Molecular Docking Studies on Methanolic Propolis Extracts Collected from Different Regions in Saudi Arabia as a Potential Inhibitor of Topoisomerase IIβ
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Sample Collection and Preparation
2.2. Cytotoxicity Assay
2.2.1. Cell Lines
2.2.2. Cell Viability
2.2.3. Statistical Analysis
2.3. Methanolic Propolis Extract Analysis by HPLC
2.4. Molecular Docking of the Propolis Extracted Compounds
3. Results
3.1. Cytotoxicity Assay
3.2. HPLC Analysis of Propolis Methanolic Extracts
3.3. Molecular Docking of the Propolis Extracted Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, J.F.; Dos Santos, H.F.; Bonamigo, T.; de Campos Domingues, N.L.; de Picoli Souza, K.; Dos Santos, E.L. Stingless Bee Propolis: New Insights for Anticancer Drugs. Oxidative Med. Cell. Longev. 2021, 2021, 2169017. [Google Scholar] [CrossRef] [PubMed]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid. -Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2010, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Propolis—Review. BeeWorld 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Coggshall, W.L.; Morse, R.A. Beeswax: Production, Harvesting, Processing and Products; Wicwas Press: Ithaca, NY, USA, 1984. [Google Scholar]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; El-Samad, G.A.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of Agronomic Traits, Nutrient Uptake, Osmolytes and Antioxidants of Maize as Influenced by Exogenous Potassium Silicate under Deficit Irrigation and Semiarid Conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Stan, L.; Liviu, A.; Dezmirean, D. Quality Criteria for Propolis Standardization. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 137–140. [Google Scholar]
- Katekhaye, S.; Fearnley, H.; Fearnley, J.; Paradkar, A. Gaps in propolis research: Challenges posed to commercialization and the need for a holistic approach. J. Apic. Res. 2019, 58, 604–616. [Google Scholar] [CrossRef]
- Abou-Shaara, H.; Alashaal, S.A.; Hosni, E.M.; Nasser, M.G.; Ansari, M.J.; Alharbi, S.A. Modeling the Invasion of the Large Hive Beetle, Oplostomus fuligineus, into North Africa and South Europe under a Changing Climate. Insects 2021, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Hosni, E.M.; Nasser, M.; Al-Khalaf, A.A.; Al-Shammery, K.A.; Al-Ashaal, S.; Soliman, D. Invasion of the Land of Samurai: Potential Spread of Old-World Screwworm to Japan under Climate Change. Diversity 2022, 14, 99. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Nasser, M.G.; Abou-Shaara, H.F.; Radwan, M.H. Modeling the Potential Global Distribution of Honeybee Pest, Galleria mellonella under Changing Climate. Insects 2022, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Hosni, E.M.; Nasser, M.G.; Al-Ashaal, S.A.; Rady, M.H.; Kenawy, M.A. Modeling current and future global distribution of Chrysomya bezziana under changing climate. Sci. Rep. 2020, 10, 4947. [Google Scholar] [CrossRef] [PubMed]
- Barbarić, M.; Mišković, K.; Bojić, M.; Lončar, M.B.; Smolčić-Bubalo, A.; Debeljak, Ž.; Medić-Šarić, M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef]
- World Health Organization. Cancer: Key Facts. 2018. Available online: https://www.who.int/newsroom/fact-sheets/detail/cancer (accessed on 1 November 2020).
- International Agency for Research on Cancer. Cancer Fact Sheets. World Health Organization, 2018. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 1 November 2020).
- International Agency for Research on Cancer. Indonesia Global Cancer Observatory. World Health Organization, 2018. Available online: https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf (accessed on 1 November 2020).
- Rohs, R.; Bloch, I.; Sklenar, H.; Shakked, Z. Molecular flexibility in ab-initio drug docking to DNA: Binding-site and binding-mode transitions in all-atom Monte Carlo simulations. Nucleic Acids Res. 2005, 33, 7048–7057. [Google Scholar] [CrossRef]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor-ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Juan-Borrás, M. Standardizing the analysis of phenolic profile in propolis. Food Res. Int. 2018, 106, 834–841. [Google Scholar] [CrossRef]
- Hussain, R.F.; Nouri, A.M.; Oliver, R.T. A new approach for measurement of cytotoxicity using colorimetric assay. J. Immunol. Methods 1993, 160, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Claeson, P.; Tuchinda, P.; Reutrakuv, V. Some empirical aspects on the practical use of flash chromatography and medium pressure uquid chromatography for the isolation of biologically active compounds from plants. ScienceAsia 1993, 19, 073. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. Beneficial Features of Biochar and Arbuscular Mycorrhiza for Improving Spinach Plant Growth, Root Morphological Traits, Physiological Properties, and Soil Enzymatic Activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, R.; Borgonovo, G.; Leoni, V.; Giupponi, L.; Ceciliani, G.; Sala, S.; Bassoli, A.; Giorgi, A. Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy. Molecules 2020, 25, 504. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, R.; Vali, L.; Watson, D.; Fearnley, J.; Seidel, V. Antimethicillin-resistant Staphylococcus aureus (MRSA) activity of ‘pacific propolis’ and isolated prenylflavanones. Phytother. Res. 2010, 24, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Kardar, M.N.; Zhang, T.; Coxon, G.D.; Watson, D.G.; Fearnley, J.; Seidel, V. Characterisation of triterpenes and new phenolic lipids in Cameroonian propolis. Phytochemistry 2014, 106, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2020-1628-41379-56382 (accessed on 1 November 2022).
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin Biopriming Positively Stimulates Antioxidants Defense, Osmolytes Metabolism and Ionic Status to Confer Salt Stress Tolerance in Soybean. Acta Physiol. Plant. 2020, 42, 114. [Google Scholar] [CrossRef]
- Chiu, H.; Han, Y.; Shen, Y.; Golovinskaia, O.; Venkatakrishnan, K.; Wang, C. Chemopreventive and Chemotherapeutic Effect of Propolis and Its Constituents:A Mini-review. J. Cancer Prev. 2020, 25, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Ramadhan, R.; Khairunnisa, B.; Amen, Y.; Matsumoto, M.; Nagata, M.; Kusuma, I.W.; Paramita, S.; Sukemi; Yadi; et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J. Biol. Sci. 2021, 28, 7182–7189. [Google Scholar] [CrossRef]
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 cells changing the course of breast cancer research and care for 45 years. J. Natl. Cancer Inst. 2015, 107, djv073. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Ichwan, S.J.A.; Soundharrajan, I.; Govindan, N. Nutraceuticals as potential therapeutic agents for coloncancer: A review. Acta Pharm. Sin. 2014, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.H.; Abdel-Kader, D.Z.; El Elish, A.M. Role of Heat Shock and Salicylic Acid in Antioxidant Homeostasis in Mungbean (Vigna radiata L.) Plant Subjected to Heat Stress. Am. J. Plant Physiol. 2007, 2, 344–355. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Phuwapraisirisan, P.; Puthong, S.; Palaga, T.; Arung, E.T.; Chanchao, C. Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 6581–6589. [Google Scholar] [CrossRef]
- Nikzad, S.; Baradaran-Ghahfarokhi, M.; Nasri, P. Dose-response modeling using MTT assay: A short review. Life Sci. J. 2014, 11, 432–437. [Google Scholar]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell proliferation and cytotoxicity assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.-D.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-based medicinal properties of stingless bee products: Recent progress and future directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef]
- Alnusairi, G.S.H.; Mazrou, Y.S.A.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.M.; Elnahhas, N. Exogenous Nitric Oxide Reinforces Photosynthetic Efficiency, Osmolyte, Mineral Uptake, Antioxidant, Expression of Stress-Responsive Genes and Ameliorates the Effects of Salinity Stress in Wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Molecular docking studies on thirteen fluoroquinolines with human topoisomerase II a and b. Silico Pharmacol. 2017, 5, 4. [Google Scholar] [CrossRef]
- Ayyamperumal, S.; Dhananjay, D.J.; Tallapaneni, V.; Mohan, S.; Basappa, S.; Selvaraj, J.; Joghee, N.M.; Chandrasekar, M.J. Molecular docking analysis of α-Topoisomerase II with δ-Carboline derivatives as potential anti-cancer agents. Bioinformation 2021, 17, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Drwal, M.N.; Agama, K.; Pommier, Y.; Griffith, R. Development of purely structure-based pharmacophores for the topoisomerase I-DNA-ligand binding pocket. J. Comput. Aided Mol. Des. 2013, 27, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, V.; Moro, S. Bridging molecular docking to molecular dynamics in exploring ligand-protein recognition process: An overview. Front. Pharmacol. 2018, 9, 923. [Google Scholar] [CrossRef] [PubMed]
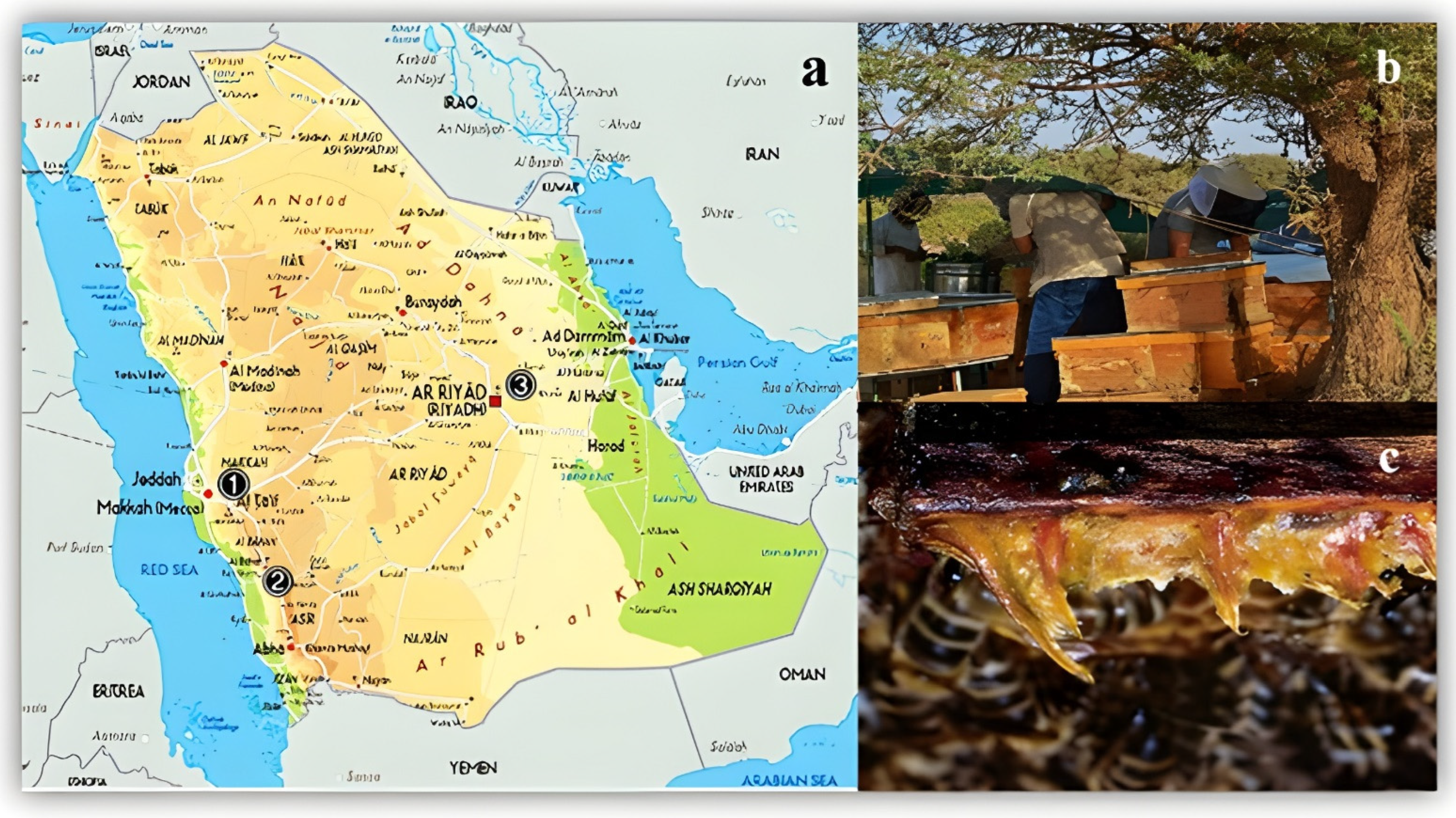
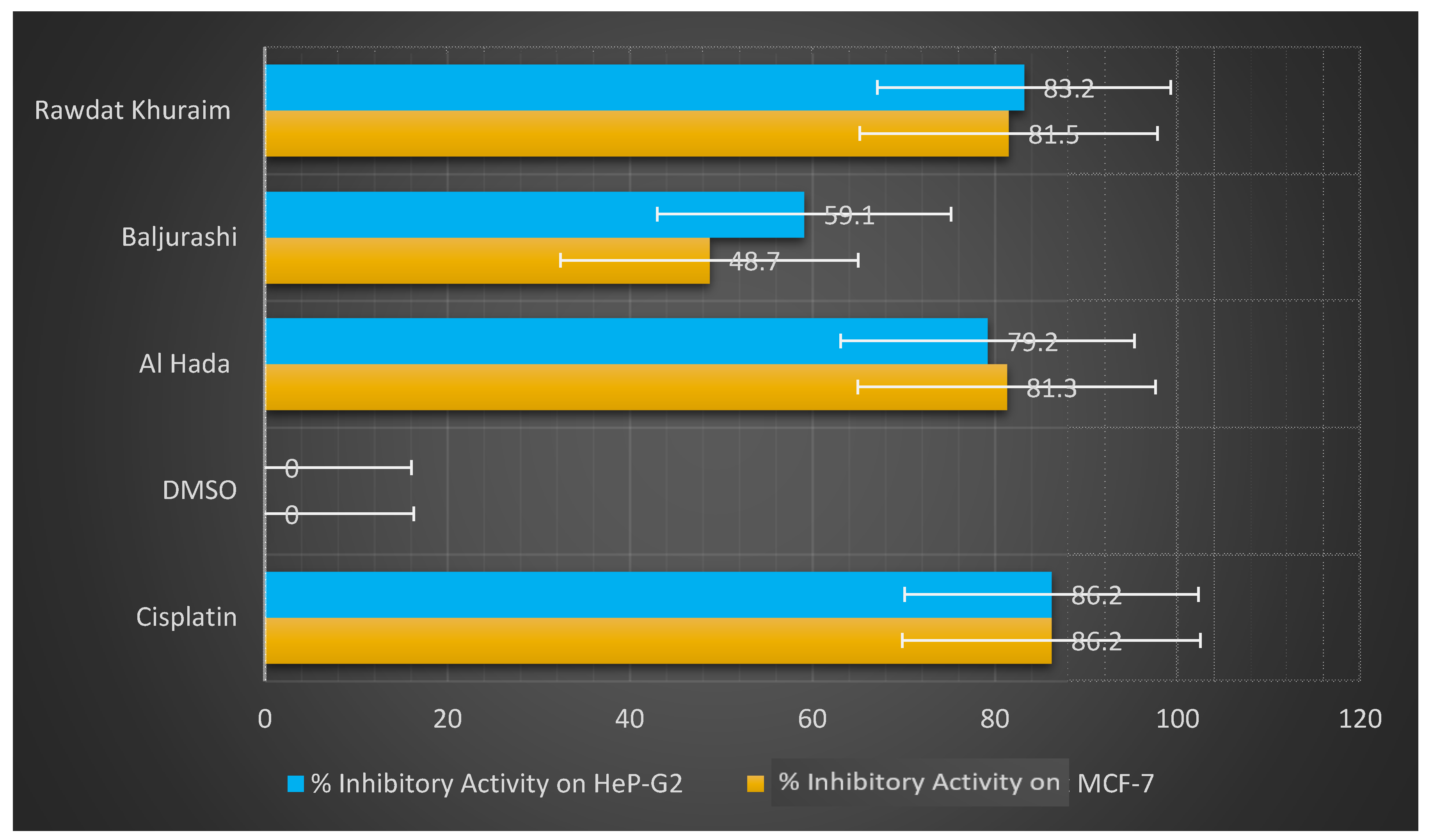
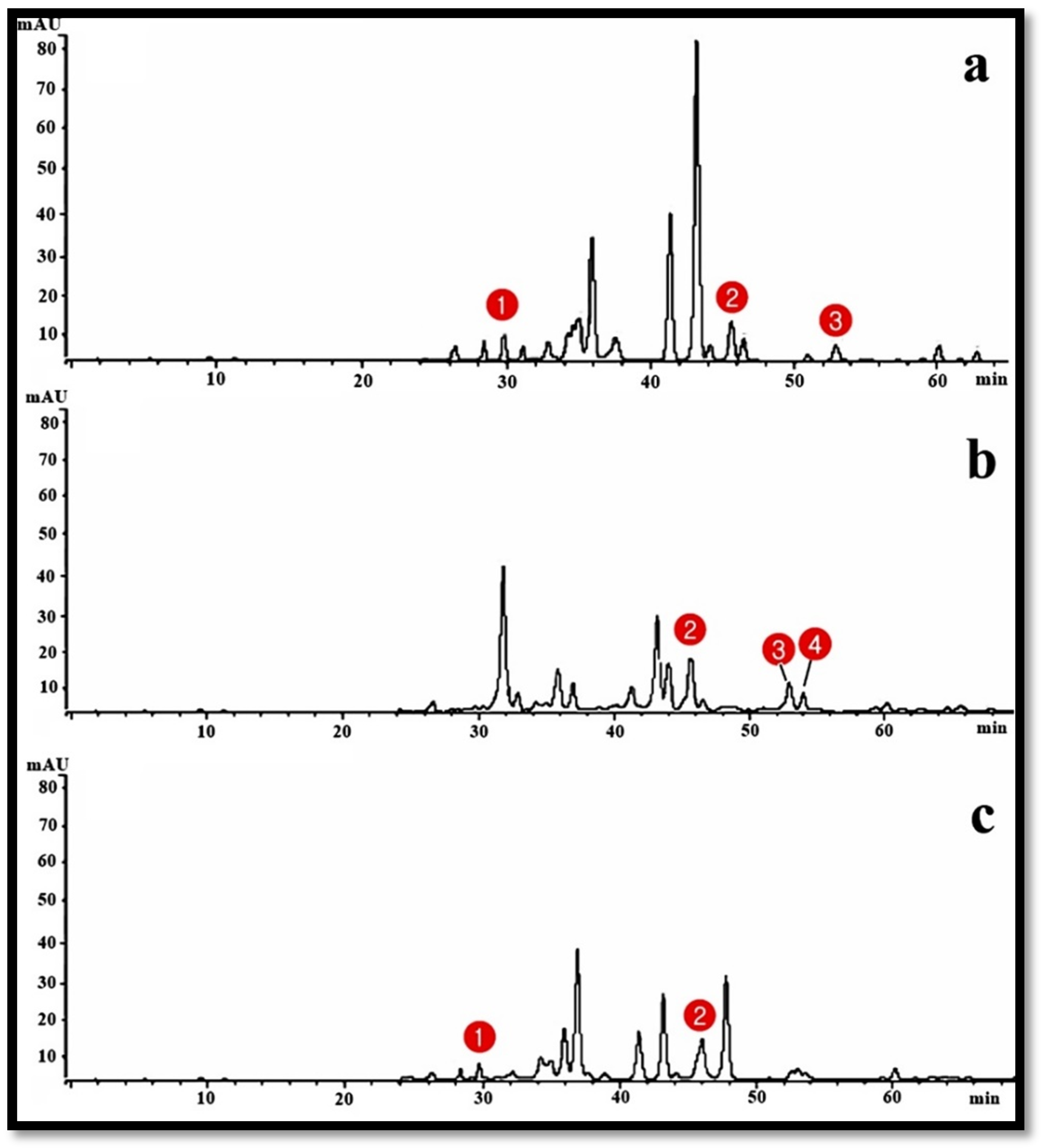
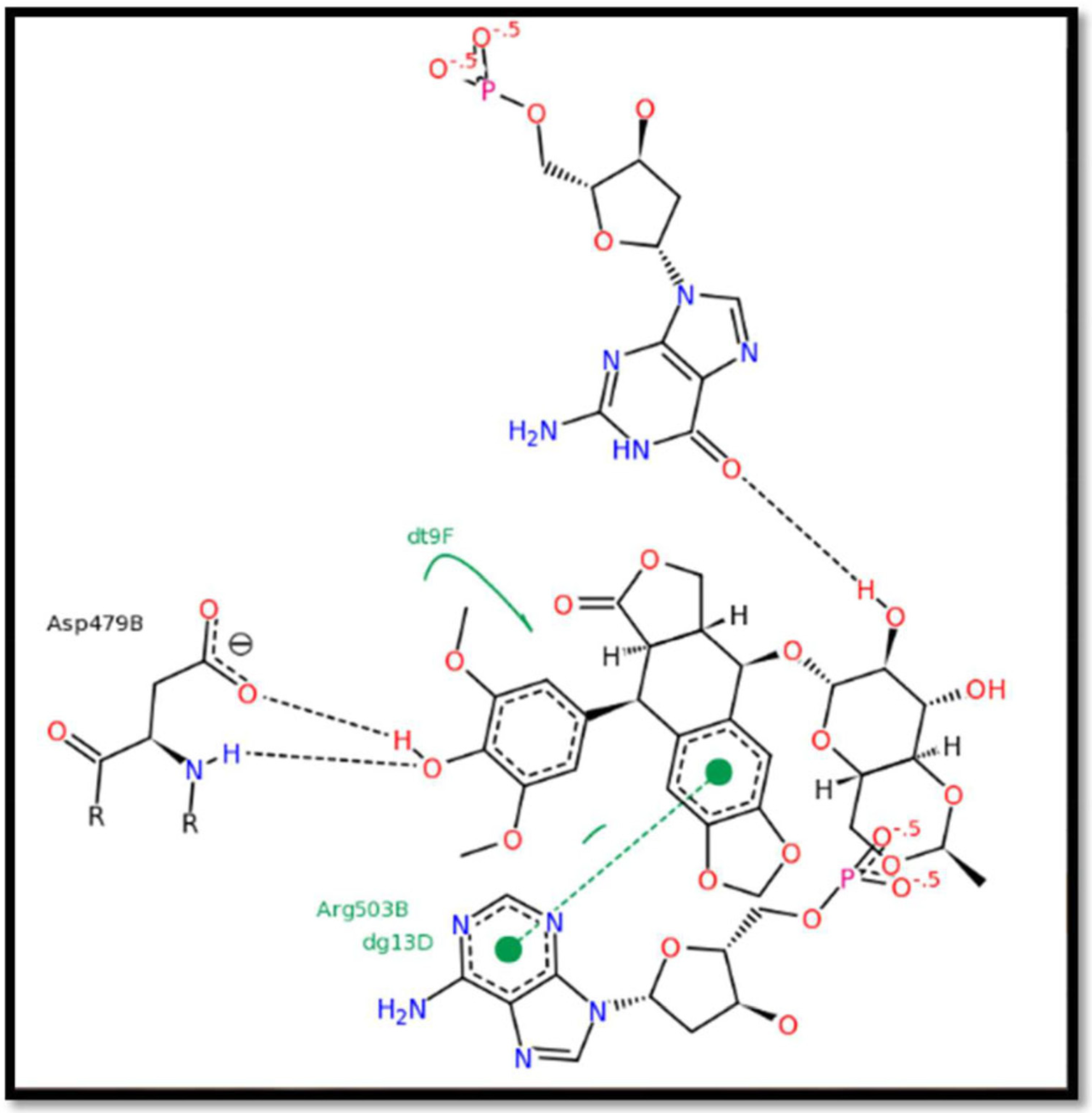
| Treatment | % Inhibitory Activity on MCF-7 | % Inhibitory Activity on HeP-G2 | |
|---|---|---|---|
| Cisplatin (positive control) | 86.2 | 86.2 | |
| DMSO (negative control) | 0 | 0 | |
| Propolis Sample | Al Hada site | 81.3 | 79.2 |
| Baljurashi site | 48.7 | 59.1 | |
| Rawdat Khuraim site | 81.5 | 83.2 | |
| Peak No. | Retention Time | Compounds | Propolis Samples | ||
|---|---|---|---|---|---|
| Al Hada | Rawdat Khuraim | Baljurashi | |||
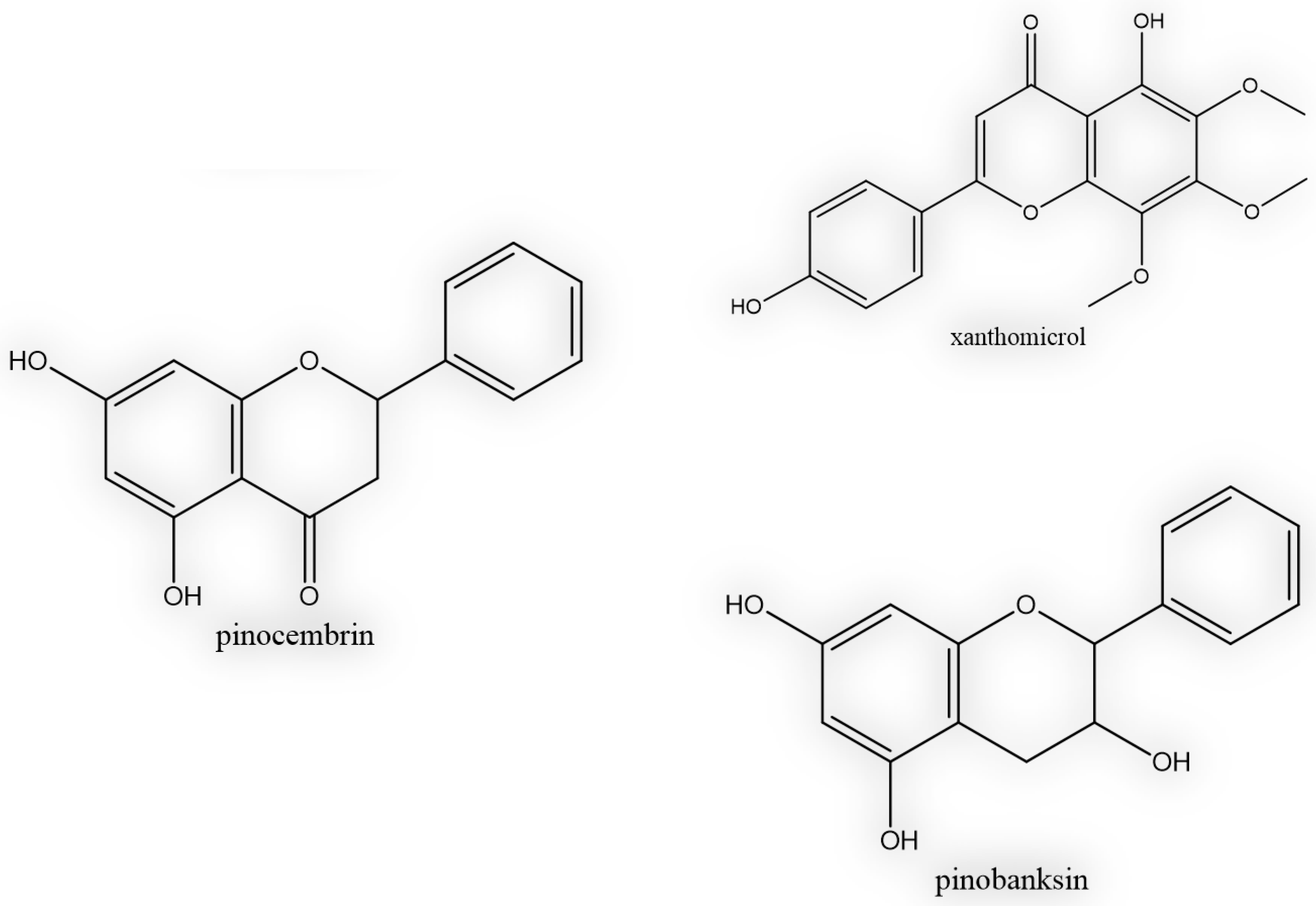
| 1 | 30 | Pinobanksin | + | - | + |
| 2 | 46 | Pinocembrin | + | + | + |
| 3 | 53 | Xanthomicrol | + | + | - |
| 4 | 54 | Galangin | - | + | - |
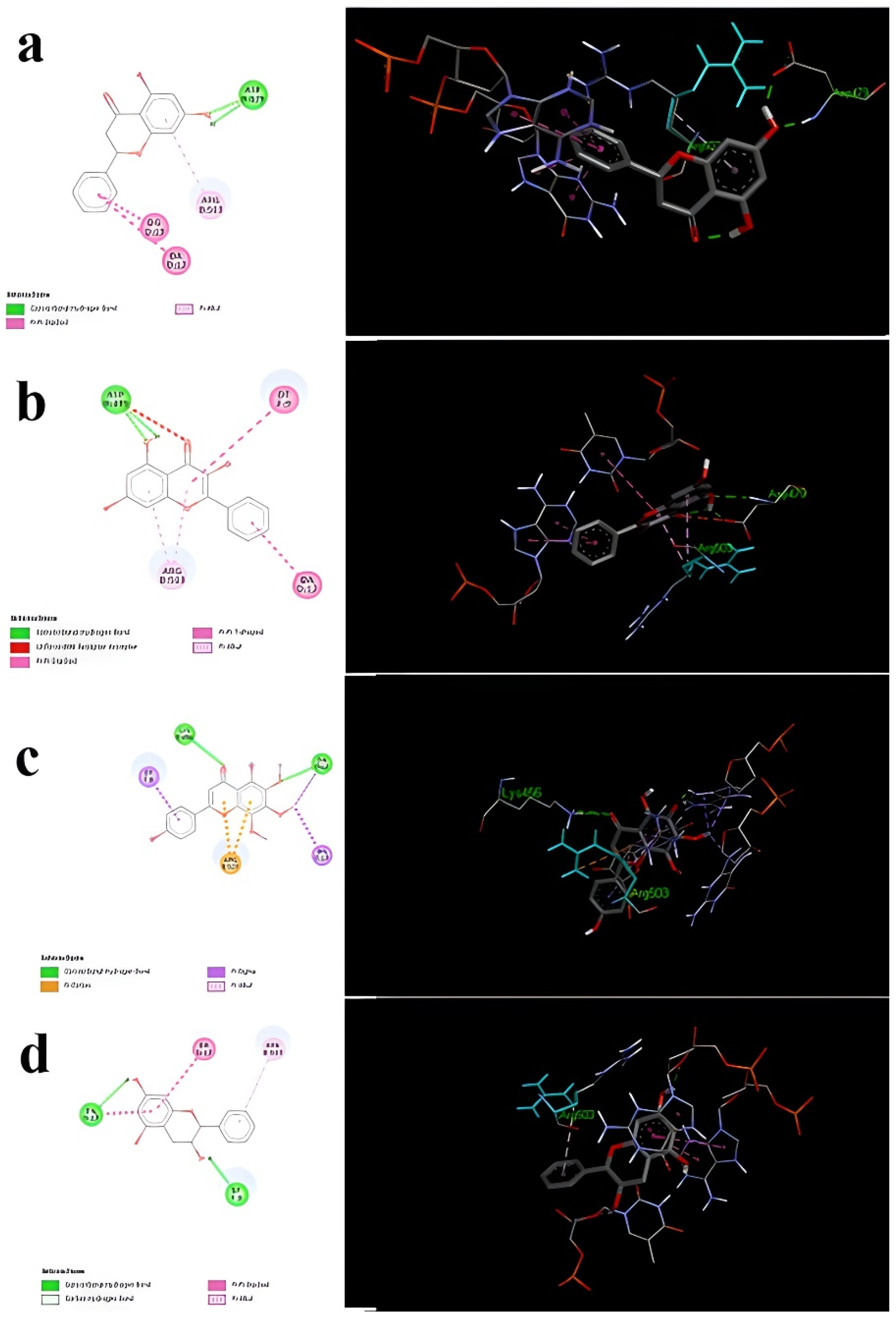
| Active Compounds in Propolis Samples | Binding Energy | Inhibition Constant (ki) | Amino Acids Involved in the Bond with the Ligand | Number of Hydrogen Bonds |
|---|---|---|---|---|
| Pinocembrin | −7.06 | 6.67 | Asp 479–Arg 503 | Formed (2) |
| Pinobanksin | −6.74 | 11.47 | Arg 503 | Formed with non-amino acids |
| Galangin | −6.25 | 26.23 | Asp479–Arg503 | Formed (2) |
| Xanthomicrol | −6.34 | 22.35 | Lys486–Arg503 | Formed (only one with crucial amino acids) |
| Control (ref original cpd. Docking only) (etoposide) | −6.43 | 19. 27 | Asp 479–Arg 503 | Formed (2) |
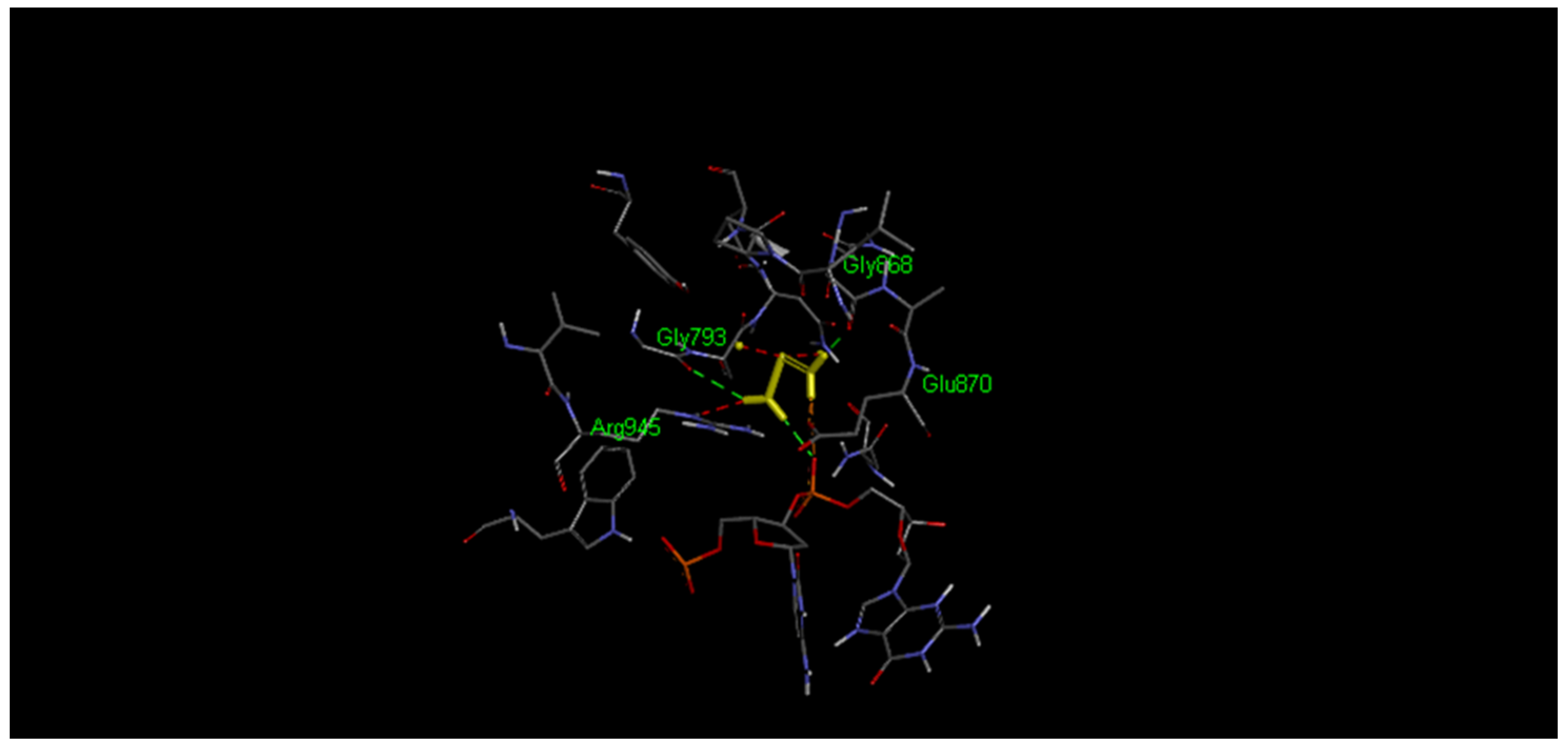
| Cisplatin (ref. experimentally) | −2.46 | 15.7 | Gly793-Gly868 | Formed with non-crucial amino acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khalaf, A.A.; Alabdelkareem, I.; Al-Rejaie, S.S.; Mohany, M.; Hozzein, W.N. Molecular Docking Studies on Methanolic Propolis Extracts Collected from Different Regions in Saudi Arabia as a Potential Inhibitor of Topoisomerase IIβ. Separations 2022, 9, 392. https://doi.org/10.3390/separations9120392
Al-Khalaf AA, Alabdelkareem I, Al-Rejaie SS, Mohany M, Hozzein WN. Molecular Docking Studies on Methanolic Propolis Extracts Collected from Different Regions in Saudi Arabia as a Potential Inhibitor of Topoisomerase IIβ. Separations. 2022; 9(12):392. https://doi.org/10.3390/separations9120392
Chicago/Turabian StyleAl-Khalaf, Areej A., Ibrahim Alabdelkareem, Salim S. Al-Rejaie, Mohamed Mohany, and Wael N. Hozzein. 2022. "Molecular Docking Studies on Methanolic Propolis Extracts Collected from Different Regions in Saudi Arabia as a Potential Inhibitor of Topoisomerase IIβ" Separations 9, no. 12: 392. https://doi.org/10.3390/separations9120392
APA StyleAl-Khalaf, A. A., Alabdelkareem, I., Al-Rejaie, S. S., Mohany, M., & Hozzein, W. N. (2022). Molecular Docking Studies on Methanolic Propolis Extracts Collected from Different Regions in Saudi Arabia as a Potential Inhibitor of Topoisomerase IIβ. Separations, 9(12), 392. https://doi.org/10.3390/separations9120392








