Comparative Study of the Phytochemical Profiles of the Rhizomes of Cultivated and Wild-Grown Polygonatum sibiricum
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Sample Extraction
2.4. Ultra-High Performance Liquid Chromatography Analysis
2.5. Mass Spectrometry Analysis
2.6. Data Processing and Multivariate Statistical Analysis
3. Results
3.1. Method Validation
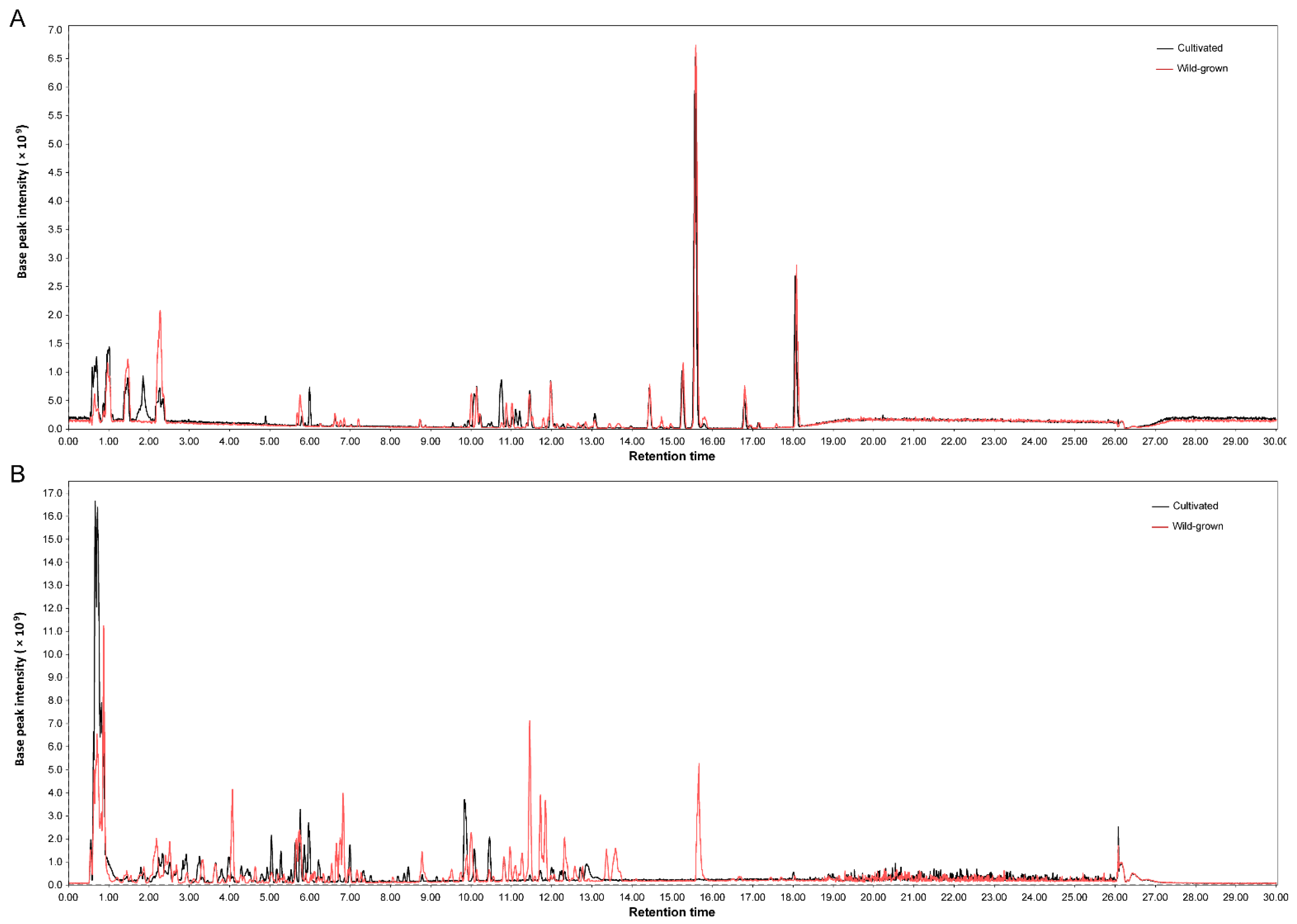
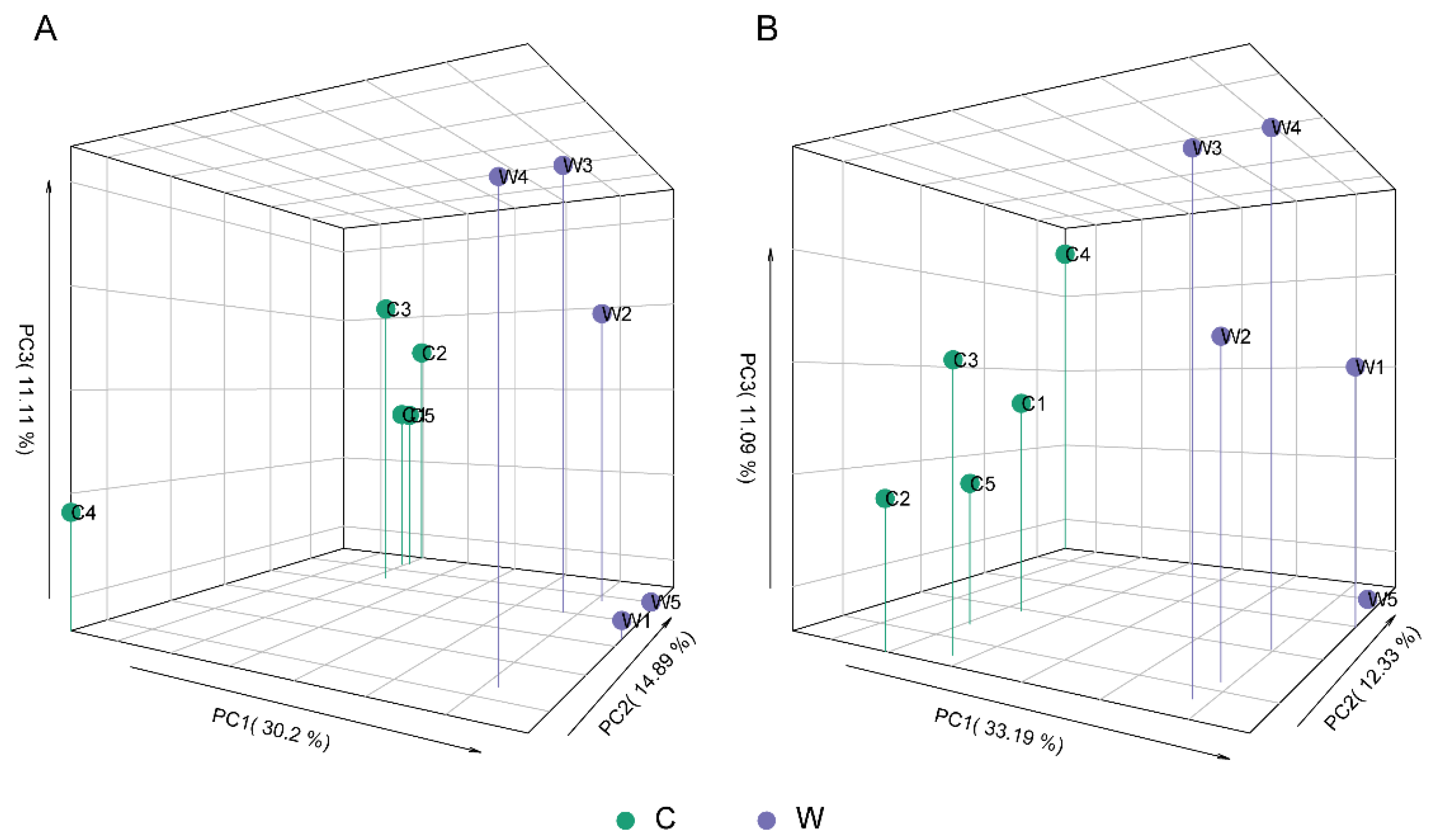
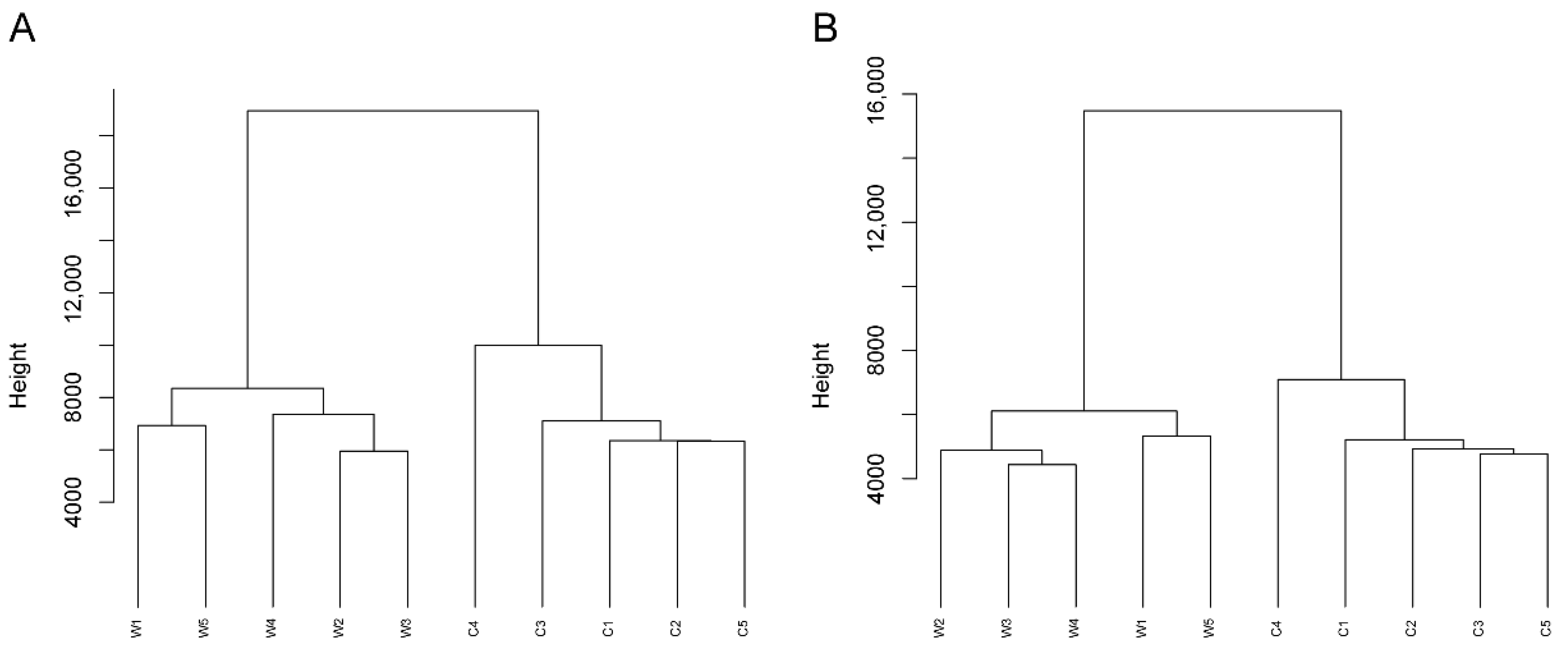
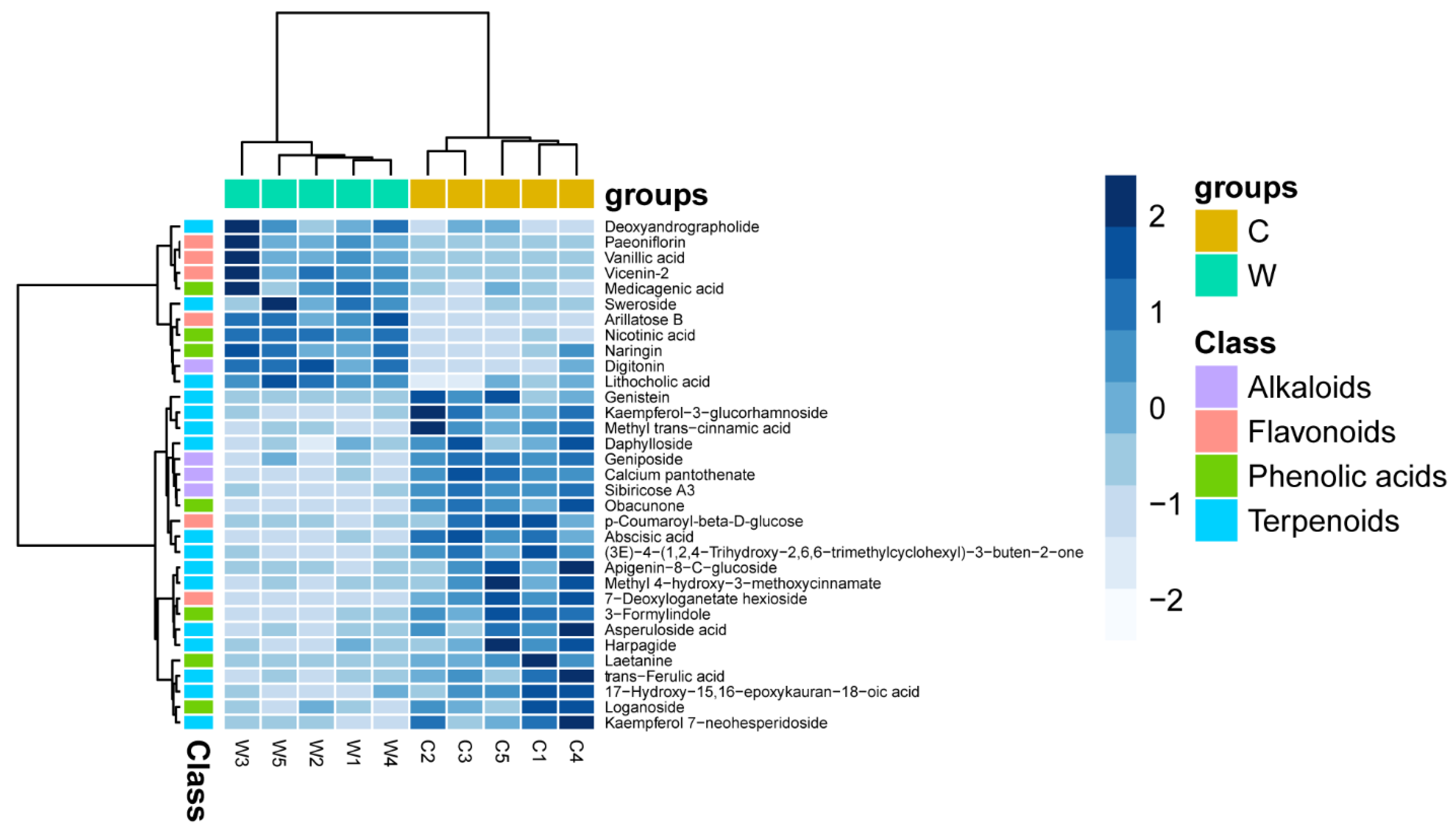
3.2. General Comparison of the Metabolic Profiles
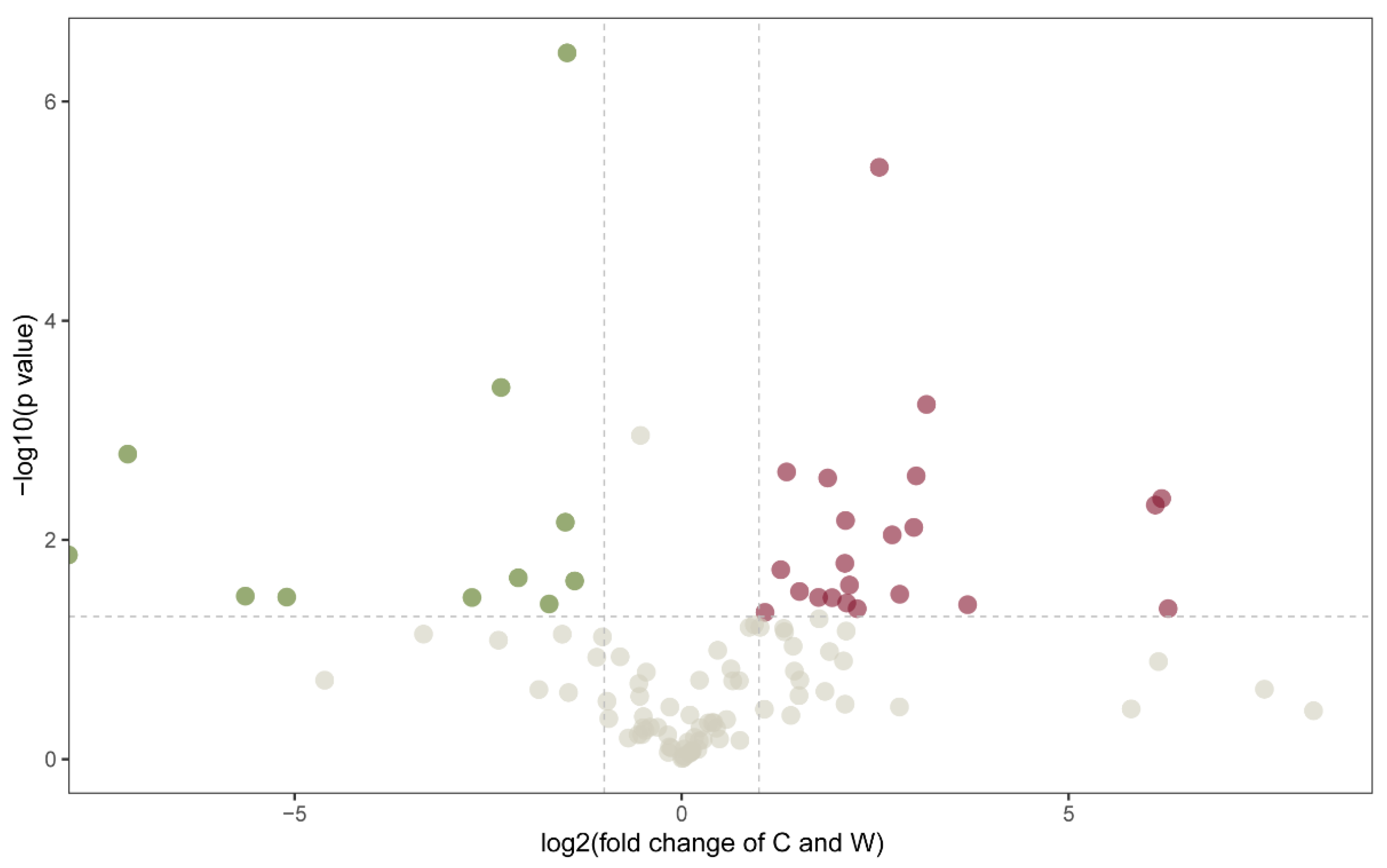
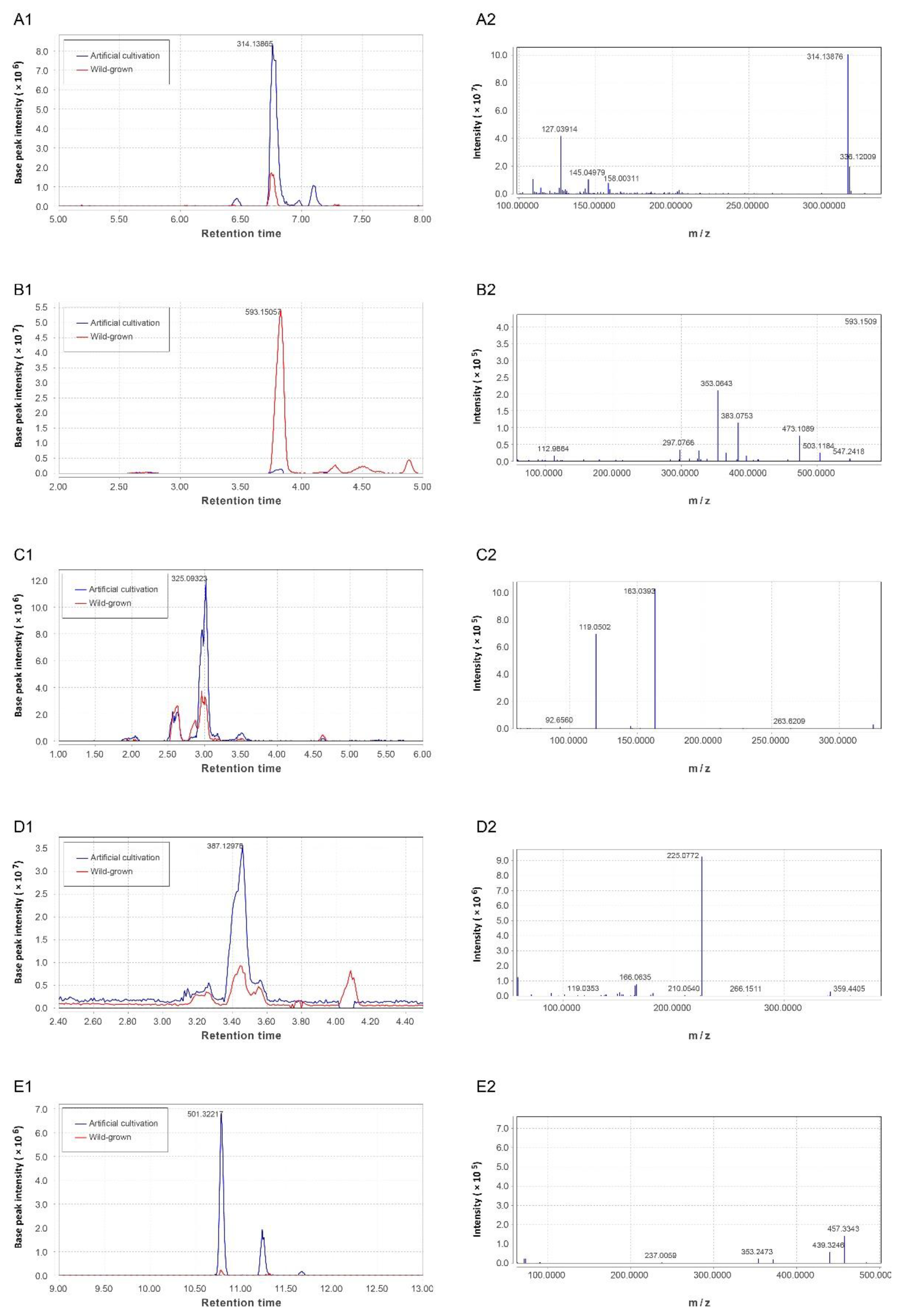
3.3. Identification of the Phytochemicals
3.4. Differentially Altered Alkaloids
3.5. Differentially Altered Flavonoids
3.6. Differentially Altered Phenolic Acids
3.7. Differentially Altered Terpenoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Ji, H.; Cheng, C.; Sun, Y.; Cheng, H.; Wang, D.; Pan, Y.; Liu, X. Comprehensive determination of the processing level of rhizome of Polygonatum sibiricum by macroscopic, micromorphological, and microscopic characterizations. Microsc. Res. Tech. 2022, 85, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jiang, J.-G. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Park, S.R.; Jo, J.E.; Lim, B.O. Antioxidant and anti-inflammatory activity of Polygonatum sibiricum rhizome extracts. Asian Pac. J. Trop. Dis. 2013, 3, 308–313. [Google Scholar] [CrossRef]
- Li, Y.-L. Isolation and identification of endophytic fungi from Polygonatum sibiricum in Mountain Tai and study on their antimicrobial activity. Chin. Tradit. Herb. Drugs 2013, 44, 1490–1494. [Google Scholar]
- Zhao, H.; Wang, Q.-L.; Hou, S.-B.; Chen, G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264. 7 macrophage cells. Nat. Prod. Res. 2019, 33, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Wu, S.; Huang, X.-L.; Hu, X.-Q.; Zhang, Y. Hypolipidemic activity and antiatherosclerotic effect of polysaccharide of Polygonatum sibiricum in rabbit model and related cellular mechanisms. Evid.-Based Complement. Altern. Med. 2015, 2015, 391065. [Google Scholar] [CrossRef]
- Chai, Y.; Luo, J.; Bao, Y. Effects of Polygonatum sibiricum saponin on hyperglycemia, gut microbiota composition and metabolic profiles in type 2 diabetes mice. Biomed. Pharmacother. 2021, 143, 112155. [Google Scholar] [CrossRef]
- Luo, J.; Chai, Y.; Zhao, M.; Guo, Q.; Bao, Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020, 11, 4327–4338. [Google Scholar] [CrossRef]
- Long, T.; Liu, Z.; Shang, J.; Zhou, X.; Yu, S.; Tian, H.; Bao, Y. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-κB signaling pathways. Int. J. Biol. Macromol. 2018, 111, 813–821. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, D.; Sun, T.; Lin, W. Quantitative Determination of Total Flavonoids from Polygonatum sibiricum by Spectrophotometry. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 10–12 October 2019; p. 022126. [Google Scholar]
- Hu, C.-Y.; Xu, D.-P.; Wu, Y.-M.; Ou, S.-Y. Triterpenoid saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2010, 12, 801–808. [Google Scholar] [CrossRef]
- Tang, C.; Yu, Y.-M.; Qi, Q.-L.; Wu, X.-D.; Wang, J.; Tang, S.-A. Steroidal saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2019, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-R.; Li, X.; Wang, S.-X. Two new alkaloids from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2005, 7, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Liu, Y.; Zhang, Y.-Q.; Huang, J.-H.; Shao, J.-H.; Zhu, J.-Z.; Zhao, C.-C. A New Phenolic Glycoside from Polygonatum sibiricum and its α-Glucosidase Inhibitory Activity. Chem. Nat. Compd. 2021, 57, 50–52. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J. Chemical constituents of the genus Polygonatum and their role in medicinal treatment. Nat. Prod. Commun. 2015, 10, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Lin, J.; Cheng, W.; Liu, Z.; Yu, T.; Yang, B. UHPLC-Q-Orbitrap-MS-Based Metabolomics Reveals Chemical Variations of Two Types of Rhizomes of Polygonatum sibiricum. Molecules 2022, 27, 4685. [Google Scholar] [CrossRef]
- Xie, H.; Wang, H.; Chen, B.; Lou, J.; Wang, H.; Xiong, Y.; Hu, Y.; Xu, X.; Jing, Q.; Jiang, M. Untargeted metabolomics analysis to unveil the chemical markers for the differentiation among three Gleditsia sinensis-derived herbal medicines by ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Arab. J. Chem. 2022, 15, 103762. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.; Vincken, J.-P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, B.; Liu, Y.; Fang, L.; Yin, B.; Sun, Y.; Ma, M.; Huang, Y.; Zhu, Y.; Zhang, Y. Unique Biomarker Characteristics in Gestational Diabetes Mellitus Identified by LC-MS-Based Metabolic Profiling. J. Diabetes Res. 2021, 2021, 6689414. [Google Scholar] [CrossRef]
- Augustijn, D.; de Groot, H.J.; Alia, A. HR-MAS NMR applications in plant metabolomics. Molecules 2021, 26, 931. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants metabolome study: Emerging tools and techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Qi, J.; Wei, J.; Liao, D.; Ding, Z.; Yao, X.; Sun, P.; Li, X. Untargeted Metabolomics Analysis Revealed the Major Metabolites in the Seeds of four Polygonatum Species. Molecules 2022, 27, 1445. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Zhang, X.; Li, G.; dong Li, X.; Chang, H.; Niu, J.; Wang, Z. Metabolomics Study of Different Germplasm Resources for Three Polygonatum Species Using UPLC-Q-TOF-MS/MS. Front. Plant Sci. 2022, 13, 826902. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, Y.; Yang, B.; Wang, X.; Huang, L. Separation of alkaloids from herbs using high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.; Fadaeinasab, M.; Taha, H.; Widyawaruyanti, A.; Nafiah, M.A.; Rachmatiah, T. Aporphine alkaloids with in vitro antiplasmodial activity from the leaves of Phoebe tavoyana. J. Asian Nat. Prod. Res. 2020, 22, 52–60. [Google Scholar] [CrossRef] [PubMed]
- E Middleton, M.D., Jr. Biological properties of plant flavonoids: An overview. Int. J. Pharmacogn. 1996, 34, 344–348. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Fossen, T.; Brede, C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2020, 309, 125678. [Google Scholar] [CrossRef]
- Marrassini, C.; Davicino, R.; Acevedo, C.; Anesini, C.; Gorzalczany, S.; Ferraro, G. Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J. Nat. Prod. 2011, 74, 1503–1507. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.b. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Luo, J.-G.; Han, C.; Xu, J.-F.; Kong, L.-Y. Bioassay-guided preparative separation of angiotensin-converting enzyme inhibitory C-flavone glycosides from Desmodium styracifolium by recycling complexation high-speed counter-current chromatography. J. Pharm. Biomed. Anal. 2015, 102, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2005; pp. 197–227. [Google Scholar]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Shan, M.; Yu, S.; Yan, H.; Guo, S.; Xiao, W.; Wang, Z.; Zhang, L.; Ding, A.; Wu, Q.; Li, S.F.Y. A review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules 2017, 22, 1689. [Google Scholar] [CrossRef]
- Costa, R.; Albergamo, A.; Pellizzeri, V.; Dugo, G. Phytochemical screening by LC-MS and LC-PDA of ethanolic extracts from the fruits of Kigelia africana (Lam.) Benth. Nat. Prod. Res. 2017, 31, 1397–1402. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Zhang, R.-Q.; Rahman, K.; Cao, Z.-X.; Zhang, H.; Peng, C. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid.-Based Complement. Altern. Med. 2019, 2019, 4925682. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Kim, C.Y.; Yoon, K.-D.; Ryu, M.Y.; Cheong, J.H.; Chin, Y.-W.; Kim, J. Steroidal Saponins from the Rhizomes of Polygonatum sibiricum. J. Nat. Prod. 2006, 69, 360–364. [Google Scholar] [CrossRef]
- Xing-Cong, L.; Chong-Ren, Y.; Makoto, I.; Hiromichi, M.; Ryoji, K.; Kazuo, Y. Steroid saponins from Polygonatum kingianum. Phytochemistry 1992, 31, 3559–3563. [Google Scholar] [CrossRef]
- Jin, J.-M.; Zhang, Y.-J.; Li, H.-Z.; Yang, C.-R. Cytotoxic steroidal saponins from Polygonatum z anlanscianense. J. Nat. Prod. 2004, 67, 1992–1995. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Zhu, W.; Zhang, J.; Peng, P. Steroidal saponins from the rhizomes of Polygonatum odoratum. Nat. Prod. Res. 2009, 23, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; de Souza Araújo, A.A.; da Silva Almeida, J.R.G.; Thangaraj, P.; Júnior, L.J.Q.; Quintans, J.D.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2021, 96, 153842. [Google Scholar] [CrossRef] [PubMed]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Cheng, W.; Liu, Z.; Wu, W.; Yang, B.; Lin, J. Comparative Study of the Phytochemical Profiles of the Rhizomes of Cultivated and Wild-Grown Polygonatum sibiricum. Separations 2022, 9, 398. https://doi.org/10.3390/separations9120398
Pan Z, Cheng W, Liu Z, Wu W, Yang B, Lin J. Comparative Study of the Phytochemical Profiles of the Rhizomes of Cultivated and Wild-Grown Polygonatum sibiricum. Separations. 2022; 9(12):398. https://doi.org/10.3390/separations9120398
Chicago/Turabian StylePan, Zhibin, Weiqing Cheng, Zhibin Liu, Weibin Wu, Bin Yang, and Junhan Lin. 2022. "Comparative Study of the Phytochemical Profiles of the Rhizomes of Cultivated and Wild-Grown Polygonatum sibiricum" Separations 9, no. 12: 398. https://doi.org/10.3390/separations9120398
APA StylePan, Z., Cheng, W., Liu, Z., Wu, W., Yang, B., & Lin, J. (2022). Comparative Study of the Phytochemical Profiles of the Rhizomes of Cultivated and Wild-Grown Polygonatum sibiricum. Separations, 9(12), 398. https://doi.org/10.3390/separations9120398






