R.; Kwon, Y.-U. Mechanisms of absorption and desorption of CO2 by molten NaNO3-promoted MgO. Phys. Chem. Chem. Phys. 2017, 19, 6224–6232. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Hu, Y.; Cheng, Z.; Fang, X. Nanosheet MgO-based CO2 sorbent promoted by mixed-alkali-metal nitrate and carbonate: Performance and mechanism. Ind. Eng. Chem. Res. 2017, 56, 5802–5812. [Google Scholar] [CrossRef]
- Pfeiffer, H. Advances on alkaline ceramics as possible CO2 captors. In Advances in CO2 Conversion and Utilization; ACS Publications: Washington, DC, USA, 2010; pp. 233–253. [Google Scholar]
- Peltzer, D.; Mùnera, J.; Cornaglia, L.; Strumendo, M. Characterization of potassium doped Li2ZrO3 based CO2 sorbents: Stability properties and CO2 desorption kinetics. Chem. Eng. J. 2018, 336, 1–11. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Castro-Díaz, M.; Drage, T.C.; Maroto-Valer, M.M. Use of small-amplitude oscillatory shear rheometry to study the flow properties of pure and potassium-doped Li2ZrO3 sorbents during the sorption of CO2 at high temperatures. Sep. Purif. Technol. 2010, 73, 415–420. [Google Scholar] [CrossRef]
- Munro, S.; Åhlén, M.; Cheung, O.; Sanna, A. Tuning Na2ZrO3 for fast and stable CO2 adsorption by solid state synthesis. Chem. Eng. J. 2020, 388, 124284. [Google Scholar] [CrossRef]
- Mendoza-Nieto, J.A.; Martínez-Hernández, H.; Pfeiffer, H.; Gómez-García, J.F. A new kinetic model for CO2 capture on sodium zirconate (Na2ZrO3): An analysis under different flow rates. J. CO2 Util. 2022, 56, 101862. [Google Scholar] [CrossRef]
- Halliday, C.; Ozbek, N.; Hatton, T.A. Understanding Material Compatibility in CO2 Capture Systems Using Molten Alkali Metal Borates. ACS Appl. Mater. Interfaces 2020, 12, 51468–51477. [Google Scholar] [CrossRef]
- Halliday, C.; Harada, T.; Hatton, T.A. Bench-Scale Demonstration of Molten Alkali Metal Borates for High-Temperature CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 8937–8945. [Google Scholar] [CrossRef]
- Kibar, M.E.; Akın, A.N. A novel process for CO2 capture by using sodium metaborate. Part I: Effects of calcination. Environ. Sci. Pollut. Res. 2018, 25, 3446–3457. [Google Scholar] [CrossRef]
- Kibar, M.E.; Akin, A.N. A Novel Process for CO2 Capture by Using Sodium Metaborate, Part II: Carbonation Reaction and Kinetic Studies. Int. J. Chem. Kinet. 2017, 49, 119–129. [Google Scholar] [CrossRef]
- Bernabé-Pablo, E.; Duan, Y.; Pfeiffer, H. Developing new alkaline ceramics as possible CO2 chemisorbents at high temperatures: The lithium and sodium yttriates (LiYO2 and NaYO2) cases. Chem. Eng. J. 2020, 396, 125277. [Google Scholar] [CrossRef]
- Cruz, D.; Bulbulian, S.; Lima, E.; Pfeiffer, H. Kinetic analysis of the thermal stability of lithium silicates (Li4SiO4 and Li2SiO3). J. Solid State Chem. 2006, 179, 909–916. [Google Scholar] [CrossRef]
- Seggiani, M.; Puccini, M.; Vitolo, S. Alkali promoted lithium orthosilicate for CO2 capture at high temperature and low concentration. Int. J. Greenh. Gas Control. 2013, 17, 25–31. [Google Scholar] [CrossRef]
- Seggiani, M.; Stefanelli, E.; Puccini, M.; Vitolo, S. CO2 sorption/desorption performance study on K2CO3-doped Li4SiO4-based pellets. Chem. Eng. J. 2018, 339, 51–60. [Google Scholar] [CrossRef]
- Sanna, A.; Ramli, I.; Maroto-Valer, M.M. Development of sodium/lithium/fly ash sorbents for high temperature post-combustion CO2 capture. Appl. Energy 2015, 156, 197–206. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wang, Z.; Song, J.; Li, G.; Xu, Q.; You, J.; Cheng, H.; Lu, X. Alkali carbonates promote CO2 capture by sodium orthosilicate. Phys. Chem. Chem. Phys. 2019, 21, 13135–13143. [Google Scholar] [CrossRef]
- Wang, Z.R.; Liu, W.H.; Tang, Z.F.; Xu, Q. In situ Raman and XRD study of CO2 sorption and desorption in air by a Na4SiO4-Na2CO3 hybrid sorbent. Phys. Chem. Chem. Phys. 2020, 22, 27263–27271. [Google Scholar] [CrossRef]
- Sanna, A.; Ramli, I.; Maroto-Valer, M.M. Novel Na-silicates CO2 sorbents from fly ash. Energy Procedia 2014, 63, 739–744. [Google Scholar] [CrossRef]
- Sanna, A.; Maroto-Valer, M.M. CO2 capture at high temperature using fly ash-derived sodium silicates. Ind. Eng. Chem. Res. 2016, 55, 4080–4088. [Google Scholar] [CrossRef]
- Rodríguez, M.T.; Pfeiffer, H. Sodium metasilicate (Na2SiO3): A thermo-kinetic analysis of its CO2 chemical sorption. Thermochim. Acta 2008, 473, 92–95. [Google Scholar] [CrossRef]
- Rodríguez-Mosqueda, R.; Pfeiffer, H. High CO2 capture in sodium metasilicate (Na2SiO3) at low temperatures (30–60 °C) through the CO2–H2O chemisorption process. J. Phys. Chem. C 2013, 117, 13452–13461. [Google Scholar] [CrossRef]
- Kwak, J.-S.; Oh, K.-R.; Kim, K.-Y.; Lee, J.-M.; Kwon, Y.-U. CO2 absorption and desorption characteristics of MgO-based absorbent promoted by triple eutectic alkali carbonate. Phys. Chem. Chem. Phys. 2019, 21, 20805–20813. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Gao, W.; Harada, T.; Qin, Q.; Zheng, Q.; Hatton, T.A.; Wang, Q. Alkali carbonate molten salt coated calcium oxide with highly improved carbon dioxide capture capacity. Energy Technol. 2017, 5, 1328–1336. [Google Scholar] [CrossRef]
- Martinez-dlCruz, L.; Pfeiffer, H. Effect of Oxygen Addition on the Thermokinetic Properties of CO2 Chemisorption on Li2ZrO3. Ind. Eng. Chem. Res. 2010, 49, 9038–9042. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Peng, K.; Wang, Z.; Zou, X.; Cheng, H.; Lu, X. Elucidating the promotion of Na2CO3 in CO2 capture by Li4SiO4. Phys. Chem. Chem. Phys. 2021, 23, 26696–26708. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Qin, C.; Brinkman, K.; Gong, Y.; Wang, S.; Huang, K. First spectroscopic identification of pyrocarbonate for high CO 2 flux membranes containing highly interconnected three dimensional ionic channels. Phys. Chem. Chem. Phys. 2013, 15, 13147–13152. [Google Scholar] [CrossRef]
- Corradini, D.; Coudert, F.-X.; Vuilleumier, R. Carbon dioxide transport in molten calcium carbonate occurs through an oxo-Grotthuss mechanism via a pyrocarbonate anion. Nat. Chem. 2016, 8, 454–460. [Google Scholar] [CrossRef]
- Jo, H.G.; Yoon, H.J.; Lee, C.H.; Lee, K.B. Citrate sol–gel method for the preparation of sodium zirconate for high-temperature CO2 sorption. Ind. Eng. Chem. Res. 2016, 55, 3833–3839. [Google Scholar] [CrossRef]
- Martínez-dlCruz, L.; Pfeiffer, H. Cyclic CO2 chemisorption–desorption behavior of Na2ZrO3: Structural, microstructural and kinetic variations produced as a function of temperature. J. Solid State Chem. 2013, 204, 298–304. [Google Scholar] [CrossRef]
- Habibi-Khorassani, S.M.; Maghsoodlou, M.T.; Shahraki, M.; Talaiefar, S.; Kazemian, M.A.; Aboonajmi, J. Full kinetics and a mechanistic investigation of three-component reaction catalyzed by sodium acetate leading to 3, 4-dihydropyrano [c] chromene. Res. Chem. Intermed. 2015, 41, 5821–5837. [Google Scholar] [CrossRef]
- Ema, K.; Hibino, Y.; Shigekawa, H.; Hyodo, S.-I. Component assignments of the Raman spectrum from highly elongated silica glass filbers. Jpn. J. Appl. Phys. 1987, 26, 649. [Google Scholar] [CrossRef]
- Richet, P.; Mysen, B.O. High-temperature dynamics in cristobalite (SiO2) and carnegieite (NaAlSiO4): A raman spectroscopy study. Geophys. Res. Lett. 1999, 26, 2283–2286. [Google Scholar] [CrossRef]
- Mysen, B.O.; Virgo, D.; Scarfe, C.M. Relations between the anionic structure and viscosity of silicate melts—A Raman spectroscopic study. Am. Mineral. 1980, 65, 690–710. [Google Scholar]
- McMillan, P.F.; Wolf, G.H.; Poe, B.T. Vibrational spectroscopy of silicate liquids and glasses. Chem. Geol. 1992, 96, 351–366. [Google Scholar] [CrossRef]
- Salazar Hoyos, L.A.; Faroldi, B.M.; Cornaglia, L.M. K-doping effect in the kinetics of CO2 capture at high temperature over lithium silicates obtained from rice husks: In situ/operando techniques. Ceram. Int. 2021, 47, 1558–1570. [Google Scholar] [CrossRef]
| Sample | T (°C) | k1 (s−1) | k2 (s−1) | R2 |
|---|---|---|---|---|
| P–Na | 200 | 1.21 × 10−3 | 1.21 × 10−4 | 0.999 |
| 250 | 1.93 × 10−3 | 1.56 × 10−4 | 0.999 | |
| 300 | 2.89 × 10−3 | 2.10 × 10−4 | 0.999 | |
| 10(LiNa)90Na | 200 | 1.97 × 10−3 | 2.21 × 10−4 | 0.999 |
| 250 | 2.73 × 10−3 | 2.66 × 10−4 | 0.999 | |
| 300 | 3.81 × 10−3 | 3.10 × 10−4 | 0.999 | |
| 20(LiNa)80Na | 200 | 1.57 × 10−3 | 1.58 × 10−4 | 0.999 |
| 250 | 2.24 × 10−3 | 1.96 × 10−4 | 0.999 | |
| 300 | 3.25 × 10−3 | 2.45 × 10−4 | 0.999 |
| Sample | T (°C) | k1 (s−1) | k2 (s−1) | R2 |
|---|---|---|---|---|
| P–Na | 500 | 2.91 × 10−3 | 2.01 × 10−4 | 0.999 |
| 550 | 3.46 × 10−3 | 2.29 × 10−4 | 0.999 | |
| 600 | 4.23 × 10−3 | 2.63 × 10−4 | 0.999 | |
| 10(LiNa)90Na | 500 | 4.56 × 10−3 | 2.96 × 10−4 | 0.999 |
| 550 | 5.13 × 10−3 | 3.21 × 10−4 | 0.999 | |
| 600 | 5.71 × 10−3 | 3.47 × 10−4 | 0.999 | |
| 20(LiNa)80Na | 500 | 3.34 × 10−3 | 2.27 × 10−4 | 0.999 |
| 550 | 3.88 × 10−3 | 2.51 × 10−4 | 0.999 | |
| 600 | 4.49 × 10−3 | 2.76 × 10−4 | 0.999 |
| Sample | Dopant | Sorption Conditions | Weight Gain, % | Reference |
|---|---|---|---|---|
| Na2CO3-Fly ash | - | Isothermally, 700 °C, 100% CO2, 120 min | 9.8 | A. Sanna [27] |
| Na2CO3-Fly ash | 5 mol% Li2CO3 | Isothermally, 700 °C, 100% CO2, 120 min | 10.2 | A. Sanna [27] |
| Na2CO3-Fly ash | 10 mol% Li2CO3 | Isothermally, 700 °C,100% CO2, 120 min | 11.4 | A. Sanna [27] |
| Na2CO3-Fly ash | 10 mol% K2CO3 | Isothermally, 700 °C,100% CO2, 120 min | 9.9 | A. Sanna [27] |
| Na4SiO4 | 33.3 wt% Li2CO3 | Dynamic thermally, 50–1000 °C, 80% CO2 | 14.9 | Liu [24] |
| Na4SiO4 | 33.3 wt% Na2CO3 | Dynamic thermally, 50–1000 °C, 80% CO2 | 7.7 | Liu [24] |
| Na4SiO4 | 33.3 wt% K2CO3 | Dynamic thermally, 50–1000 °C, 80% CO2 | 10.8 | Liu [24] |
| Na4SiO4 | 33.3 mol% Na2CO3 | Isothermally, 550 °C, Air, 190 min | 3.2 | Wang [25] |
| Na4SiO4 | - | Isothermally, 300 °C, 80% CO2, 120 min | 7.9 | This work |
| Na4SiO4 | - | Isothermally, 600 °C, 80% CO2, 120 min | 13.8 | This work |
| Na4SiO4 | 10 mol% (Li2CO3-Na2CO3) | Isothermally, 300 °C, 80% CO2, 120 min | 13.3 | This work |
| Na4SiO4 | 10 mol% (Li2CO3-Na2CO3) | Isothermally, 600 °C, 80% CO2, 120 min | 16.7 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, C.; Xu, Q.; Zou, X.; Cheng, H.; Lu, X. In Situ XRD, Raman Characterization, and Kinetic Study of CO2 Capture by Alkali Carbonate-Doped Na4SiO4. Separations 2022, 9, 428. https://doi.org/10.3390/separations9120428
Wang Z, Sun C, Xu Q, Zou X, Cheng H, Lu X. In Situ XRD, Raman Characterization, and Kinetic Study of CO2 Capture by Alkali Carbonate-Doped Na4SiO4. Separations. 2022; 9(12):428. https://doi.org/10.3390/separations9120428
Chicago/Turabian StyleWang, Zhen, Chenteng Sun, Qian Xu, Xingli Zou, Hongwei Cheng, and Xionggang Lu. 2022. "In Situ XRD, Raman Characterization, and Kinetic Study of CO2 Capture by Alkali Carbonate-Doped Na4SiO4" Separations 9, no. 12: 428. https://doi.org/10.3390/separations9120428
APA StyleWang, Z., Sun, C., Xu, Q., Zou, X., Cheng, H., & Lu, X. (2022). In Situ XRD, Raman Characterization, and Kinetic Study of CO2 Capture by Alkali Carbonate-Doped Na4SiO4. Separations, 9(12), 428. https://doi.org/10.3390/separations9120428






