In-Tube Solid-Phase Microextraction Directly Coupled to Mass Spectrometric Systems: A Review
Abstract
1. Introduction
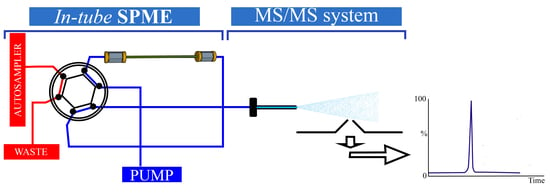
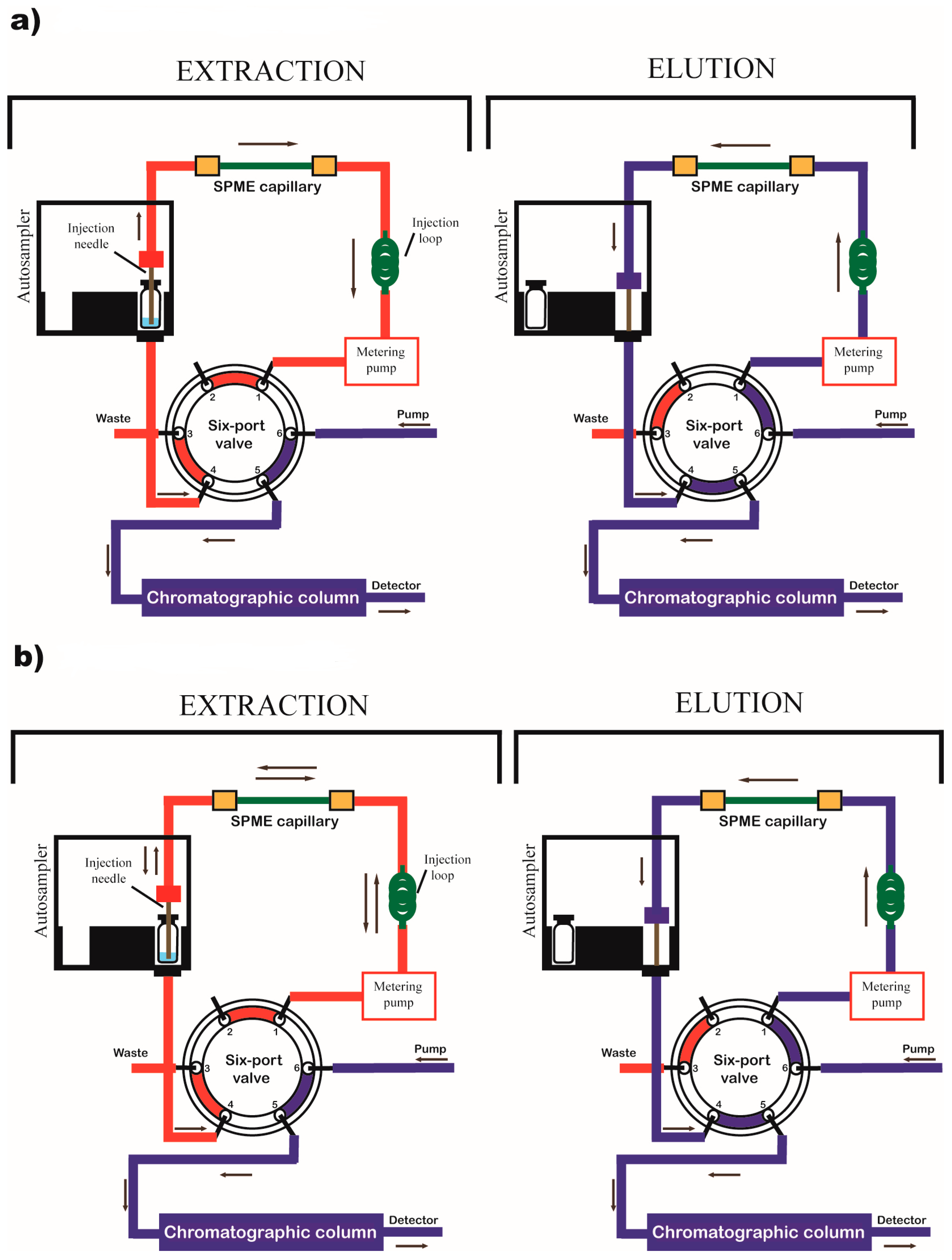
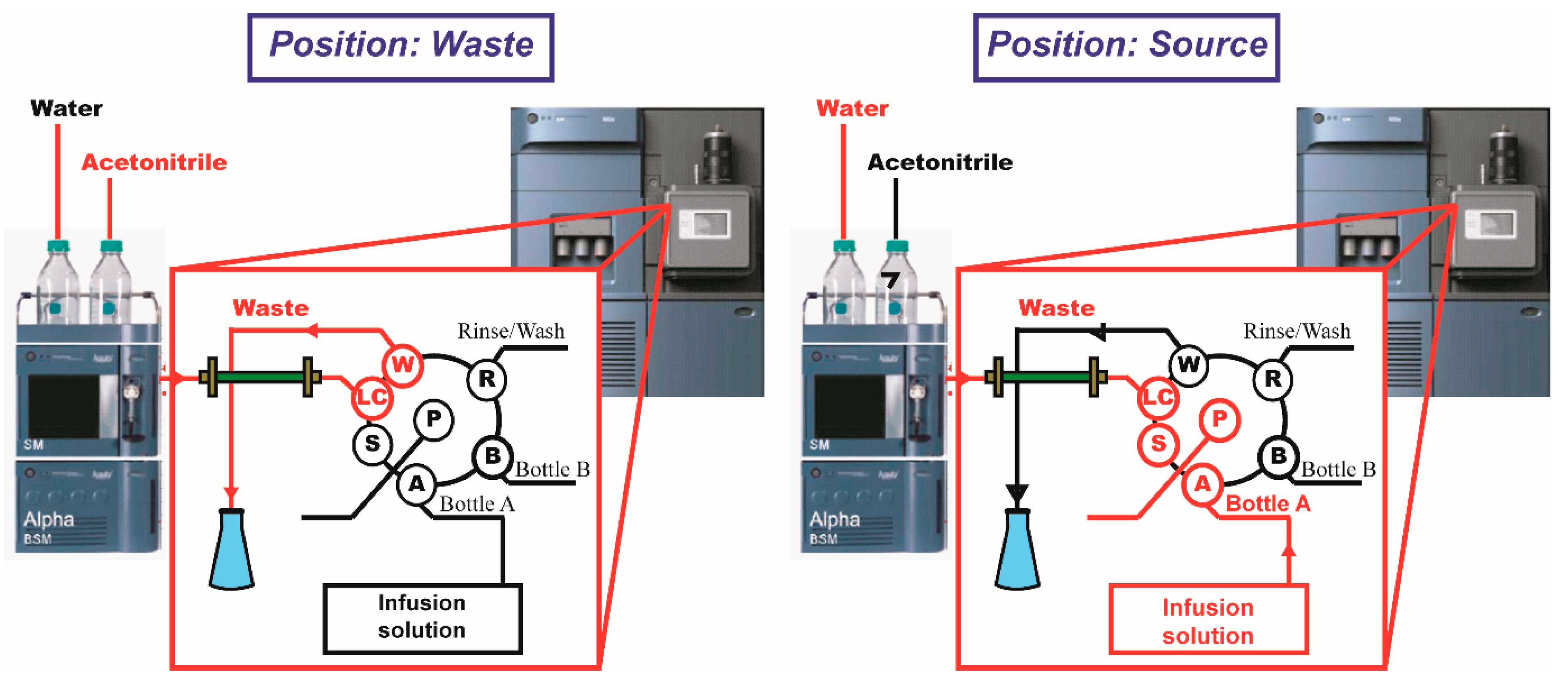
2. Instrumental Configuration of the In-Tube SPME System
In-Tube SPME Directly Coupled to MS/MS System
3. Optimization of the In-Tube SPME Parameters
3.1. Sample Pre-Treatment
3.2. Sorption Conditions
3.3. Desorption Conditions
3.4. Capillary Column Geometry
3.5. Parameters Optimization for In-Tube SPME Direclty Coupled to MS/MS System
4. Innovative Stationary Phases
4.1. Commercial Porous-Layer Open-Tubular (PLOT) Columns
4.2. Monolithic Stationary Phase Columns
4.3. Particle-Based Stationary Phases
4.4. Fiber-Coated Stationary Phases (Fiber In-Tube SPME)
4.5. Stationary Phases for In-Tube SPME Directly Coupled to MS System
5. Applications
6. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Yamini, Y.; Rezazadeh, M.; Seidi, S. Liquid-phase microextraction—The different principles and configurations. TrAC Trends Anal. Chem. 2019, 112, 264–272. [Google Scholar] [CrossRef]
- Jon, C.-S.; Meng, L.-Y.; Li, D. Recent review on carbon nanomaterials functionalized with ionic liquids in sample pretreatment application. TrAC Trends Anal. Chem. 2019, 120, 115641. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; Melo, L.P. Selective capillary coating materials for in-tube solid-phase microextraction coupled to liquid chromatography to determine drugs and biomarkers in biological samples: A review. Anal. Chim. Acta 2014, 826, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beloti, L.; Miranda, L.; Queiroz, M. Butyl Methacrylate-Co-Ethylene Glycol Dimethacrylate Monolith for Online in-Tube SPME-UHPLC-MS/MS to Determine Chlopromazine, Clozapine, Quetiapine, Olanzapine, and Their Metabolites in Plasma Samples. Molecules 2019, 24, 310. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.; Oliveira, I.G.C.; Queiroz, M.E.C. Innovative extraction materials for fiber-in-tube solid phase microextraction: A review. Anal. Chim. Acta 2021, 1165, 238110. [Google Scholar] [CrossRef]
- Grecco, C.F.; Souza, I.D.; Queiroz, M.E.C. Novel materials as capillary coatings for in-tube solid-phase microextraction for bioanalysis. J. Sep. Sci. 2021, 44, 1662–1693. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; de Souza, I.D.; Marchioni, C. Current advances and applications of in-tube solid-phase microextraction. TrAC Trends Anal. Chem. 2019, 111, 261–278. [Google Scholar] [CrossRef]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef]
- Eisert, R.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. Anal. Chem. 1997, 69, 3140–3147. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Reyes-Garcés, N.; Bojko, B.; Pawliszyn, J. Biocompatible Solid-Phase Microextraction Nanoelectrospray Ionization: An Unexploited Tool in Bioanalysis. Anal. Chem. 2016, 88, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.F.C.; Gonçalves, R.R.; Queiroz, M.E.C. A Dual Ligand Sol–Gel Organic-Silica Hybrid Monolithic Capillary for In-Tube SPME-MS/MS to Determine Amino Acids in Plasma Samples. Molecules 2019, 24, 1658. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.G.; Tavares, I.M.C.; Barbosa, A.F.; Bettini, J.; Figueiredo, E.C. Analysis of tricyclic antidepressants in human plasma using online-restricted access molecularly imprinted solid phase extraction followed by direct mass spectrometry identification/quantification. Talanta 2017, 163, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ishizaki, A.; Nonaka, Y.; Saito, K. Developments and applications of capillary microextraction techniques: A review. Anal. Chim. Acta 2009, 655, 8–29. [Google Scholar] [CrossRef]
- Kataoka, H. Automated sample preparation using in-tube solid-phase microextraction and its application—A review. Anal. Bioanal. Chem. 2002, 373, 31–45. [Google Scholar] [CrossRef]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent advances of in-tube solid-phase microextraction. TrAC Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Fernández-Amado, M.; Prieto-Blanco, M.C.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Strengths and weaknesses of in-tube solid-phase microextraction: A scoping review. Anal. Chim. Acta 2016, 906, 41–57. [Google Scholar] [CrossRef]
- Safari, M.; Yamini, Y. Application of magnetic nanomaterials in magnetic in-tube solid-phase microextraction. Talanta 2021, 221, 121648. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Z.-S.; Duan, R.; Wang, X.; Yang, Y.-S.; Dong, L.-Y.; Wang, X.-H. Advances and applications of in-tube solid-phase microextraction for analysis of proteins. J. Chromatogr. A 2021, 1640, 461962. [Google Scholar] [CrossRef]
- Oliveira, I.G.C.; de Souza, I.D.; Nascimento, G.C.d.; del Bel, E.; Queiroz, M.E.C. In-tube solid-phase microextraction directly coupled to tandem mass spectrometry for anandamide and 2-arachidonoylglycerol determination in rat brain samples from an animal model of Parkinson’s disease. J. Chromatogr. A 2021, 1636, 461766. [Google Scholar] [CrossRef]
- Kataoka, H.; Kaji, S.; Moai, M. Risk Assessment of Passive Smoking Based on Analysis of Hair Nicotine and Cotinine as Exposure Biomarkers by In-Tube Solid-Phase Microextraction Coupled On-Line to LC-MS/MS. Molecules 2021, 26, 7356. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Kataoka, H. Online In-Tube Solid-Phase Microextraction Coupled to Liquid Chromatography–Tandem Mass Spectrometry for the Determination of Tobacco-Specific Nitrosamines in Hair Samples. Molecules 2021, 26, 2056. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Nakayama, D. Online In-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography–Tandem Mass Spectrometry for Automated Analysis of Four Sulfated Steroid Metabolites in Saliva Samples. Molecules 2022, 27, 3225. [Google Scholar] [CrossRef] [PubMed]
- Morisue Sartore, D.; Costa, J.L.; Lanças, F.M.; Santos-Neto, Á.J. Packed in-tube SPME–LC–MS/MS for fast and straightforward analysis of cannabinoids and metabolites in human urine. Electrophoresis 2022, 43, 1555–1566. [Google Scholar] [CrossRef]
- Souza, I.D.; Anderson, J.L.; Queiroz, M.E.C. Crosslinked zwitterionic polymeric ionic liquid-functionalized nitinol wires for fiber-in-tube solid-phase microextraction and UHPLC-MS/MS as an amyloid beta peptide binding protein assay in biological fluids. Anal. Chim. Acta 2022, 1193, 339394. [Google Scholar] [CrossRef]
- Song, X.; Wu, J.; Pang, J.; Wu, Y.; Huang, X. Task specific monolith for magnetic field-reinforced in-tube solid phase microextraction of mercury species in waters prior to online HPLC quantification. J. Hazard. Mater. 2021, 411, 125141. [Google Scholar] [CrossRef]
- Sun, M.; Feng, J.; Ji, X.; Li, C.; Han, S.; Sun, M.; Feng, Y.; Feng, J.; Sun, H. Polyaniline/titanium dioxide nanorods functionalized carbon fibers for in-tube solid-phase microextraction of phthalate esters prior to high performance liquid chromatography-diode array detection. J. Chromatogr. A 2021, 1642, 462003. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Luan, C.-C.; Lu, Z.-Y.; Chen, N.; Zhang, Y.-J.; Cui, C.-X. Brass wires with different surface wettability used for in-tube solid-phase microextraction. J. Chromatogr. A 2022, 1670, 462948. [Google Scholar] [CrossRef]
- Nasrollahi, S.S.; Yamini, Y.; Mani-Varnosfaderani, A. A green approach for in-tube solid phase microextraction of acidic red dyes from juice samples using chitosan/poly vinyl alcohol electrospun nanofibers. J. Food Compos. Anal. 2022, 106, 104339. [Google Scholar] [CrossRef]
- Pang, J.; Chen, H.; Huang, X. Magnetism-assisted in-tube solid phase microextraction for the on-line chromium speciation in environmental water and soil samples. Microchem. J. 2021, 164, 105956. [Google Scholar] [CrossRef]
- Wei, L.; Liu, J.; Liu, Q.; Chen, X.; Li, Z.; Xu, Y.; Gao, X.; Lu, X.; Guo, Z.; Zhao, J. Polyhedral Oligomeric Silsesquioxane–Based Hybrid Monolithic Column On-line In-Tube Solid-Phase Microextraction Coupled with High-Performance Liquid Chromatography for the Determination of Five Phthalate Esters in Bottled Water. Food Anal. Methods 2022, 15, 1107–1117. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Wei, L.; Chen, X.; Li, Z.; Xu, Y.; Gao, X.; Lu, X.; Zhao, J. A novel polyhedral oligomeric silsesquioxane-based hybrid monolith as a sorbent for on-line in-tube solid phase microextraction of bisphenols in milk prior to high performance liquid chromatography-ultraviolet detection analysis. Food Chem. 2022, 374, 131775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ding, X.; Lin, C.; Lin, X.; Xie, Z. In situ photo-initiated polymerized oligonucleotide-functionalized hydrophilic capillary affinity monolith for highly selective in-tube microextraction of ochratoxin A mycotoxin. Microchim. Acta 2021, 188, 341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tong, S.; Zhou, S.; Lin, C.; Lin, X.; Xie, Z. A facile aptamer immobilization strategy to fabricate a robust affinity monolith for highly specific in-tube solid-phase microextraction. Analyst 2021, 146, 5732–5739. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, L.; Guan, P.; Tang, K.; Yu, S.; Ding, C.-F. Simultaneous enrichment and analysis of benzimidazole by in-tube SPME-MS based on poly (3-Acrylamidophenylboronic acid-co-divinylbenzene-co-N,N′-methylenebisacrylamide) monolithic column. Talanta 2021, 224, 121402. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, S.; Wang, L.; Wei, W.; Ding, C.-F. Simultaneous enrichment and analysis of tobacco alkaloids by microextraction coupled with mass spectrometry using a poly (N-isopropyl-acrylamide-co-divinyl-benzene-co-N, N’-methylene diacrylamide) monolithic column. Talanta 2019, 198, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Pichon, V.; Brothier, F.; Combès, A. Aptamer-based-sorbents for sample treatment—A review. Anal. Bioanal. Chem. 2015, 407, 681–698. [Google Scholar] [CrossRef]

| In-Tube SPME Steps | Parameters Evaluated |
|---|---|
| Sample pre-treatment | Sample pH Addition of complex agent Ionic strength Dilution |
| Sorption | Draw/eject mode: Sample volume (few microliters) Number of cycles and sampling flow = rate of each cycle |
| Flow-through mode: Sample volume (few microliters to milliliters) Flow rate of mobile phase and pre-concentration time | |
| Desorption | Composition and volume of desorption solvent Desorption time Flow rate of desorption solvent |
| Capillary column dimensions | Inner diameters and length |
| Matrix | Analytes | Sample Pre-Treatment | In-Tube SPME Procedure | Stationary Phase | Separation/Detection System | Linear Range (ng mL−1) Reproducibility (RSD, %) Durability (Extractions) | Ref. |
|---|---|---|---|---|---|---|---|
| Biological | |||||||
| Hair (5 mg) | Tobacco-Specific Nitrosamines | Washing with 0.1% sodium dodecyl sulfate, water, and methanol. Stored in amber glass desiccator at room temperature until use. Addition of 0.1 mL of water and heated and extracted at 80 °C for 30 min. The extract was cooled to room temperature and filtered through a 45 µm hydrophilic PTFE syringe filter. Dilution of 1.0 mL of the filtrate with 0.5 mL of water. | Twenty-five repeated draw/eject cycles of 40 µL sample at a flow rate of 0.2 mL min−1 | Supel-Q PLOT capillary (60 cm × 0.32 mm i.d.) | LC-MS/MS | 0.0005–0.1 2.1–9.2% NM | Ishizaki and Kataoka, 2021 [22] |
| Hair (0.2 mg) | Nicotine and Cotinine | Washing with 3 mL of DCM by sonication to remove external nicotine and cotinine from the hair surface and stored in amber glass desiccator at room temperature until use. Addition of 1.0 mL of distilled water and extraction at 80 °C for 30 min. Dilution of 1.0 mL of the filtrate with 1.0 mL of distilled water. | Twenty repeated draw/eject cycles of 40 µL sample at a flow rate of 0.2 mL min−1 | Carboxen 1006 PLOT capillary column (60 cm × 0.32 mm i.d., 17 µm film thickness) | LC-MS/MS | 0.005–1 1.62–5.97% NM | Kataoka et al., 2021 [21] |
| Saliva (50 µL) | Sulfated Steroid Metabolites | Centrifugation at 2500× g for 1 min followed by ultrafiltration using an Amicon Ultra 0.5 mL 3K regenerated cellulose 3000 molecular weight cutoff centrifugal filter device at 15,000 rpm for 20 min. Addition of 0.05 mL of 0.2 M potassium hydrogen phthalate-HCl buffer (pH 3). The total volume was made up to 1.0 mL of distilled water. | Twenty-five repeated draw/eject cycles of 40 µL sample at a flow rate of 0.2 mL min−1 | Supel-Q PLOT capillary (60 cm × 0.32 mm i.d.) | LC-MS/MS | 0.01–10 2.1–11.1% NM | Kataoka and Nakayama, 2022 [23] |
| Urine (250 µL) | Cannabinoids and metabolites | Dilution with ACN (1:2, v/v) and centrifugation | Loading flow rate: 0.25 mL min−1 Loading time: 2.5 min | LichroPrep RP-18 (508 µm i.d. ×50 mm) | LC-MS/MS | 10–1000 NM 150 | Sartore et al., 2022 [24] |
| Plasma (300 µL) CSF (300 µL) | Amyloid beta peptides | Incubation process for 1 h at 37 °C with gentle agitation followed by dilution (1:1, v/v) with 1% formic acid aqueous solution | Wash and conditioning: 600 µL of methanol + 600 µL of formic acid aqueous solution 1% Sampling: 6 draw/eject cycles of sample. Clean up: 500 µL of: water/ACN (95:5, v/v) containing 1% of formic acid. Desorption: 100 µL of water/ACN (20:80, v/v) +1% of ammonium hydroxide. Sampling rate: 1.0 mL min−1 | Crosslinked zwitterionic polymeric ionic liquid-functionalized nitinol wires (0.120 × 200 mm) | UHPLC-MS/MS | 0.3–15 2.1–7.3% 90 | Souza et al., 2022 [25] |
| Ambiental | |||||||
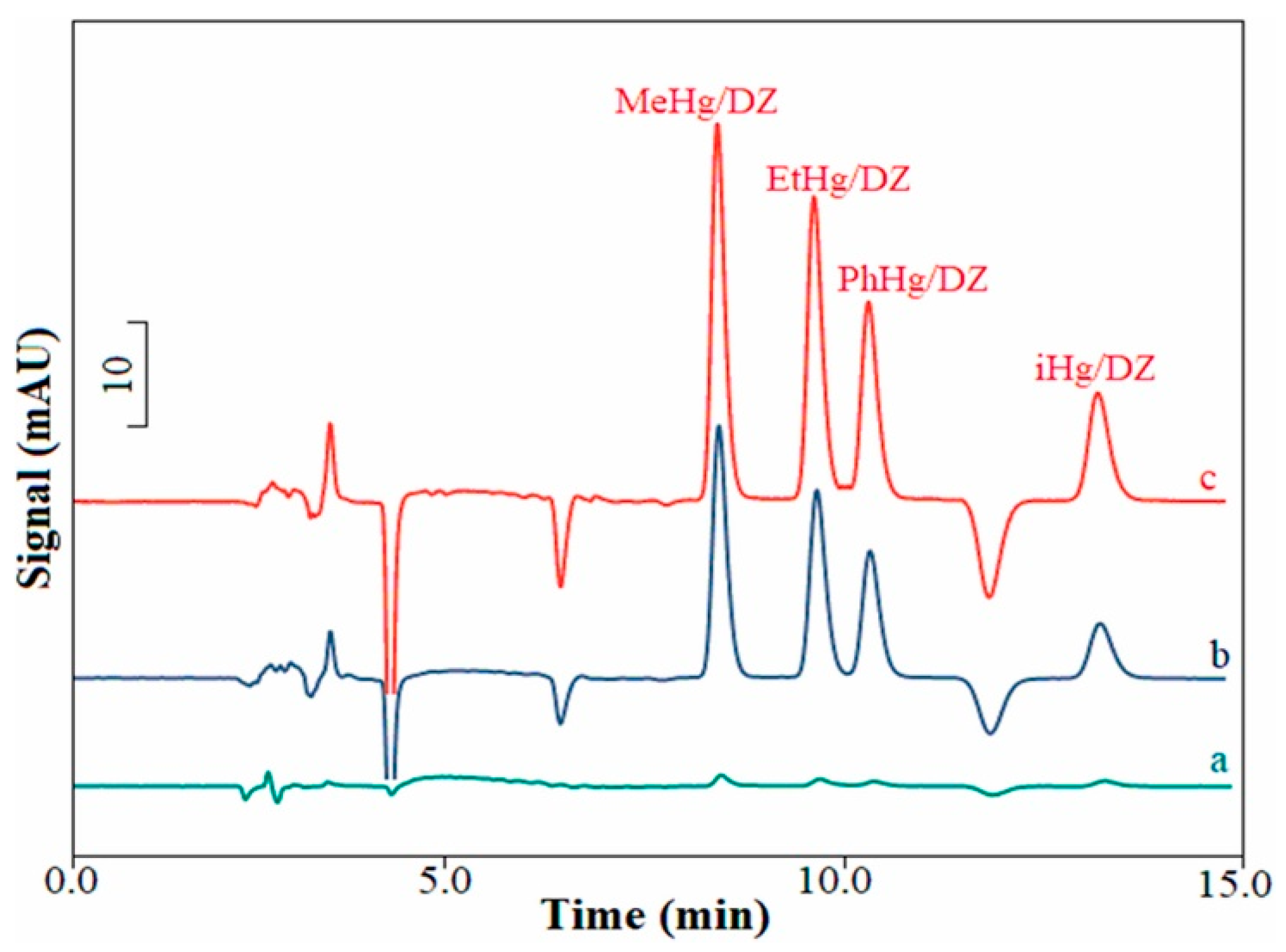
| Sea, tap, river and lake waters (3.0 mL) | Mercury species (coordinated with dithizone) | Water samples were filtered, pH adjusted at 4.0. Dithizone was added to sample to form Hg complexes. EDTA was added to avoid the interference of co-existing ions. | Sampling (3.0 mL) at 0.10 mL min−1, 20 Gs magnetic field with the identical direction of the flow of sample solution; Desorption: 80 μL MeOH at 40 μL min−1, 30 Gs reverse magnetic field. | Task-specific monolith doped with Fe3O4 (20 cm × 320 µm i.d.) | MFR/IT-SPME-HPLC-DAD | 0.05–300 0.8–6.7% 60 | Song et al., 2021 [26] |
| Sea, tap, river and lake waters (5 mL) and soil samples (5.0 g) | Cr(III) and Cr(VI) species (coordinate with APD) | Water samples were filtered, pH adjusted at 5.5. APD was added to sample to form Cr(III) and Cr(VI) complexes. Soil samples were crushed and dried to a constant weight at 80 °C. The samples were fully ground and passed through a 100-mesh sieve. The sample was extracted with ultrapure water. The pH of supernatant was adjusted (5.5) and filtered (0.22 µm). | Sampling (5.0 mL) at 0.14 mL min−1, 30 Gs magnetic field with the identical direction of the flow of sample solution; Desorption: 60 μL ACN at 0.01 μL min−1, 40 Gs reverse magnetic field. | Porous monolith doped with magnetic nanoparticles (20 cm × 320 µm i.d.) | MFR/IT-SPME-HPLC-DAD | 0.010–100 1.4–11% 50 | Pang et al., 2021 [30] |
| Plastic bottled water (18.0 mL) | Phthalate esters | NM | Sampling (18.0 mL) at 0.75 mL min−1 for enrichment. Desorption with 100% ACN at 0.2 mL min−1 for 5 min | POSS-co-BMA monolith (10 mm × 3 mm i.d.) | HPLC-UV | 0.1–60 2.0–9.4% 60 | Wei et al., 2022 [31] |
| Bottled water and water sample from Disposable lunch box (50 mL) | Phthalate esters | Samples were filtered (0.45 μm). | Sampling (50 mL) at 1.25 mL min−1 using 1.0% (v/v) MeOH containing 2.0% (w/v) of NaCl. Desorption with methanol-water (75:15, v/v) at 1.0 mL min−1 for 0.6 min. | Polyaniline/TiO2 nanorods functionalized carbon fibers (35 cm × 0.75 mm i.d.) | HPLC-DAD | 0.03–30 3.5–13.9% 40 | Sun et al., 2021 [27] |
| Food | |||||||
| Fish feed (60 mL of filtrate) | Polar estrogens and non-polar PAHs | Fish feed was soaked in distilled water, and the solution was then filtered through a 0.45-µm membrane. | Injection rate: 2 mL min−1. Desorption time: 2 min at a flow rate of 1 mL min−1 | Brass wires modified with 2-naphthalenethiol (30 cm wires packed inside a stainless-steel tube of 0.75 i.d.) | HPLC-DAD | 0.5–10.0 1.9–6.5% NM | Zhang et al., 2022 [28] |
| Milk (10 mL) | Bisphenols | A total of 10.0 mL milk sample was precipitated with 10.0 mL acetonitrile added (twice). The supernatant was concentrated to 1.0 mL and then redissolved with 100.0 mL ultrapure water, which was filtered (Nylon60 0.22 μm syringe). | Sampling at a flow rate of 1.0 mL min−1. Desorption: 0.3% phosphoric acid–water:ACN (from 30.0–55.0% of ACN) for 2 min. | Poly (POSS-co-AM-co-EDMA) monolith (10 mm × 3 mm i.d.) | HPLC-UV | 0.2–200.0 <2.7% 60 | Liu et al., 2022 [32] |
| Beer and red wine (10 mL) | Ochratoxin A | Samples were degassed; pH adjusted to 8.50, filtration (0.22-μm filter membrane), and diluted with binding buffer solution at a ratio of 1:1 (v/v). | Sampling (20 μL) at flow rate of 0.1 mL min−1; Cleanup with binding buffer solution; Desorption: 20 μL ACN: Tris-HCl/EDTA buffer (40:60, v/v). Eluent was collected in a sample loop and injected to be measured with HPLC-FLD system. | Aptamer-based affinity monolith | HPLC-FLD | 0.05–50.00 2.4–6.0% 30 days | Zhao et al., 2021 [33] |
| Beer (10 mL) | Ochratoxin A | Samples were degassed; pH adjusted to 8.50, filtration (0.22 μm filter membrane) | Sampling (20 μL) at flow rate of 0.1 mL min−1; Cleanup with binding buffer solution (40 µL); Desorption: 20 μL ACN: Tris-HCl/EDTA buffer (30:70, v/v). | Aptamer-based affinity monolith | HPLC-FLD | 0.05–50.00 <2.8% 30 days | Zhao et al., 2021 [34] |
| Pomegranate, red grape, and sour cherry juice (20 mL) | Acidic red dyes (Amaranth, Ponceau 4R, Allura red, Carmoisine, and Erythrosine) | Samples filtered (0.45 μm) and diluted (30 times for the pomegranate and red grape juices and 10 times for the sour cherry juice). | Sampling (20 mL) at 500 μg L−1 in a circulating path. Desorption (offline): 358.7 μL of ethanol (96%) with NaNO3. Desorption solvent was dried and reconstituted in 50 μL. | Stainless steel wires coated with chitosan and polyvinyl alcohol | HPLC-UV | 1.0–750.0 2.5–12.7% 85 | Nasrollahi et al., 2022 [29] |
| Matrix | Analytes | Sample Pre-Treatment | In-Tube SPME Procedure | Stationary Phase | Separation/Detection System | Linear range (ng mL−1) Reproducibility (RSD, %) Durability (Extractions) | Ref. |
|---|---|---|---|---|---|---|---|
| Human plasma (NM) | Tricyclic antidepressants | Dilution 1:4 with water, followed by filtration | Loading and reconditioning: water Desorption: 0.01% acetic acid aqueous solution: ACN (30:70, v/v) | RAMIP-BSA (10 cm ×4.6 mm i.d.) | MS/MS | 15.0–500.0 4.1–13.6% NM | Santos et al., 2017 [13] |
| Human plasma (200 µL) | Amino acids and neurotransmitters | Protein precipitation (ACN, 400 µL), centrifugation (30 min, 9000 rpm), supernatant dried and reconstituted (50 µL ACN + 0.1% formic acid v/v). | Sample volume: 10 µL. Desorption: water at 0.1 mL min−1 | Cyano-Aminopropyl (10 cm × 530 µm i.d.) | MS/MS | 6–300 <15% 40 | Miranda et al., 2019 [12] |
| Cigarette tobacco (2.0 g) | Tobacco alkaloids | The cigarette tobacco filing of cigarettes was placed into a centrifuge tube containing 2 mL of diethyl ether and 5 wt% aqueous NaOH (10 mL). The tube was shaken and vortexed for 15 min at 4000 rpm, and the layers were separated. The ethyl layer was collected and dried under stream of N2 to obtain a residue that was reconstituted in 1% of ACN solution (v/v) and filtered through a 0.45 µm nylon filter prior to analysis. | Pre-conditioning: 1% of ACN in water for 5 min Sampling solution: 300 µL at a flow rate of 30 µL min−1 for 10 min. Desorption: at a flow rate of 10 µL min−1 for 10 min | Poly (NIPAAm-co-DVB-co-MBAA) (15 cm) | MS | 1–100 ** 0.34–1.22% 40 | Wu et al., 2019 [36] |
| Rat brain (1.0 mg) | AEA and 2-AG | Homogenization with 4.5 µL of 0.1 mol L−1 formic acid solution. After homogenization, addition of 150 µL of ACN and 100 µL of 5.0 mol L−1 ammonium formate, followed by agitation at 3000 rpm for 30 s in a vortex mixer and centrifugation at 9000 rpm and 5 °C for 15 min for protein precipitation. The upper organic phase was collected (50 µL), dried, and reconstituted with 50 µL of water/ACN (30:70, v/v) | Sampling: water/ACN (80:20, v/v) at 0,2 mL min−1. Desorption: 0.5% formic acid aqueous solution:ACN (30:70, v/v) at 0.2 mL min−1. Washing: ACN at 0.2 mL min−1 | Stainless steel tube packed with RAM phase (diol external surface and octyl inner surface) (25 mm × 4 mm × 25 µm) | MS/MS | 6.0–30.0 1.9–15.7% NM | Oliveira et al., 2021 [20] |
| Animal products (2.0 g) | Benzimidazole | Homogenization with 5 mL of ACN + 1% formic acid followed by agitation for 3 min. Whereafter, 1.0 g of NaCl and 2.0 g of MgSO4 was added, followed by agitation for 2 min and centrifugation at 3000 rpm for 5 min. The supernatant was collected and dried under N2 stream at room temperature. Then, the dried residues were reconstituted with a solution containing water:ACN (99:1, v/v) | Equilibration: 2% of ACN in water for 5 min; | Poly (AAPBA-co-DVB-co-MBAA) (20 cm) | MS | 514–1000 * <2.45% 60 | Wu et al., 2021 [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grecco, C.F.; de Souza, I.D.; Oliveira, I.G.C.; Queiroz, M.E.C. In-Tube Solid-Phase Microextraction Directly Coupled to Mass Spectrometric Systems: A Review. Separations 2022, 9, 394. https://doi.org/10.3390/separations9120394
Grecco CF, de Souza ID, Oliveira IGC, Queiroz MEC. In-Tube Solid-Phase Microextraction Directly Coupled to Mass Spectrometric Systems: A Review. Separations. 2022; 9(12):394. https://doi.org/10.3390/separations9120394
Chicago/Turabian StyleGrecco, Caroline Fernandes, Israel Donizeti de Souza, Igor Gustavo Carvalho Oliveira, and Maria Eugênia Costa Queiroz. 2022. "In-Tube Solid-Phase Microextraction Directly Coupled to Mass Spectrometric Systems: A Review" Separations 9, no. 12: 394. https://doi.org/10.3390/separations9120394
APA StyleGrecco, C. F., de Souza, I. D., Oliveira, I. G. C., & Queiroz, M. E. C. (2022). In-Tube Solid-Phase Microextraction Directly Coupled to Mass Spectrometric Systems: A Review. Separations, 9(12), 394. https://doi.org/10.3390/separations9120394