Assessment of Anti-Obesity Potential and Techno-Functional Properties of Bougainvillea spectabilis Willd. Bracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
2.3. Moisture Content
2.4. Techno-Functional Properties of Dried Bougainvillea Spectabilis Willd. Bracts Powder
2.5. Extraction Process
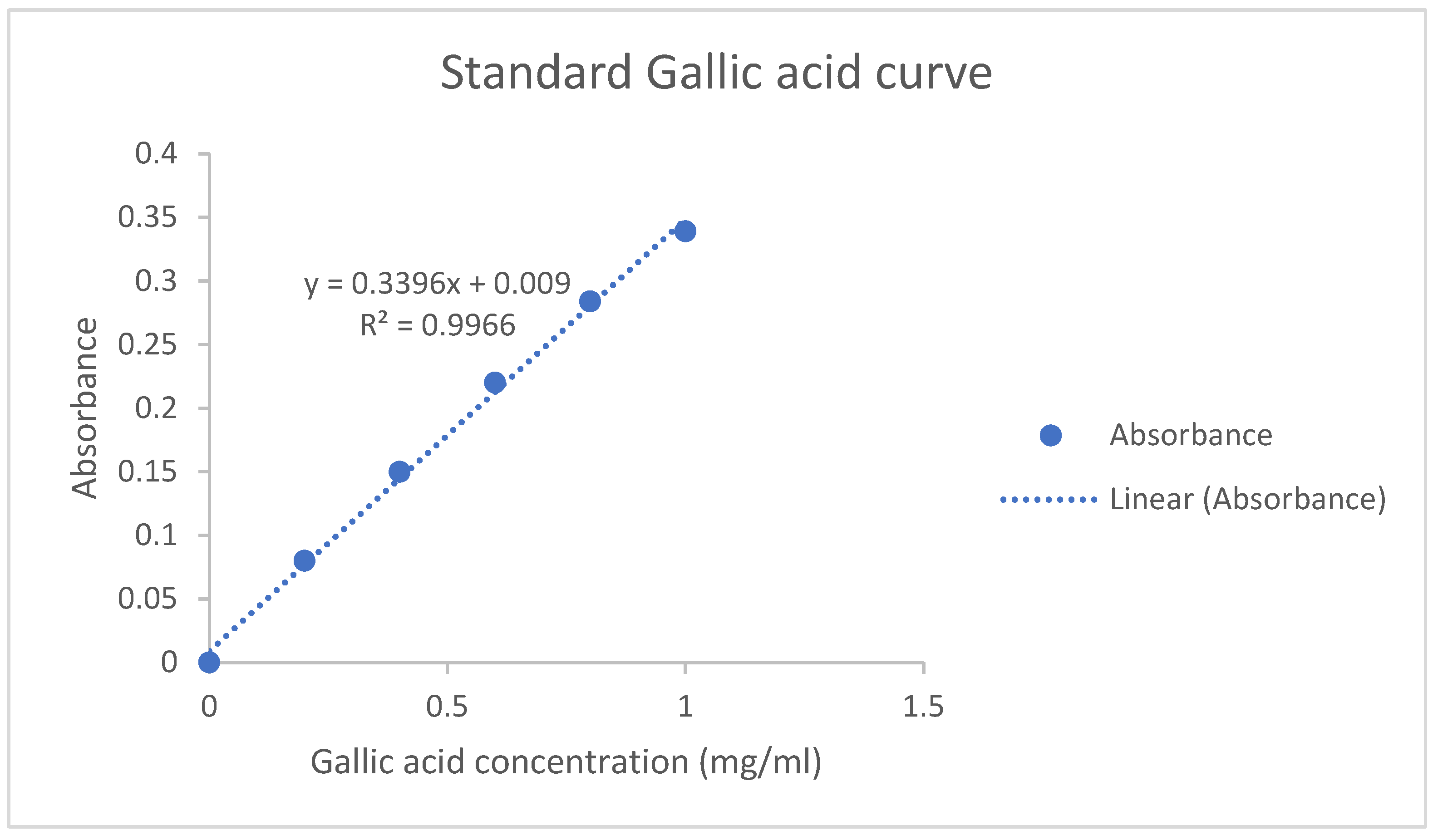
2.6. Total Phenolic Content
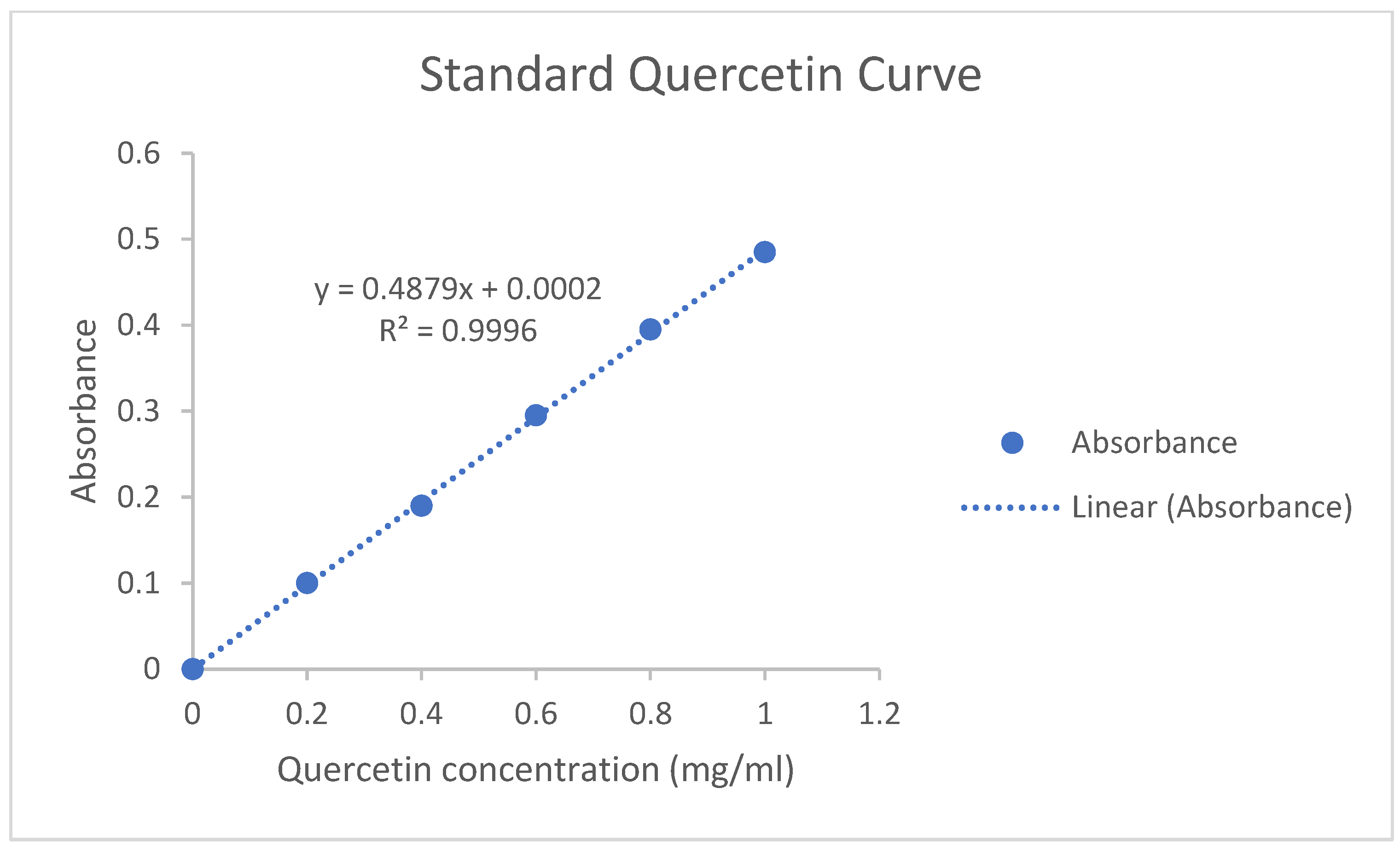
2.7. Total Flavonoid Content
2.8. Antioxidant Efficacy Estimation
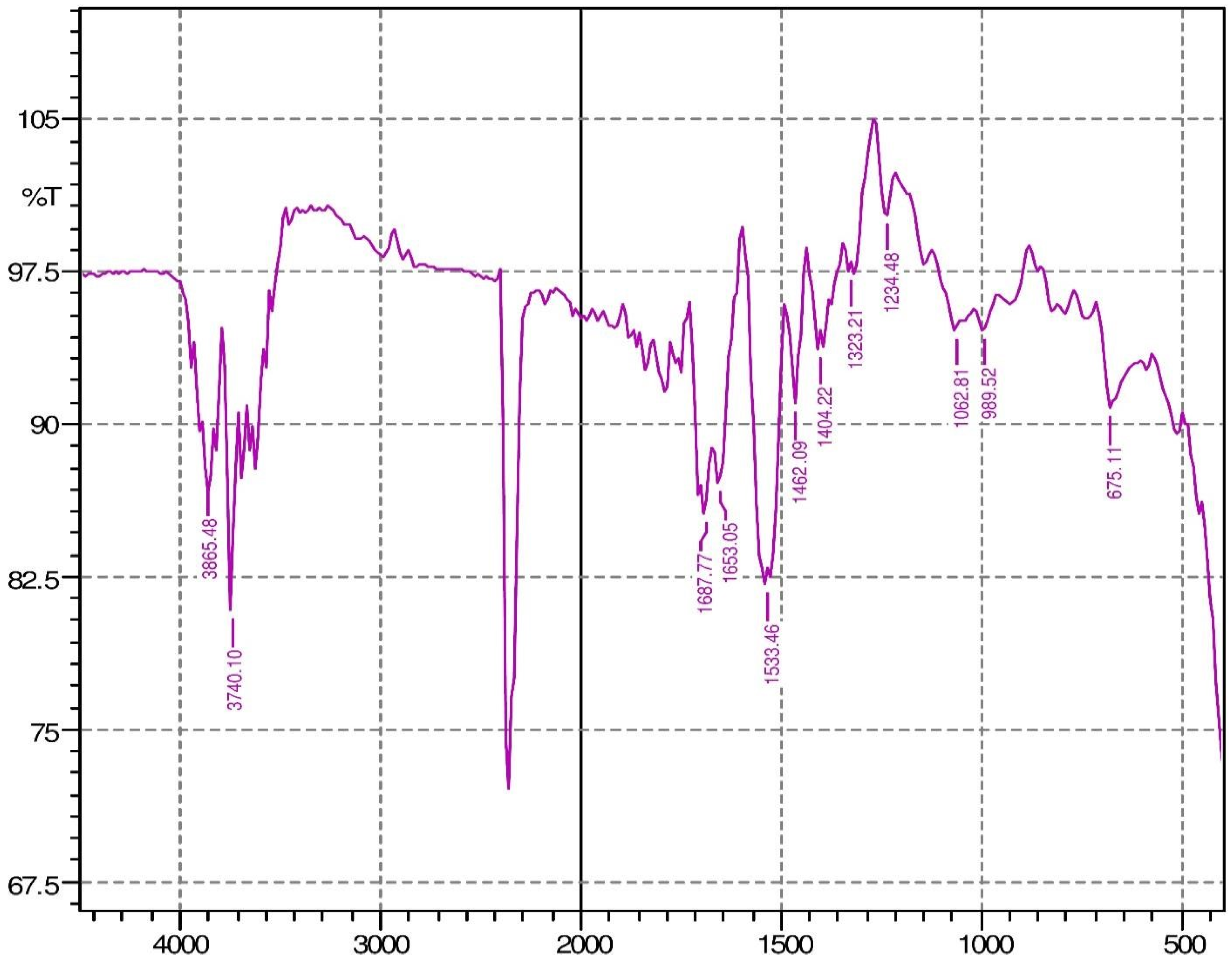
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Lipase and Amylase Inhibition Assay
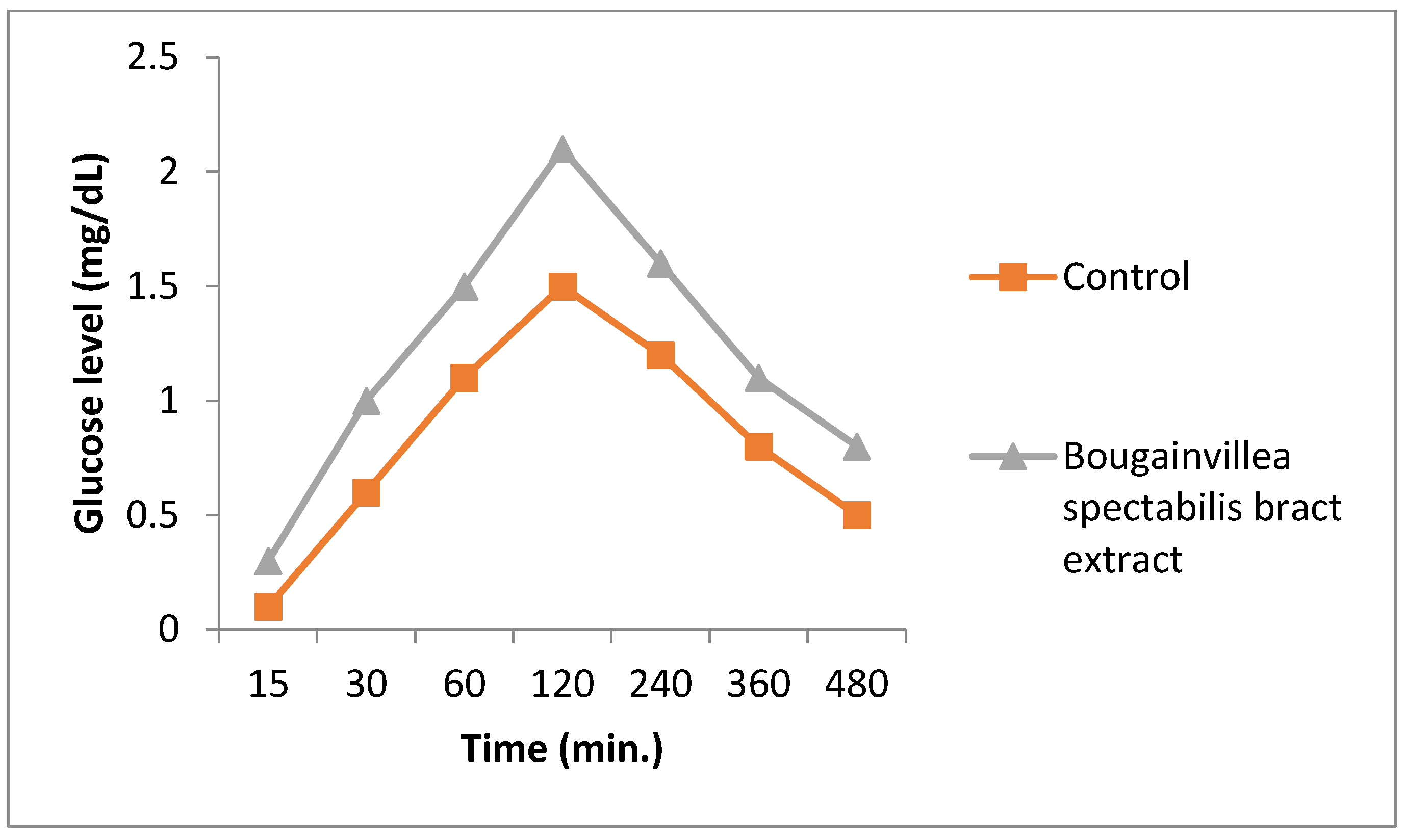
2.11. Glucose Uptake Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Techno-Functional Properties of Dried Bougainvillea Spectabilis Willd. Bracts Powder
3.2. Phytochemical Analysis of Bougainvillea Spectabilis Willd. Bracts Powder
3.3. FTIR Analysis
3.4. Lipase Inhibition Assay
3.5. Amylase Inhibition Assay
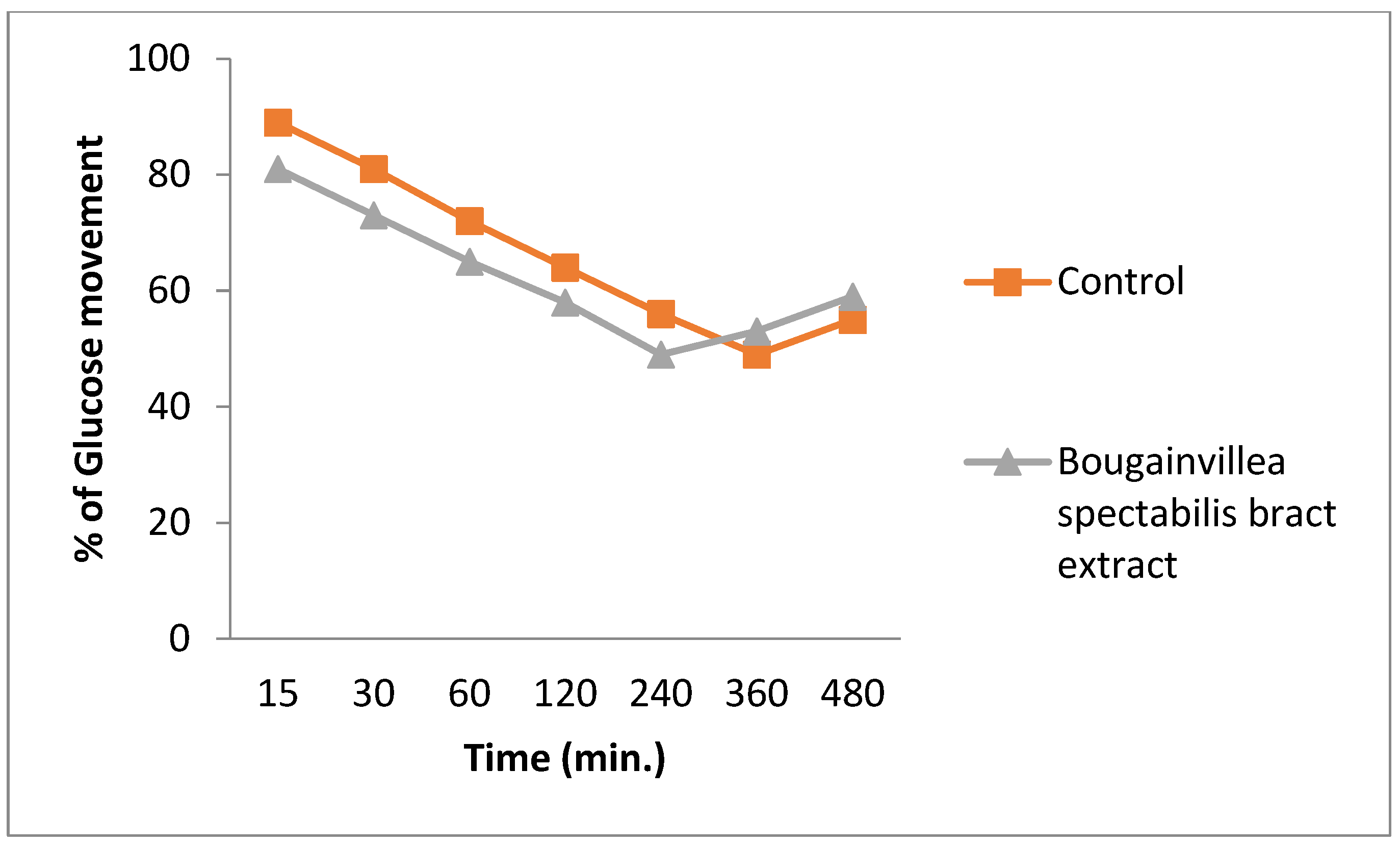
3.6. Glucose Uptake Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse. Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Guleria, S.; Chawla, P.; Khan, A.; Modi, V.K.; Kumar, N.; Kaushik, R. Anti-obesity efficacy of the selected high altitude Himalayan herbs: In vitro studies. J. Food Sci. Technol. 2020, 57, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kaushik, D.; Kaur, J.; Proestos, C.; Oz, F.; Oz, E.; Gupta, P.; Kundu, P.; Kaur, A.; Anisha, A.; et al. A Critical Review on Obesity: Herbal Approach, Bioactive Compounds, and Their Mechanism. Appl. Sci. 2022, 12, 8342. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. The importance of herbal medicine use in the German health-care system: Prevalence, usage pattern, and influencing factors. BMC Health Serv. Res. 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Tradit. Complement. Med. 2017, 7, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea genus: A review on phytochemistry, pharmacology, and toxicology. eCAM 2018, 2018, 9070927. [Google Scholar] [CrossRef] [PubMed]
- Nitesh, C.J.; Ambika, N.J.; Alkama, G.F. Efficiency of Bougainvillea spectabilis Willd in Monitoring Dust. Int. J. Appl. Environ. Sci. 2017, 12, 773–785. [Google Scholar]
- Ghogar, A.; Jiraungkoorskul, K.; Jiraungkoorskul, W. Paper Flower, Bougainvillea spectabilis: Update properties of traditional medicinal plant. J. Nat. Remedies. 2016, 16, 82–87. [Google Scholar] [CrossRef]
- Venkatachalam, R.N.; Singh, K.; Marar, T. Bougainvillea spectabilis, a good source of antioxidant phytochemicals. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 605–613. [Google Scholar]
- Salam, P.; Bhargav, V.; Gupta, Y.C.; Nimbolkar, P.K. Evolution in Bougainvillea (Bougainvillea Commers.)-A review. J. Appl. Nat. Sci. 2017, 9, 1489–1494. [Google Scholar] [CrossRef]
- Jawla, S.; Kumar, Y.; Khan, M.S.Y. Isolation of anti-diabetic principle from Bougainvillea spectabilis willd (Nyctaginaceae) stem bark. Trop. J. Pharm. Res. 2013, 12, 761–765. [Google Scholar]
- Dhankhar, S.; Sharma, M.; Ruhil, S.; Balhara, M.; Kumar, M.; Chhillar, A.K. Evaluation of antimicrobial and antioxidant activities of Bougainvillea spectabilis. Int. J. Pharm. Pharm. Sci. 2013, 5, 178–182. [Google Scholar]
- Vukovic, N.; Kacaniova, M.; Hleba, L.; Sukdolak, S. Chemical Composition of the Essential oil of Bougainvillea spectabilis from Montenegro. J. Essent. Oil Bear. Plants 2013, 16, 212–215. [Google Scholar] [CrossRef]
- Sudipta, K.M.; Lokesh, P.; Rashmi, W.; Vijay, R.; Ssn, K. Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. Int. J. Phytomedicine 2012, 4, 375–379. [Google Scholar]
- Do, L.T.; Aree, T.; Siripong, P.; Pham, T.N.; Nguyen, P.K.; Tip-pyang, S. Bougainvinones A–H, peltogynoids from the stem bark of purple Bougainvillea spectabilis and their cytotoxic activity. J. Nat. Prod. 2016, 79, 939–945. [Google Scholar] [CrossRef]
- Mandal, G.; Chatterjee, C.; Chatterjee, M. Evaluation of anti inflammatory activity of methanolic extract of leaves of Bougainvillea spectabilis in experimental animal models. Pharmacogn. Res. 2015, 7, 18–22. [Google Scholar]
- Do, L.T.; Aree, T.; Siripong, P.; Vo, N.T.; Nguyen, T.T.; Nguyen, P.K.; Tip-pyang, S. Cytotoxic flavones from the stem bark of Bougainvillea spectabilis willd. Planta Med. 2018, 84, 129–134. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kulshrestha, A.; Shrivastava, S.; Sharma, B.; Goswamy, H.M.; Prasad, G.B.K.S. Bougainvillea spectabilis exhibits antihyperglycemic and antioxidant activities in experimental diabetes. eCAM 2016, 21, 177–185. [Google Scholar] [CrossRef]
- Bhat, M.; Kothiwale, S.K.; Tirmale, A.R.; Bhargava, S.Y.; Joshi, B.N. Antidiabetic properties of Azardiracta indica and Bougainvillea spectabilis: In vivo studies in murine diabetes model. eCAM 2011, 2011, 561625. [Google Scholar]
- Killedar, S.; Pawar, A.; Nadaf, S.; Nale, A.; Tamboli, U.; Pishawikar, S. Novel analytical method development for some amide group containing drugs using Bougainvillea spectabilis bract extracts. Asian Pac. J. Trop. Med. 2014, 7, S560–S567. [Google Scholar] [CrossRef]
- Hajare, C.N.; Inamdar, F.R.; Patil, R.V.; Shete, C.S.; Wadkar, S.S.; Patil, K.S.; Ghosh, J.S. Anti-bacterial activity of the leaves of Bougainvillea spectabilis against E. coli NCIM 2832 and M. aureus NCIM 5021. Int. J. Pharm. Sci. Rev. Res. 2015, 34, 194–196. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005; Method 935.14 and 992.24. [Google Scholar]
- Singh, J.; Rasane, P.; Kaur, S.; Nanda, V. Comparative analysis of antioxidant potential and techno-functional properties of selected corn silk varieties at different developmental stages. J. Food Meas. Charact. 2022, 16, 2685–2698. [Google Scholar] [CrossRef]
- Tze, N.L.; Han, C.P.; Yusof, Y.A.; Ling, C.N.; Talib, R.A.; Taip, F.S.; Aziz, M.G. Physicochemical and nutritional properties of spray-dried pitaya fruit powder as natural colorant. Food Sci. Biotechnol. 2012, 21, 675–682. [Google Scholar] [CrossRef]
- Brar, I.S.; Dixit, A.K.; Khurana, R.; Gautam, A. Studies on physical properties of maize (Zea mays L.) seeds. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 963–970. [Google Scholar] [CrossRef]
- Mokhtar, S.M.; Swailam, H.M.; Embaby, H.E. Physicochemical properties, nutritional value and techno-functional properties of goldenberry (Physalis peruviana) waste powder concise title: Composition of goldenberry juice waste. Food Chem. 2018, 248, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ishara, J.R.; Sila, D.N.; Kenji, G.M.; Buzera, A.K. Nutritional and functional properties of mushroom (Agaricus bisporus & Pleurotus ostreatus) and their blends with maize flour. Am. J. Food Technol. 2018, 6, 33–41. [Google Scholar]
- Baljeet, S.Y.; Ritika, B.Y.; Reena, K. Effect of incorporation of carrot pomace powder and germinated chickpea flour on the quality characteristics of biscuits. Int. Food Res. J. 2014, 21, 217–222. [Google Scholar]
- Sadh, P.K.; Chawla, P.; Duhan, J.S. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018, 22, 113–120. [Google Scholar] [CrossRef]
- Chawla, P.; Kumar, V.; Bains, A.; Singh, R.; Sadh, P.K.; Kaushik, R.; Kumar, N. Improvement of Mineral Absorption and Nutritional Properties of Citrullus vulgaris Seeds Using Solid-State Fermentation. J. Am. Coll. Nutr. 2020, 39, 628–635. [Google Scholar] [CrossRef]
- Shashikant, M.; Bains, A.; Chawla, P.; Sharma, M.; Kaushik, R.; Kandi, S.; Kuhad, R.C. In-vitro antimicrobial and anti-inflammatory activity of modified solvent evaporated ethanolic extract of Calocybe indica: GCMS and HPLC characterization. Int. J. Food Microbiol. 2022, 376, 109741. [Google Scholar] [CrossRef]
- Patil, M.M.; Anand, T.; Ilaiyaraja, N.; Khanum, F. Invitro antioxidant and anti-obesity properties of Bauhinia variegate. Def. Life Sci. J. 2017, 2, 128–132. [Google Scholar] [CrossRef]
- Souza, S.D.; Murata, H.; Jose, M.V.; Askarova, S.; Yantsen, Y.; Andersen, J.D.; Edington, C.D.; Clafshenkel, W.P.; Koepsel, R.R.; Russell, A.J. Engineering of cell membranes with a bisphosphonate-containing polymer using ATRP synthesis for bone targeting. Biomaterials 2014, 35, 9447–9458. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.A.; Rehmani, F.S.; Arman, M.; Ibrahimand, M.; Shafique, S. Estimation of Moisture Content & Metal Ions in White Flowers of Bougainvillea spectabilis and Purple Flowers of Bougainvillea glabra in Pakistan. Pak. J. Chem. 2011, 1, 190–192. [Google Scholar]
- Compaore, M.; Bakasso, S.; Nacoulma, A.P.; Meda, R.N.; Kiendrebeogo, M.; Millogo, J.F.; Nacoulma, O.G. Polyphenolic Contents of Bougainvillea spectabilis Wild and Bougainvillea glabra Choisy (Nictagynaceae) with Potential Antioxidant and Antiproliferative Activities. Asian J. Pharm. Biol. Res. 2013, 3, 24–28. [Google Scholar]
- Singh, J.; Kaur, S.; Rasane, P. Evaluation of the nutritional and quality characteristics of black carrot fortified instant noodles. Curr. Nutr. Food Sci. 2018, 14, 442–449. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.; Barroca, M.J.; Gonçalves, F.J.; Alves, M.; Oliveira, S.; Correia, P.M. Effect of drying on total phenolic compounds, antioxidant activity, and kinetics decay in pears. Int. J. Fruit Sci. 2015, 15, 173–186. [Google Scholar] [CrossRef]
- Yen, G.C.; Cheng, H.L.; Lin, L.Y.; Chen, S.C.; Hsu, C.L. The potential role of phenolic compounds on modulating gut microbiota in obesity. J. Food Drug Anal. 2020, 28, 195–205. [Google Scholar] [CrossRef]
- de la Garza, A.L. The role of flavonoids in the effort to prevent obesity: Nutrition 4.0. Curr. Res. Nutr. Food Sci. J. 2018, 6, 573–575. [Google Scholar] [CrossRef]
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic efficacy of antioxidants in ameliorating obesity phenotype and associated comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Singh, J.; Inbaraj, B.S.; Kaur, S.; Rasane, P.; Nanda, V. Phytochemical Analysis and Characterization of Corn Silk (Zea mays, G5417). Agronomy 2022, 12, 777. [Google Scholar] [CrossRef]
- Sahu, N.; Saxena, J. Phytochemical analysis of Bougainvillea glabra Choisy by FTIR and UV-VIS spectroscopic analysis. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 196–198. [Google Scholar]
- Saikia, H.; Lama, A. Effect of Bougainvillea spectabilis leaves on serum lipids in albino rats fed with high fat diet. Int. J. Pharm. Sci. Drug Res. 2011, 3, 141–145. [Google Scholar]
- Lim, T.K. Bougainvillea spectabilis. In Edible Medicinal and Non-Medicinal Plants, 1st ed.; Lim, T.K., Ed.; Springer Dordrecht: Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2014; Volume 8, pp. 489–496. [Google Scholar]
| Techno-Functional Properties | Bougainvillea spectabilis Willd. Bracts Powder |
|---|---|
| Bulk density (g/cm3) | 0.401 ± 0.098 |
| Tapped density (g/cm3) | 0.510 ± 0.22 |
| Carr Index (CI) | 21.37 ± 0.94 |
| Hausner ration (HR) | 1.271 ± 0.17 |
| Angle of repose (°) | 32.09 ± 0.92 |
| Water absorption index (WAI) (g/g) | 9.78 ± 0.67 |
| Water solubility index (WSI) (%) | 20.45 ± 0.94 |
| Swelling capacity (mL/g) | 6.82 ± 0.57 |
| Foaming capacity (%) | 13.45 ± 0.82 |
| Foam stability (%) | 57.72 ± 0.16 |
| Oil absorption capacity (OAC) (g/g) | 4.03 ± 0.23 |
| Sample | Time (min) | Moisture (%) | TPC (mg GAE/g) | TFC (mg QE/g) | Antioxidant (%) |
|---|---|---|---|---|---|
| Bougainvillea spectabilis Willd. bracts powder | 60 | 80 ± 0.002 a | 3.5 ± 0.002 a | 23.6 ± 0.002 a | 87.5 ± 0.002 a |
| 120 | 63.7 ± 0.002 a | 3.4 ± 0.007 b | 21.3 ± 0.002 a | 88.3 ± 0.006 b | |
| 180 | 47.7 ± 0.002 a | 3.2 ± 0.001 a | 14.1 ± 0.002 a | 86.7 ± 0.005 b | |
| 240 | 30 ± 0.001 a | 3.0 ± 0.002 a | 13.0 ± 0.002 a | 87.5 ± 0.001 a | |
| 300 | 14.7 ± 0.002 a | 2.9 ± 0.002 a | 12.3 ± 0.002 a | 89.9 ± 0.007 b |
| Sample | % of Inhibition of Lipase | IC50 | % of Inhibition of Amylase | IC50 |
|---|---|---|---|---|
| Bougainvillea spectabilis Willd. bracts extract | 81.09 ± 0.92 | 68.21 | 74.24 ± 0.88 | 60.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Kaushik, D.; Kaur, J.; Proestos, C.; Oz, F.; Kumar, A.; Anjali, A.; Elobeid, T.; Terzioğlu, M.E.; Xiao, J. Assessment of Anti-Obesity Potential and Techno-Functional Properties of Bougainvillea spectabilis Willd. Bracts. Separations 2022, 9, 399. https://doi.org/10.3390/separations9120399
Kumar M, Kaushik D, Kaur J, Proestos C, Oz F, Kumar A, Anjali A, Elobeid T, Terzioğlu ME, Xiao J. Assessment of Anti-Obesity Potential and Techno-Functional Properties of Bougainvillea spectabilis Willd. Bracts. Separations. 2022; 9(12):399. https://doi.org/10.3390/separations9120399
Chicago/Turabian StyleKumar, Mukul, Deepika Kaushik, Jasjit Kaur, Charalampos Proestos, Fatih Oz, Ashwani Kumar, Anjali Anjali, Tahra Elobeid, Murat Emre Terzioğlu, and Jianbo Xiao. 2022. "Assessment of Anti-Obesity Potential and Techno-Functional Properties of Bougainvillea spectabilis Willd. Bracts" Separations 9, no. 12: 399. https://doi.org/10.3390/separations9120399
APA StyleKumar, M., Kaushik, D., Kaur, J., Proestos, C., Oz, F., Kumar, A., Anjali, A., Elobeid, T., Terzioğlu, M. E., & Xiao, J. (2022). Assessment of Anti-Obesity Potential and Techno-Functional Properties of Bougainvillea spectabilis Willd. Bracts. Separations, 9(12), 399. https://doi.org/10.3390/separations9120399









