In Vitro Anti-Colorectal Cancer and Anti-Microbial Effects of Pinus roxburghii and Nauplius graveolens Extracts Modulated by Apoptotic Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts
2.2. Determination of Phytochemicals
2.2.1. Total Phenolic Content
2.2.2. Total Flavonoid Content
2.2.3. Total Carotenoids
2.3. Determination of Antioxidant Activity
2.3.1. DPPH Activity
- A control: Negative control absorbance.
- A sample: The tested compound absorbance.
2.3.2. ABTS Radical Scavenging Activity
- Abs0: Control sample absorbance
- Abs1: Extract sample absorbance
2.4. Assessment of Polyphenolic Compounds by HPLC
2.5. Cell Culture
2.5.1. Cell Viability Assay
2.5.2. Determination of IC50 Values
2.5.3. Selectivity Index (SI)
2.6. DNA Extraction
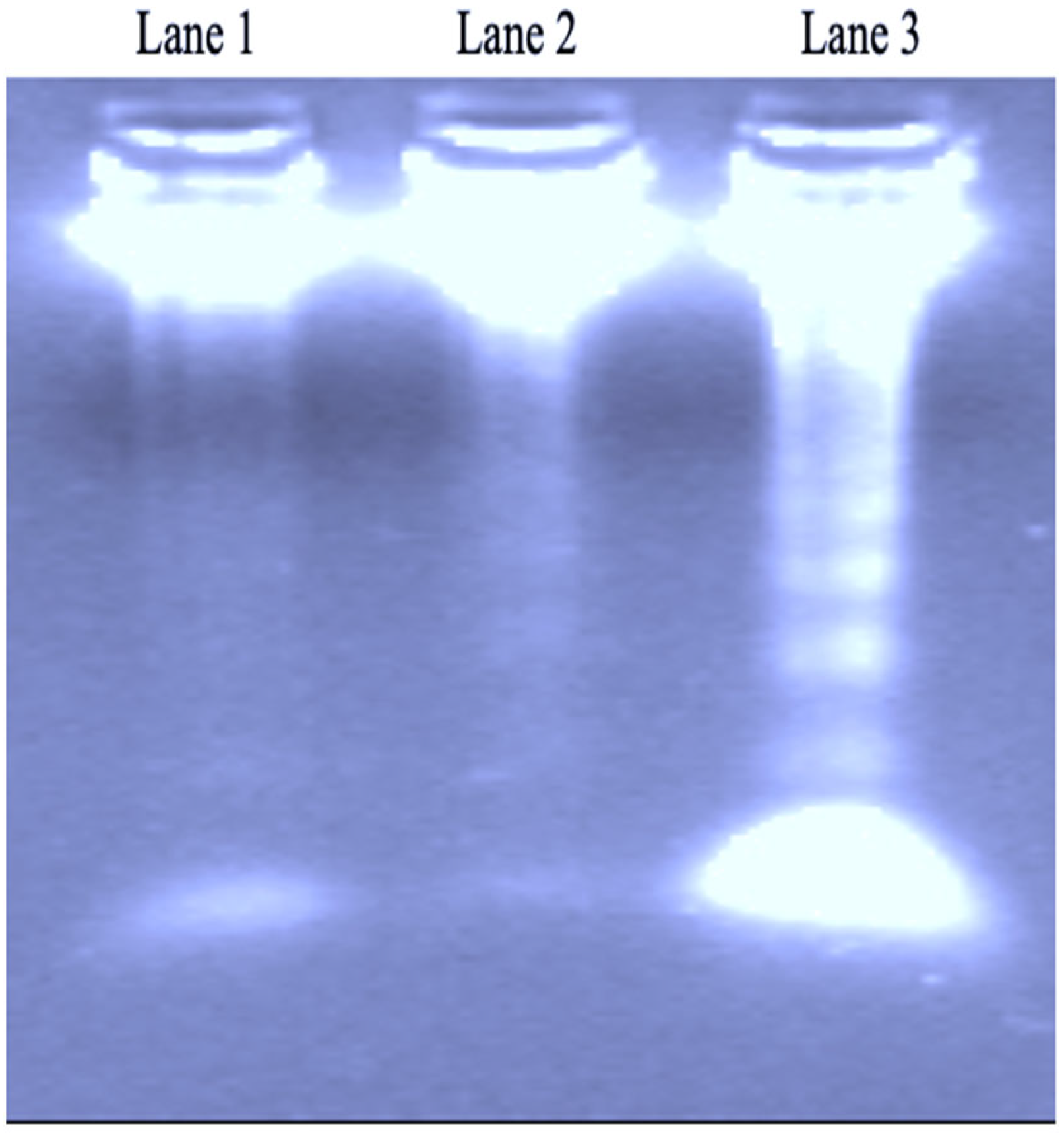
DNA Fragmentation Analysis
2.7. RNA Extraction and cDNA Synthesis
2.8. Quantitative Real-Time PCR
2.9. Antimicrobial Activity
2.9.1. Tested Microorganisms
2.9.2. Disc Diffusion Technique
2.9.3. Determination of Minimum Inhibitory Concentration (MIC)
2.10. Statistical Analyses
3. Results and Discussion
3.1. Total Phenolic Content and Antioxidant Activity
3.2. Identification of Bioactive Compounds Using HPLC
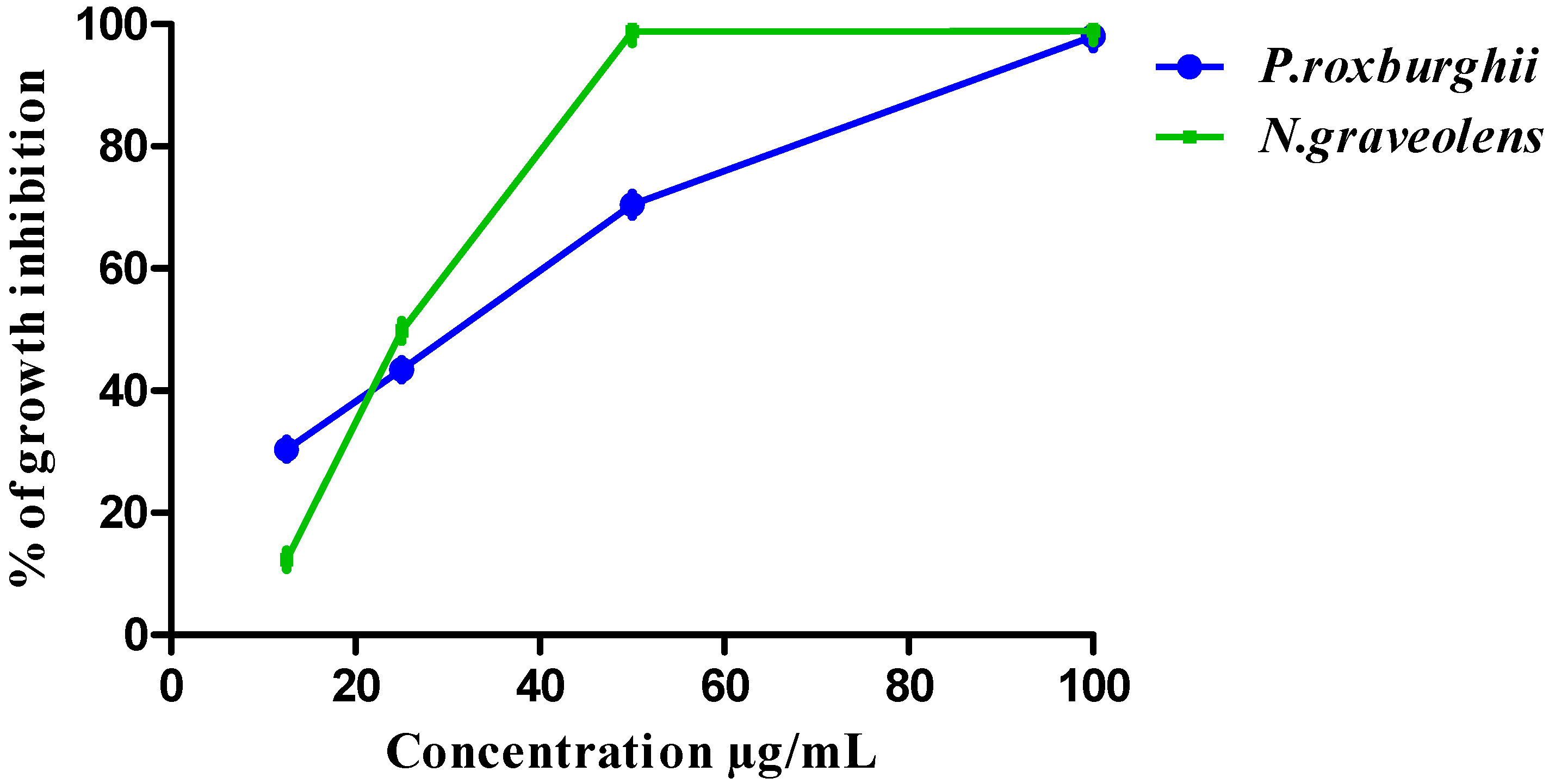
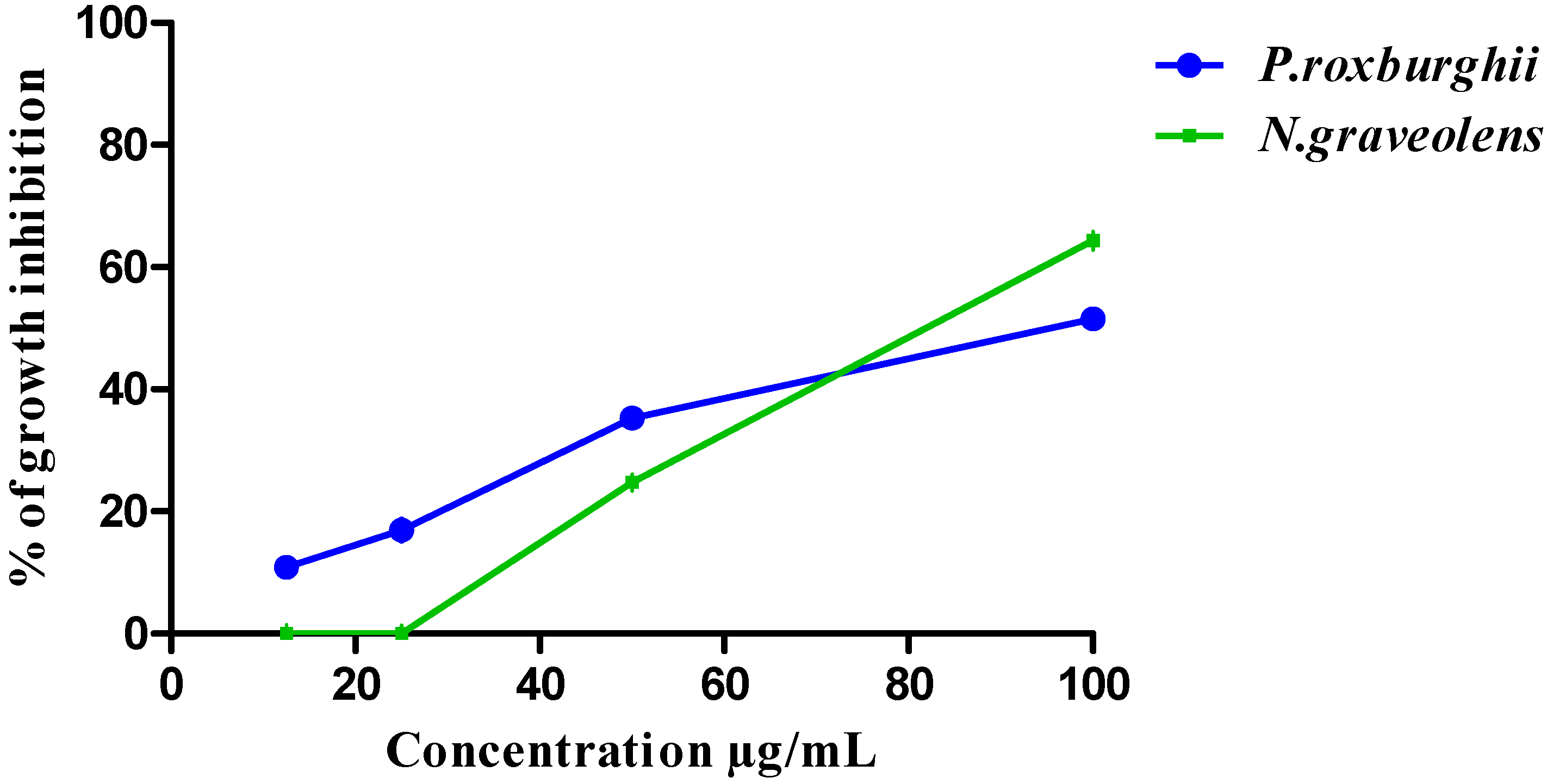
3.3. HCT-116 and BJ-1 Cytotoxicity of P. roxburghii and N. graveolens Extracts
3.4. DNA Fragmentation Activity
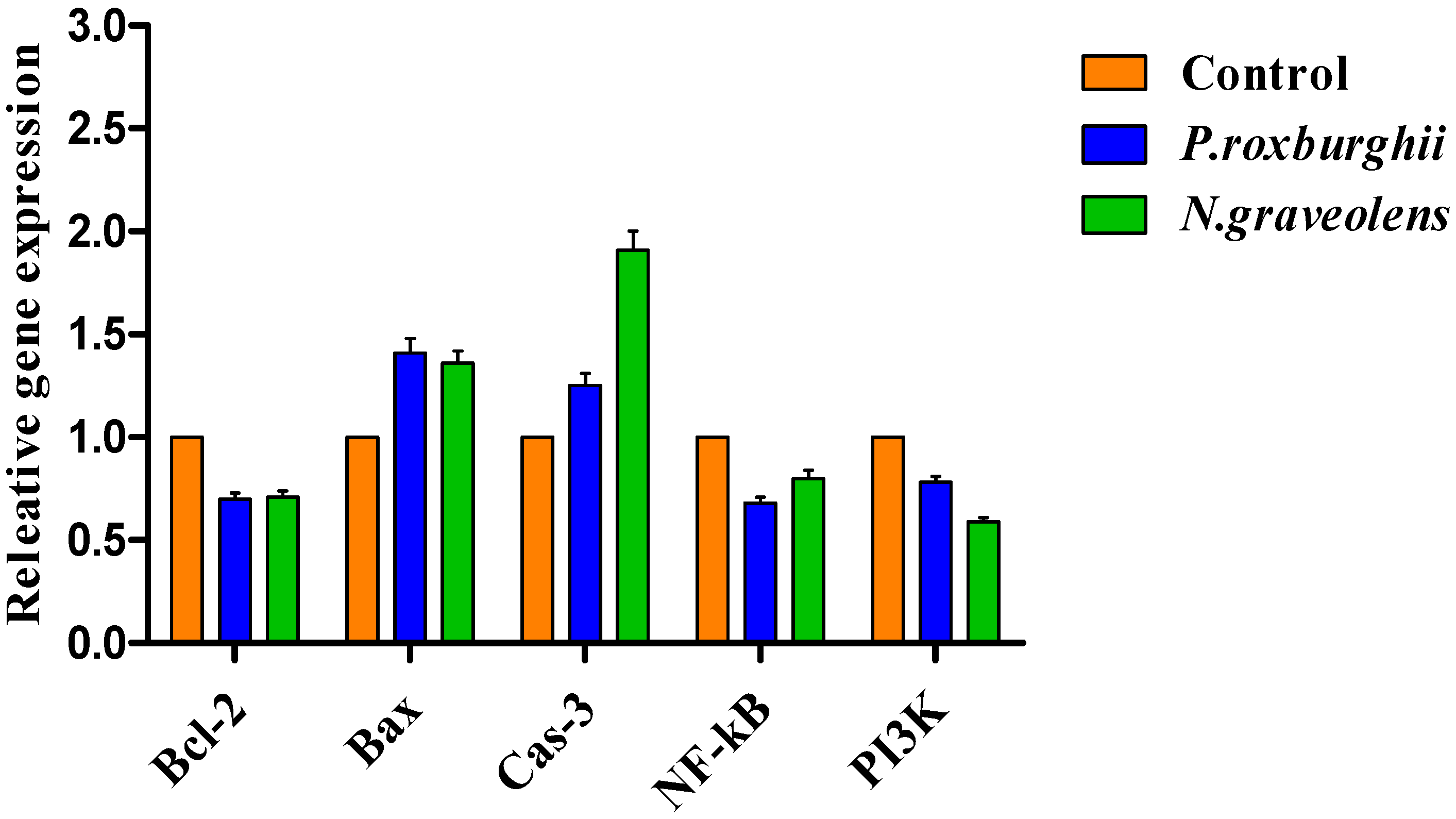
3.5. Gene Expression
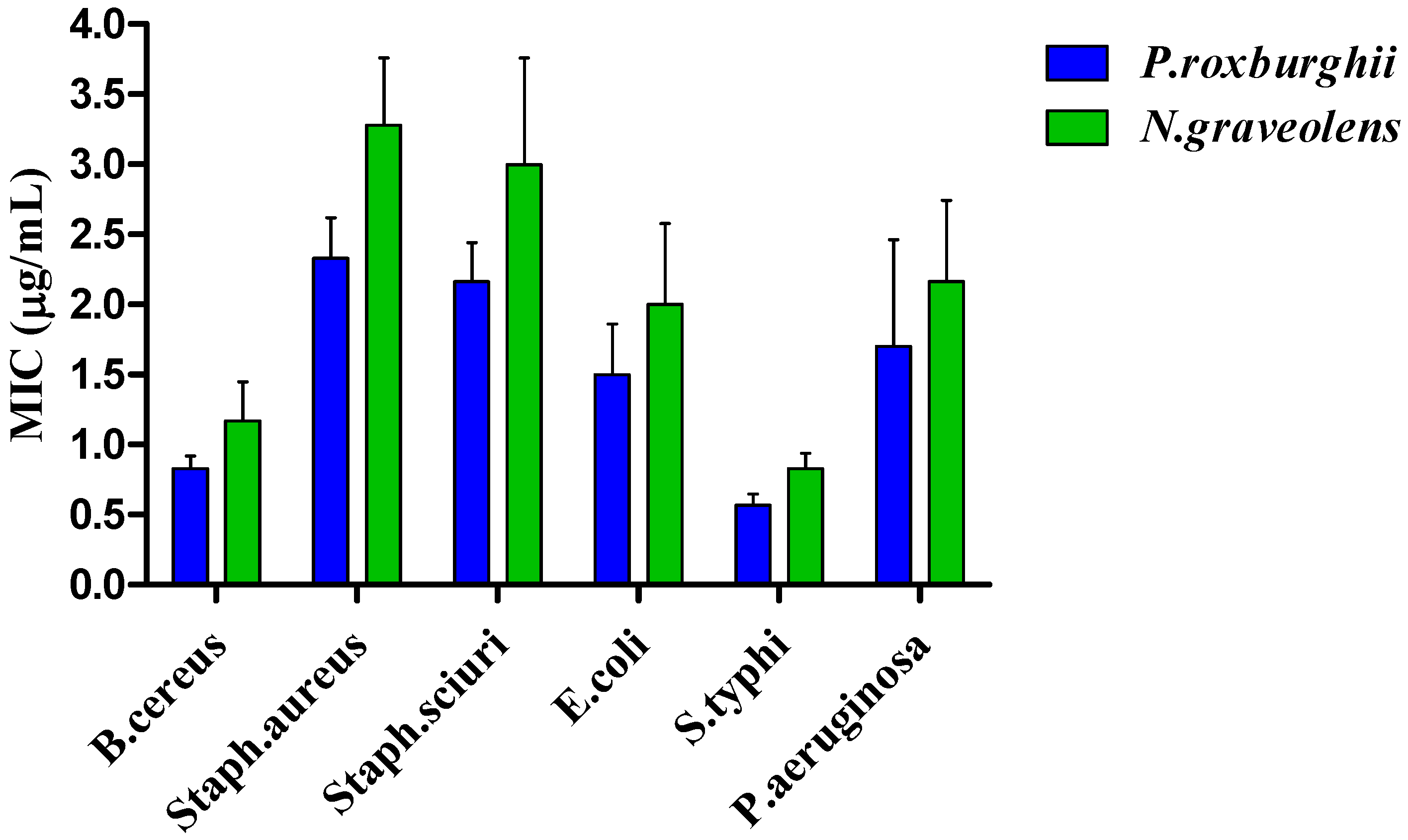
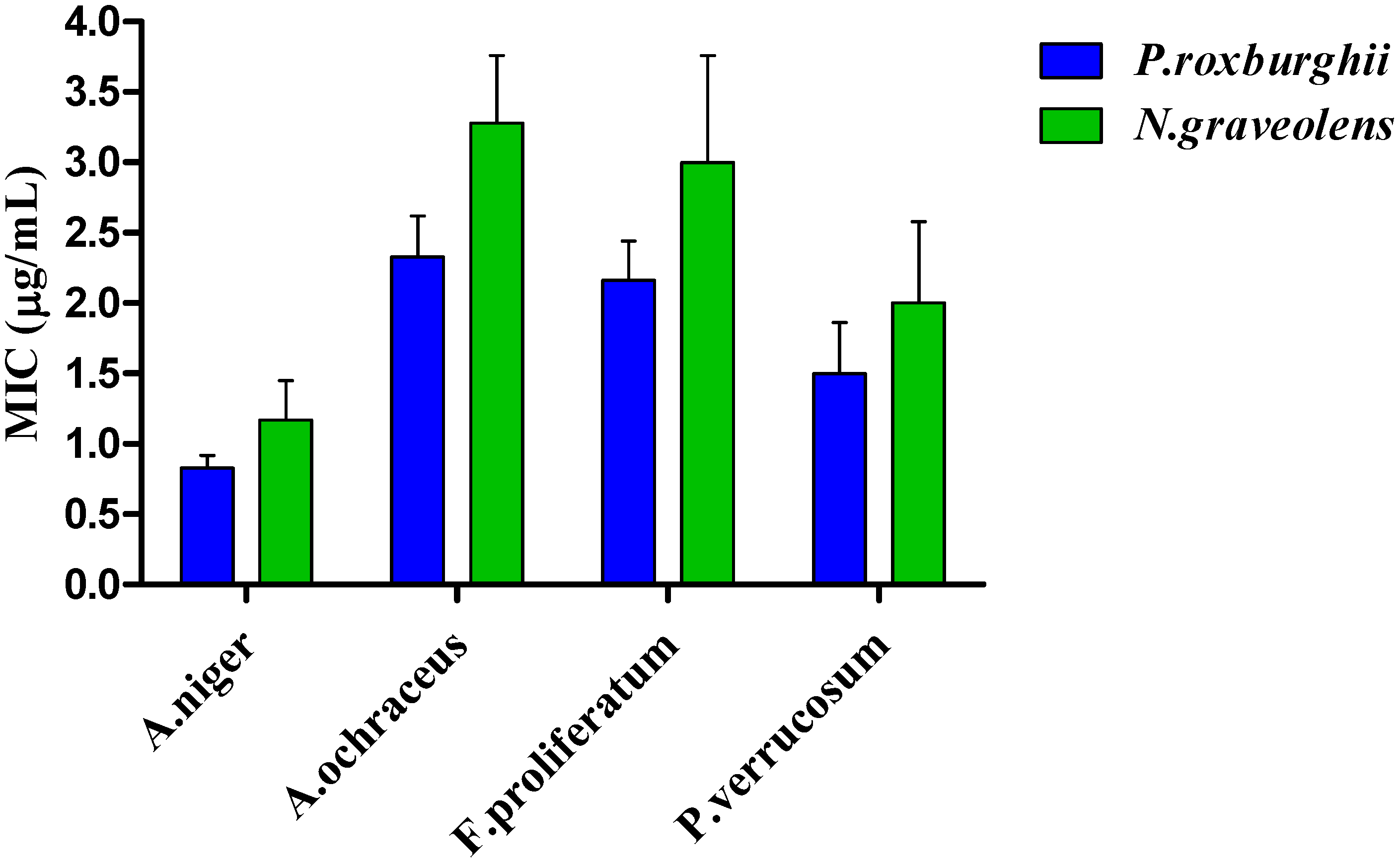
3.6. Antimicrobial Activity
3.6.1. Antibacterial Activity
3.6.2. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 1 December 2020).
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; WHO: Geneva, Switzerland, 2020; Available online: who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 11 December 2020).
- Cheng, L.; Eng, C.; Nieman, L.Z.; Kapadia, A.S.; Du, X.L. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am. J. Clin. Oncol. 2011, 34, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M. The Second World Cancer Research Fund/American Institute for Cancer Research Expert Report. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Proc. Nutr. Soc. 2008, 67, 253–256. [Google Scholar] [CrossRef]
- Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer; American Institute for Cancer Research: Washington, DC, USA, 2011. [Google Scholar]
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of Cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Shim, J.H.; Abd El-Aty, A.M. A Review on extraction, characterization, and applications of bioactive peptides from pressed black cumin seed cake. Front. Nutr. 2021, 8, 743909. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; El-Aty, A.M.A. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Bahnasy, M.I.; Khamis, M.H. Distribution pattern of trees diversity in Orman Botanical Garden. Middle East J. Agric. Res. 2019, 8, 1–11. [Google Scholar]
- Kaushik, D.; Kumar, A.; Kaushik, P.; Rana, A.C. Analgesic and Anti-Inflammatory Activity of Pinus roxburghii Sarg. Adv. Pharmacol. Sci. 2012, 2012, 245431. [Google Scholar] [CrossRef]
- Puri, A.; Srivastava, A.K.; Singhal, B.; Mishra, S.K.; Srivastava, S.; Lakshmi, V. Antidyslipidemic and antioxidant activity of Pinus roxburghii needles. Med. Chem. Res. 2011, 20, 1589–1593. [Google Scholar] [CrossRef]
- Shah, W.A.; Qadir, M.; Banday, J.A. GC-MS analysis, antibacterial, antioxidant and anticancer activity of essential oil of Pinus roxburghii from Kashmir, India. Int. J. Pharm. Res. 2014, 4, 228–232. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Raut, J.; Deo, A.; Dosoky, N.S.; Setzer, W.N. Volatile constituents of Pinus roxburghii from Nepal. Pharmacogn. Res. 2013, 5, 43–48. Available online: http://www.phcogres.com/text.asp?2013/5/1/43/105650 (accessed on 16 January 2021).
- Cheriti, A.; Saad, A.; Belboukhari, N.; Ghezali, S. The essential oil composition of Bubonium graveolens (Forssk.) Maire from the Algerian Sahara. Flav. Frag. J. 2007, 22, 286–288. [Google Scholar] [CrossRef]
- Ramdane, F.; Essid, R.; Mkadmini, K.; Hammami, M.; Fares, N.; Mahammed, M.H.; El Ouassis, D.; Tabbene, O.; Limam, F.; Hadj, M.D.O. Phytochemical composition and biological activities of Asteriscus graveolens (Forssk) extracts. Process Biochem. 2017, 56, 186–192. [Google Scholar] [CrossRef]
- Chaib, F.; Allali, H.; Bennaceur, M.; Flamini, G. Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Asteriscus graveolens (Forssk.) and Pulicaria incisa (LAM.) DC. Two Asteraceae Herbs Growing Wild in the Hoggar. Chem. Biodiversity 2017, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Aouissi, H.; Gourine, N.; Wang, H.; Chen, X.; Bombarda, I.; Boudjeniba, M.; Yousfi, M. Chemical composition, antioxidative, antimicrobial and anti-cancer activities of Asteriscus graveolens (Forssk) essential oil. Orient. Pharm. Exp. Med. 2018, 18, 217–223. [Google Scholar] [CrossRef]
- Guo, X.X.; Li, Y.; Sun, C.; Jiang, D.; Lin, Y.J.; Jin, F.X.; Lee, S.K.; Jin, Y.H. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein Cell 2014, 5, 224–234. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.W.; Reed, J.C. Bcl-2 family proteins and cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, A.A.P.; Henkel, T. Function and Activation of NF-kappaB in the Immune System. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Hassouna, H.Z.; Mahmoud, K.; Abd-Rabou, A.A.; Abdel-Azeem, A.S.; Hegazy, A.M.; Lattife, M.S.A.; Ahmed, F.A. Preliminary study on the toxicological impacts of Pinus roxburghii and Nauplius graveolens extracts on albino mice. Egypt. J. Chem. 2021, 64, 3489–3498. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt 3: Compositae-Verbenaceae; Al Hadara Publishing: Cairo, Egypt, 2002. [Google Scholar]
- Zaky, A.A.; Chen, Z.; Liu, Y.; Li, S.; Jia, Y. Preparation and assessment of bioactive extracts having antioxidant activity from rice bran protein hydrolysates. J. Food Meas. Charact. 2019, 13, 2542–2548. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, Tocopherol, Phenolic Acid, and Antioxidant Properties of Maryland-Grown Soft Wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef]
- Zaky, A.A.; Liu, Y.; Han, P.; Ma, A.; Jia, Y. Effect of flavorzyme digestion on the antioxidant capacities of ultra-filtrated rice bran protein hydrolyzates. J. Food Process. Preserv. 2020, 44, e14551. [Google Scholar] [CrossRef]
- Zaky, A.A.; Liu, Y.; Han, P.; Chen, Z.; Jia, Y. Effect of Pepsin–Trypsin In Vitro Gastro-Intestinal Digestion on the Antioxidant Capacities of Ultra-Filtrated Rice Bran Protein Hydrolysates (Molecular Weight > 10 kDa; 3–10 kDa, and < 3 kDa). Int. J. Pept. Res. Ther. 2020, 26, 1661–1667. [Google Scholar] [CrossRef]
- Zaky, A.A.; Chen, Z.; Qin, M.; Wang, M.; Jia, Y. Assessment of antioxidant activity, amino acids, phenolic acids and functional attributes in defatted rice bran and rice bran protein concentrate. Prog. Nutr. 2020, 22, e2020069. [Google Scholar] [CrossRef]
- Thabrew, M.I.; Hughes, R.D.; Mcfarlane, I.G. Screening of Hepatoprotective Plant Components using a HepG2 Cell Cytotoxicity Assay. J. Pharm. Pharmacol. 1997, 49, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef]
- Gad, M.; Hassouna, H.Z.; Mahmoud, K.; Abd-Rabou, A.A.; Abdel-Azeem, A.S.; Hegazy, A.M.; Abdel-Lattife, M.S.; Ahmed, F.A.; Oz, F.; Proestos, C.; et al. Pinus roxburghii and Nauplius graveolens Extracts Elevate Apoptotic Gene Markers in C26 Colon Carcinoma Cells Induced in a BALB/c Mouse Model. Separations 2022, 9, 277. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Medeiros, M.A.N.; De Oliveira, D.C.N.; Dos Prazeres Rodrigues, D.; De Freitas, D.R.C. Prevalence and antimicrobial resistance of Salmonella in chicken carcasses at retail in 15 Brazilian cities. Rev. Panam. Salud Publica 2011, 30, 555–560. [Google Scholar] [CrossRef]
- Irith, W.; Kai, H.; Robert, H. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Marrez, D.A.; Naguib, M.M.; Sultan, Y.Y.; Higazy, A.M. Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon 2019, 5, e01404. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 2004, 15, 627–634. [Google Scholar] [CrossRef]
- Sultan, Y.Y.; Ali, M.A.; Darwesh, O.M.; Embaby, M.A.; Marrez, D.A. Influence of nitrogen source in culture media on antimicrobial activity of Microcoleus lacustris and Oscillatoria rubescens. J. Pharm. Biol. Chem. Sci. 2016, 7, 1444–1452. Available online: https://www.rjpbcs.com/pdf/2016_7(2)/[197].pdf (accessed on 6 April 2022).
- Kumar, G.; Karthik, L.; Rao, K.V.B. Phytochemical composition and in vitro antioxidant activity of aqueous extract of Aerva lanata (L.) Juss. ex Schult. Stem (Amaranthaceae). Asian Pac. J. Trop. Med. 2013, 6, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, R.; Sharma, L. Potential biological efficacy of Pinus plant species against oxidative, inflammatory and microbial disorders. BMC Complement. Altern. Med. 2015, 16, 35. [Google Scholar] [CrossRef]
- Rawat, U.; Srivastava, B.; Semwal, S.; Sati, O.P. Xanthones from Pinus roxburghii. J. Indian Chem. Soc. 2009, 38, 391–392. [Google Scholar] [CrossRef]
- Haddouchi, F.; Chaouche, T.M.; Halla, N. Screening phytochimique, activités antioxydantes et pouvoir hémolytique de quatre plantes sahariennes d’Algérie. Phytothérapie 2016, 16, S254–S262. [Google Scholar] [CrossRef]
- Alilou, H.; Asdadi, A.; Hassani, L.M.I.; Gonzalez- Mas, M.C.; Blazquez, M.A.; Akssira, M. Antifungal and Antioxidant Activity of Asteriscus graveolens subsp. odorus Essential Oil. J. Nat. Sci. Res. 2014, 4, 10. [Google Scholar]
- Oskoueian, E.; Abdullah, N.; Saad, W.Z.; Omar, A.R.; Kuan, W.B.; Zolkifli, N.A.; Hendra, R.; Ho, Y.W. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J. Med. Plants Res. 2011, 5, 49–57. [Google Scholar]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Al-Rashidi, W.; Mat Supri, N.N.; Manshoor, N. Cytotoxic activity of crude extract from Costus malortieanus (Costaceae). Am. Eurasian J. Toxicol. Sci. 2011, 3, 63–66. [Google Scholar]
- Kulshrestha, K.; Khan, H.S. Evaluation of anticancer activity of Pinus roxburghii Sarg against A549 human lung cancer cell line. J. Pharmacogn. Phytochem. 2019, 8, 2268–2272. [Google Scholar]
- Sajid, A.; Manzoor, Q.; Iqbal, M.; Tyagi, A.K.; Sarfraz, R.A.; Sajid, A. Pinus roxburghii essential oil anticancer activity and chemical composition evaluation. EXCLI J. 2018, 17, 233–245. [Google Scholar] [CrossRef]
- Achoub, H.; Mencherini, T.; Esposito, T.; Luca, R.; Aquino, R.; Gazzerro, P.; Zaiter, L.; Benayache, F.; Benayache, S. New sesquiterpenes from Asteriscus graveolens. Nat. Prod. Res. 2019, 35, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, Z.; Dudai, N.; Schechter, A.; Chalifa-Caspi, V.; Barak, S.; Ofir, R. Molecular Mode of Action of Asteriscus graveolens as an Anticancer Agent. Int. J. Mol. Sci. 2018, 19, 2162. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of Biologically Synthesized Silver Nanoparticles in MDA-MB-231 Human Breast Cancer Cells. BioMed Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Saini, A.K.; Kumar, A.; Saini, R.V. Apoptosis induction in lung and prostate cancer cells through silver nanoparticles synthesized from Pinus roxburghii bioactive fraction. JBIC J. Biol. Inorg. Chem. 2019, 25, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.; Koshy, C.; Dhayabaran, V.; Perumalsamy, L.R.; Sowdhamini, R.; Sarin, A. The N-terminus and alpha-5, alpha-6 helices of the pro-apoptotic protein Bax, modulate functional interactions with the anti-apoptotic protein Bcl-xL. BMC Cell Biol. 2007, 8, 16. [Google Scholar] [CrossRef]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Orfanos, C.E.; Geilen, C.C.; Wieder, T.; Sturm, I.; Daniel, P.T. The Bax/Bcl-2 Ratio Determines the Susceptibility of Human Melanoma Cells to CD95/Fas-Mediated Apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Kim, W.; Wang, Q.C.; Kim, D.-C.; Tan, S.N.; Yong, J.W.H.; Kim, K.-T.; Yoon, H.S. Kinetin riboside preferentially induces apoptosis by modulating Bcl-2 family proteins and caspase-3 in cancer cells. Cancer Lett. 2008, 261, 37–45. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, W.K.; Jeong, M.H.; Yoon, T.R.; Kim, H.K. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int. J. Toxicol. 2012, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.S.; Deb, G.; Babcook, M.A.; Gupta, S. Plant phytochemicals as epigenetic modulators: Role in cancer chemoprevention. AAPS J. 2014, 16, 151–163. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; De Molina, A.R. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef]
- Escárcega, R.; Fuentes-Alexandro, S.; García-Carrasco, M.; Gatica, A.; Zamora, A. The Transcription Factor Nuclear Factor-kappa B and Cancer. Clin. Oncol. 2007, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Niero, E.L.D.O.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.-Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.-H.; Chung, H.Y.; et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.T.; Gupta, S. Suppression of NF-κB and NF-κB-Regulated Gene Expression by Apigenin through IκBα and IKK Pathway in TRAMP Mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ni, F.; Zhang, Y.; Chen, H.-H.; Huang, E.; Zhuang, H.; Li, D. Rosmarinic acid inhibits stem-like breast cancer through hedgehog and Bcl-2/Bax signaling pathways. Pharmacogn. Mag. 2019, 15, 600. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; De Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Li, Z.; DePriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Supplementation of p-coumaric acid exhibits chemopreventive effect via induction of Nrf2 in a short-term preclinical model of colon cancer. Eur. J. Cancer Prev. 2019, 28, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Alrawashdeh, I.; Qaralleh, H.; Al-Limoun, M.; Khleifat, K. Antibactrial Activity of Asteriscus graveolens Methanolic Extract: Synergistic Effect with Fungal Mediated Nanoparticles against Some Enteric Bacterial Human Pathogens. J. Basic Appl. Res. Biomed. 2019, 5, 89–98. [Google Scholar] [CrossRef]
- Znini, M.; Cristofari, G.; Majidi, L.; Mazouz, H.; Tomi, P.; Paolini, J.; Costa, J. Antifungal Activity of Essential Oil from Asteriscus graveolens against Postharvest Phytopathogenic Fungi in Apples. Nat. Prod. Commun. 2011, 6, 1763–1768. [Google Scholar] [CrossRef]
| Primers | Sequences |
|---|---|
| Bcl-2 | F:5′CTGCACCTGACGCCCTTCACC3′ R:5′CACATGACCCCACCGAACTCAAAGA3′ |
| BAX | F:5′TCCCCCCGAGAGGTCTTTT3′ R:5′CGGCCCCAGTTGAAGTTG3′ |
| Cas-3 | F:5′GTGGAACTGACGATGATATGGC3′ R:5′CGCAAAGTGACTGGATGAACC3′ |
| NF-κB | F:5′ATGGCTTCTATGAGGCTGAG3′ R: 5′GTTGTTGTTGGTCTGGATGC3′ |
| PI3k | F: 5′-GCTCTCGGTTGATTCCAACGT-3′ R: 5′-ATGGCTTCTATGAGGCTGAG3′ |
| GAPDH | F:5′GTCTCCTCTGACTTCAACAGCG3′ R:5′ACCACCCTGTTGCTGTAGCCAA3′ |
| Plant Name | Total Phenolics (µg GAE/g) | Total Flavonoids (Catechin µg/g) | Total Carotenoids (µg/g) | DPPH (μg TE/g) | ABTS (μg TE/g) |
|---|---|---|---|---|---|
| P. roxburghii | 32.70 ± 0.26 b | 11.67 ± 0.21 b | 0.11 ± 0.01 b | 17.66 ± 0.09 b | 20.95 ± 0.02 b |
| N. graveolens | 73.86 ± 0.17 a | 41.55 ± 0.12 a | 0.24 ± 0.02 a | 37.81 ± 0.07 a | 25.81 ± 0.07 a |
| Compounds | RT (Min.) | Concentration (µg/g Extract) | |
|---|---|---|---|
| P. roxburghii Extract | N. graveolens Extract | ||
| Gallic acid | 4.04 | 48.92 | ND |
| Protocatechuic acid | 7.87 | 47.10 | ND |
| p-hydroxybenzoic acid | 12.19 | 13.98 | 8.25 |
| Catechin | 15.08 | 1976.49 | 61.86 |
| Chlorogenic acid | 16.07 | 42.83 | 2458.46 |
| Caffeic acid | 17.05 | 17.45 | 47.93 |
| Syringic acid | 18.66 | 18.55 | 39.35 |
| Vanillic acid | 20.61 | 9.88 | 37.87 |
| Ferulic acid | 28.09 | 11.26 | ND |
| p-coumaric | 34.10 | 26.62 | 85.00 |
| Rutin | 33.38 | ND | 474.00 |
| Apigenin-7-glucoside | 37.19 | ND | 712.49 |
| Rosmarinic acid | 37.99 | ND | 168.46 |
| Cinnamic acid | 44.48 | ND | 81.47 |
| Kaempferol | 54.17 | 7.54 | 5.04 |
| Bacteria | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Negative Control | Positive Control | P. roxburghii | N. graveolens | |
| B. cereus | 0 | 27.2 ± 1.89 a | 7.5 ± 0.50 b | 7.2 ± 0.28 b |
| S. aureus | 0 | 28.7 ± 0.58 a | 9.5 ± 0.86 b | 8.0 ± 0.50 c |
| S. sciuri | 0 | 26.5 ± 1.32 a | 10.2 ± 0.76 b | 8.3 ± 0.58 c |
| E. coli | 0 | 11.5 ± 0.86 ab | 12.0 ± 0.50 a | 11.2 ± 1.02 b |
| S. typhi | 0 | 25.2 ± 2.52 a | 11.2 ± 1.25 b | 7.2 ± 0.28 c |
| P. aeruginosa | 0 | 13.0 ± 0.70 a | 10.3 ± 0.76 b | 8.3 ± 0.58 c |
| Fungi | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Negative Control | Positive Control | P. roxburghii | N. graveolens | |
| A. niger | 0 | 20.2 ± 1.53 a | 8.0 ± 0.50 b | 9.2 ± 1.04 c |
| A. ochraceus | 0 | 13.2 ± 1.04 a | 8.7 ± 0.76 b | 7.7 ± 0.28 c |
| F. proliferatum | 0 | 10.7 ± 0.76 a | 7.3 ± 0.58 b | 7.8 ± 0.28 b |
| P. verrucosum | 0 | 19.8 ± 2.56 a | 9.8 ± 0.76 b | 8.7 ± 0.58 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, M.; Hassouna, H.Z.; Mahmoud, K.; Abd-Rabou, A.A.; Abdel-Azeem, A.S.; Hegazy, A.M.; Abdel-Lattife, M.S.; Abdel-Rahim, E.A.; Ahmed, F.A.; Shim, J.-H.; et al. In Vitro Anti-Colorectal Cancer and Anti-Microbial Effects of Pinus roxburghii and Nauplius graveolens Extracts Modulated by Apoptotic Gene Expression. Separations 2022, 9, 393. https://doi.org/10.3390/separations9120393
Gad M, Hassouna HZ, Mahmoud K, Abd-Rabou AA, Abdel-Azeem AS, Hegazy AM, Abdel-Lattife MS, Abdel-Rahim EA, Ahmed FA, Shim J-H, et al. In Vitro Anti-Colorectal Cancer and Anti-Microbial Effects of Pinus roxburghii and Nauplius graveolens Extracts Modulated by Apoptotic Gene Expression. Separations. 2022; 9(12):393. https://doi.org/10.3390/separations9120393
Chicago/Turabian StyleGad, Mosab, Hassan Z. Hassouna, Khaled Mahmoud, Ahmed A. Abd-Rabou, Amal S. Abdel-Azeem, Amany M. Hegazy, Mohamed S. Abdel-Lattife, Emam A. Abdel-Rahim, Fouad A. Ahmed, Jae-Han Shim, and et al. 2022. "In Vitro Anti-Colorectal Cancer and Anti-Microbial Effects of Pinus roxburghii and Nauplius graveolens Extracts Modulated by Apoptotic Gene Expression" Separations 9, no. 12: 393. https://doi.org/10.3390/separations9120393
APA StyleGad, M., Hassouna, H. Z., Mahmoud, K., Abd-Rabou, A. A., Abdel-Azeem, A. S., Hegazy, A. M., Abdel-Lattife, M. S., Abdel-Rahim, E. A., Ahmed, F. A., Shim, J.-H., & Zaky, A. A. (2022). In Vitro Anti-Colorectal Cancer and Anti-Microbial Effects of Pinus roxburghii and Nauplius graveolens Extracts Modulated by Apoptotic Gene Expression. Separations, 9(12), 393. https://doi.org/10.3390/separations9120393