Extraction, Separation, Antitumor Effect, and Mechanism of Alkaloids in Sophora alopecuroides: A Review
Abstract
:1. Introduction
2. Phytochemistry
2.1. Alkaloids
2.2. Extraction and Separation of Alkaloids in S. alopecuroides
3. In Vitro Antitumor Activity
3.1. Effect on Tumor Cell Growth
3.2. Antimetastatic and Anti-Invasive Effects
3.3. Effects on Apoptosis and Autophagy
3.4. Modulation of Cell Cycle
4. In Vivo Effects
5. Antitumor Mechanism
5.1. Research on the Cellular Mechanism of Antitumor Effect
5.1.1. Inhibiting Tumors by Affecting the Cell Cycle
5.1.2. Alter Cell Signal to Impede Tumor
5.1.3. Altering the Cytoskeleton to Impede Tumors
5.2. Research on the Molecular Mechanism of Antitumor Effect
5.2.1. Regulate the Expression of Apoptosis-Related Genes
5.2.2. Inhibition of Tumor Drug Resistance by Regulating Drug-Resistant Genes
5.3. Impede Tumor Invasion and Metastasis
5.4. Inhibition of Telomerase Activity
5.5. Enhanced Immunity
5.6. Delaying Induced Canceration and Preventing Chronic Inflammation from Developing into Cancer
6. Products in Clinical Trials
7. Network Pharmacology
7.1. Network Pharmacology Construction and Analysis of Alkaloids in S. alopecuroides
7.2. ADME Screening of Alkaloids in S. alopecuroides
7.3. Identification of Potential Related-Tumor Targets
7.4. Pathway Analysis and Network Building
8. Toxicity of S. alopecuroides
9. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Wang, R.; Deng, X.; Gao, Q.; Wu, X.; Han, L.; Gao, X.; Zhao, S.; Chen, W.; Zhou, R.; Li, Z.; et al. Sophora alopecuroides L.: An ethnopharmacological, phytochemical, and pharmacological review. J. Ethnopharmacol. 2020, 248, 112172. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.N.; Li, S.X.; Zhai, K.F.; Kou, J.P.; Yu, B.Y. Network pharmacology-based prediction and verification of the molecular targets and pathways for schisandrin against cerebrovascular disease. Chin. J. Nat. Med. 2014, 12, 251–258. [Google Scholar] [CrossRef]
- You, J.; Li, Y.; Sha, B.; Yin, X. Advances in Studies on the Alkaloid of Sophora alopecuroides L. J. Jiangxi Univ. Tradit. Chin. Med. 2015, 27, 109–113, 116. [Google Scholar]
- Shan, X.J.; Di, M.L.; Tao, Z.W. Research progress on the chemical constituents and pharmacology of Sophora flavescens. Chin. J. Inform. Tradit. Chin. Med. 2011, 18, 105–107. [Google Scholar]
- Yang, J.; Yu, Z. Advance on Sophora alopecuraides. Tianjin Pharm. 1998, 10, 43–46. [Google Scholar]
- Wang, Y.; Xia, Z.; Liu, Z.; Wan, C.; Luo, X.; Zhang, L. Streptomyces carminius sp. nov., a novel actinomycete isolated from Sophora alopecuroides in Xinjiang, China. Antonie Leeuwenhoek 2018, 111, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Zhang, X.L.; Chen, N.H.; Wu, Z.N.; Ye, W.C.; Li, Y.L.; Wang, G.C. Four matrine-based alkaloids with antiviral activities against HBV from the seeds of Sophora alopecuroides. Org. Lett. 2017, 19, 424–427. [Google Scholar] [CrossRef]
- Zhao, W.C.; Song, L.J.; Deng, H.Z. Protective effect of total alkaloids of Sophora alopecuroides on dextran sulfate sodium-induced chronic colitis. Chin. J. Integr. Med. 2011, 17, 616–624. [Google Scholar] [CrossRef]
- Li, J.-G.; Yang, X.-Y.; Huang, W. Total alkaloids of Sophora alopecuroides inhibit growth and induce apoptosis in human cervical tumor hela cells in vitro. Pharmacogn. Mag. 2016, 12, S253–S256. [Google Scholar]
- Ling, Z.; Guan, H.; You, Z.; Wang, C.; Hu, L.; Zhang, L.; Wang, Y.; Chen, S.; Xu, B.; Chen, M. Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer in vitro and in vivo. Onco. Targets Ther. 2018, 11, 2735–2743. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yang, L.; Luo, J.; Zhang, C.; Xia, Y.; Ma, T.; Kong, L. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264. 7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2016, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.X.; Luo, J.G.; Ren, X.P.; Kong, L.Y. Interconverting flavonostilbenes with antibacterial activity from Sophora alopecuroides. Phytochemistry 2015, 116, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, S.J.; Wang, H.L.; Li, Y.-X.; Du, J.; Zhou, R.; Zheng, J.; Ma, L.; Zhao, C.J.; Niu, Y. Effects of aloperine on acute and inflammatory pain models in mice. Scand. J. Pain 2015, 8, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.X.; Wang, H.L.; Jin, S.J.; Zhou, R.; Qiao, H.Q.; Du, J.; Wu, J.; Zhao, C.J.; Niu, Y. Oxysophocarpine ameliorates carrageenan-induced inflammatory pain via inhibiting expressions of prostaglandin E2 and cytokines in mice. Planta. Med. 2015, 81, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, L.; Guan, G.; Wang, J.; Qiu, C.; Yang, T.; Guo, Y.; Liu, Z. Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad pathway in experimental diabetic cardiomyopathy. Mol. Med. Rep. 2018, 17, 1775–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, M.; Shi, F.; Dai, F.; Song, R.; Wang, S.; You, Y.; Zhao, B. A reactive oxygen species activation mechanism contributes to Sophoridine-induced apoptosis in rat liver BRL-3A cells. J. Ethnopharmacol. 2018, 213, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Li, H.Q. Anti-arrhythmic effects of sophoridine and oxysophoridine. Acta Pharmacol. Sin. 1999, 20, 517–520. [Google Scholar]
- Wang, H.; Li, Y.; Jiang, N.; Chen, X.; Zhang, Y.; Zhang, K.; Wang, T.; Hao, Y.; Ma, L.; Zhao, C. Protective effect of oxysophoridine on cerebral ischemia/reperfusion injury in mice. Neural. Regen. Res. 2013, 8, 1349–1359. [Google Scholar]
- Zhao, P.; Chang, R.Y.; Liu, N.; Wang, J.; Zhou, R.; Qi, X.; Liu, Y.; Ma, L.; Niu, Y.; Sun, T. Neuroprotective Effect of Oxysophocarpine by Modulation of MAPK Pathway in Rat Hippocampal Neurons Subject to Oxygen-Glucose Deprivation and Reperfusion. Cell Mol. Neurobiol. 2018, 38, 529–540. [Google Scholar] [CrossRef]
- Qiu, X.; Jia, J. Research advances on TCM anti-tumor effects and the molecular mechanisms. J. Cancer Res. Ther. 2014, 10, 8. [Google Scholar]
- Xu, J.; Song, Z.; Guo, Q.; Li, J. Synergistic effect and molecular mechanisms of traditional Chinese medicine on regulating tumor microenvironment and cancer cells. BioMed. Res. Int. 2016, 2016, 1490738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mérarchi, M.; Sethi, G.; Fan, L.; Mishra, S.; Arfuso, F.; Ahn, K.S. Molecular targets modulated by fangchinoline in tumor cells and preclinical models. Molecules 2018, 23, 2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Jin, Z.; Dai, L.; Wu, H.; Wang, J.; Wang, L.; Zhou, Z.; Yang, L.; Gao, W. Aloperine induces apoptosis and inhibits invasion in MG-63 and U2OS human osteosarcoma cells. Biomed. Pharmacother. 2018, 97, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jiang, B.; Chen, Z.; Wang, X.; Shang, D.; Zhang, X.; Sun, Y.; Yang, J.; Ji, Y. Cytisine induces endoplasmic reticulum stress caused by calcium overload in HepG2 cells. Oncol. Rep. 2018, 39, 1475–1484. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, X.; Chen, Z.F.; Jiang, B.; Shang, D.Y.; Sun, Y.X.; Yang, J.H.; Zhang, L.F.; Ji, Y.B. Cytisine induces apoptosis of HepG2 cells. Mol. Med. Rep. 2017, 16, 3363–3370. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Liu, S.P.; Fang, C.H.; He, R.S.; Wang, Z.; Zhu, Y.Q.; Jiang, S.W. Effects of matrine on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol. Lett. 2013, 6, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Q.; Li, Y.; Qin, J.; Wang, Q.; She, Y.L.; Luo, Y.L.; He, J.X.; Li, J.Y.; Xie, X.D. Matrine reduces proliferation of human lung cancer cells by inducing apoptosis and changing miRNA expression profiles. Asian Pac. J. Cancer Prev. 2014, 15, 2169–2177. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Jiang, J.; Cui, H. Antitumor effects of matrine on cancer stem like cells isolated from the human liver cancer SMMC-7721 cell line. Oncol. Lett. 2018, 15, 1777–1782. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Li, Y.M.; Liu, T.; He, W.T.; Chen, Y.T.; Chen, X.H.; Li, X.; Zhou, W.C.; Yi, J.F.; Ren, Z.J. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J. Gastroenterol. 2010, 16, 4281–4290. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, L.; Bi, T.; Dai, W.; Liu, W.; Gao, Q.; Shen, G. Oxymatrine synergistically potentiates the antitumor effects of cisplatin in human gastric cancer cells. J. Cancer 2018, 9, 4527–4535. [Google Scholar] [CrossRef]
- Wang, B.; Han, Q.; Zhu, Y. Oxymatrine inhibited cell proliferation by inducing apoptosis in human lung cancer A549 cells. BioMed. Mater. Eng. 2015, 26, S165–S172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Cai, Y.; Li, M.; Zhang, Y.; Li, H.; Tan, Z. Oxymatrine promotes S-phase arrest and inhibits cell proliferation of human breast cancer cells in vitro through mitochondria-mediated apoptosis. Biol. Pharm. Bull. 2017, 40, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Wang, X.Y.; Zhang, X.H.; Ji, B.; Yan, H.C.; Deng, H.Z.; Wu, X.R. Sophoridine exerts an anti-colorectal carcinoma effect through apoptosis induction in vitro and in vivo. Life Sci. 2012, 91, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, F.; Bai, C.; Yao, C.; Zhong, H.; Zou, C.; Chen, X. Sophoridine induces apoptosis and S phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.J.; Yang, Y.; Ma, L.; Ma, B.H.; Ren, L.P.; Guo, L.C.; Wang, W.B.; Zhang, Y.X.; Zhao, Z.J.; Cui, M. In vivo and in vitro induction of the apoptotic effects of oxysophoridine on colorectal cancer cells via the Bcl-2/Bax/caspase-3 signaling pathway. Oncol. Lett. 2017, 14, 8000–8006. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Chen, G.; Wu, W.; Zhou, L.; Shi, Y.; Zeng, Q.; Li, Y.; Sun, Y.; Deng, X. Targeting miR-21 with sophocarpine inhibits tumor progression and reverses epithelial-mesenchymal transition in head and neck cancer. Mol. Ther. 2017, 25, 2129–2139. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Xu, Y.; Zuo, W.; Su, Z. MicroR-542-3p can mediate ILK and further inhibit cell proliferation, migration and invasion in osteosarcoma cells. Aging Albany 2019, 11, 18–32. [Google Scholar] [CrossRef]
- Yu, Y.T. Extraction, Separation and Purification of Alkaloids from Sophora alopecuroides L. Master’s Thesis, Xinjiang Agricultural University, Urumchi, China, 2007. [Google Scholar]
- Xiao, X.; Ao, M.; Xu, F.; Li, X.; Hu, J.; Wang, Y.; Li, D.; Zhu, X.; Xin, C.; Shi, W. Effect of matrine against breast cancer by downregulating the vascular endothelial growth factor via the Wnt/β-catenin pathway. Oncol. Lett. 2018, 15, 1691–1697. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Guo, Y.J.; Yang, X.L.; Ou, Z.L. Anti-cervical cancer role of matrine, oxymatrine and Sophora flavescens alkaloid gels and its mechanism. J. Cancer 2018, 9, 1357–1364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cheng, B.; Li, H.; Xu, W.; Zhai, B.; Pan, S.; Wang, L.; Liu, M.; Sun, X. Matrine inhibits proliferation and induces apoptosis of human colon cancer LoVo cells by inactivating Akt pathway. Mol. Biol. Rep. 2014, 41, 2101–2108. [Google Scholar] [CrossRef]
- Duan, L.; Deng, L.; Wang, D.; Ma, S.; Li, C.; Zhao, D. Treatment mechanism of matrine in combination with irinotecan for colon cancer. Oncol. Lett. 2017, 14, 2300–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Zhang, S.; Ma, H.; Wang, Y.; Liu, D.; Wang, X.; Wang, Z. Matrine reduces the proliferation and invasion of colorectal cancer cells via reducing the activity of p38 signaling pathway. Acta Biochimica. Biophysica. Sin. 2014, 46, 1049–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, J.; Tang, X.; Zhuang, X.; Hu, Z.; He, K.; Wu, Y.; Dai, T. Matrine induces apoptosis via targeting CCR7 and enhances the effect of anticancer drugs in non-small cell lung cancer in vitro. Innate. Immun. 2018, 24, 394–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Lu, J.; Lu, Q.; Wang, X.; Long, H.; Huang, J.; Guo, Z. Matrine inhibits the proliferation and migration of lung cancer cells through regulation of the protein kinase B/glycogen synthase kinase-3β signaling pathways. Exp. Ther. Med. 2018, 16, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.; Zhang, Y.; Wu, B.; Zhang, Y.; Jiang, H.; He, P. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol. Rep. 2014, 32, 1087–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Qian, X.; Jia, R.; Wu, M.; Liang, Z. Matrine induction of reactive oxygen species activates p38 leading to caspase-dependent cell apoptosis in non-small cell lung cancer cells. Oncol. Rep. 2013, 30, 2529–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Chen, T.; Yu, X.; Luo, M.; Chen, X.; Lin, C.; Lai, Y.; Huang, H. The specific killing effect of matrine on castration-resistant prostate cancer cells by targeting the Akt/FoxO3a signaling pathway. Oncol. Rep. 2017, 37, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, Z.; Wang, T.; Guo, H.; Ye, Z. Inhibitory effect of matrine on the expression of PSA and AR in prostate cancer cell line LNCaP. J. Huazhong Univ. Sci. Technol. 2008, 28, 697–699. [Google Scholar] [CrossRef]
- Li, Q.; Huang, H.; He, Z.; Sun, Y.; Tang, Y.; Shang, X.; Wang, C. Regulatory effects of antitumor agent matrine on FOXO and PI3K-AKT pathway in castration-resistant prostate cancer cells. Sci. Chin. Life Sci. 2018, 61, 550–558. [Google Scholar] [CrossRef]
- Liao, X.Z.; Tao, L.T.; Liu, J.H.; Gu, Y.Y.; Xie, J.; Chen, Y.; Lin, M.G.; Liu, T.L.; Wang, D.M.; Guo, H.-Y. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Li, W.; Yu, X.; Tan, S.; Liu, W.; Zhou, L.; Liu, H. Oxymatrine inhibits non–small cell lung cancer via suppression of EGFR signaling pathway. Cancer Med. 2018, 7, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, R.; Cao, J.; Yao, S. Matrine promotes liver cancer cell apoptosis by inhibiting mitophagy and PINK1/Parkin pathways. Cell Stress Chapero. 2018, 23, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Cui, S. Matrine inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/Akt pathway. Oncol. Lett. 2018, 16, 2965–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Du, H.; Geng, G.; Zhou, H.; Xu, M.; Cao, H.; Zhang, B.; Song, G.; Hu, T. Matrine inhibits proliferation and induces apoptosis via BID-mediated mitochondrial pathway in esophageal cancer cells. Mol. Biol. Rep. 2014, 41, 3009–3020. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhu, Y.; Jiang, T.; Lu, X.; Zhang, W.; Jing, Q.; Li, J.; Pang, L.; Chen, K.; Qiu, F. Matrine induced gastric cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules of Bcl-2 family. Toxicology 2007, 229, 245–252. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, X.; Liu, T.; Chen, Y.; He, W.; Zhang, Q.; Liu, S. Autophagy is involved in anticancer effects of matrine on SGC-7901 human gastric cancer cells. Oncol. Rep. 2011, 26, 115–124. [Google Scholar]
- Guo, B.; Zhang, T.; Su, J.; Wang, K.; Li, X. Oxymatrine targets EGFR p-Tyr845 and inhibits EGFR-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 353–363. [Google Scholar] [CrossRef]
- Tian, D.; Li, Y.; Li, X.; Tian, Z. Aloperine inhibits proliferation, migration and invasion and induces apoptosis by blocking the Ras signaling pathway in human breast cancer cells. Mol. Med. Rep. 2018, 18, 3699–3710. [Google Scholar] [CrossRef]
- Li, H.; Tan, G.; Jiang, X.; Qiao, H.; Pan, S.; Jiang, H.; Kanwar, J.R.; Sun, X. Therapeutic Effects of Matrine on Primary and Metastatic Breast Cancer. Am. J. Chin. Med. 2010, 38, 1115–1130. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Wang, G.; Chen, X.; Zhang, R.; Liu, H.; Zhu, J. Oxymatrine exhibits anti-tumor activity in gastric cancer through inhibition of IL-21R-mediated JAK2/STAT3 pathway. Int. J. Immunopathol. Pharmacol. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Liang, L.; Huang, J. Oxymatrine inhibits epithelial-mesenchymal transition through regulation of NF-κB signaling in colorectal cancer cells. Oncol. Rep. 2016, 36, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngabire, D.; Kim, G.D. Autophagy and inflammatory response in the tumor microenvironment. Int. J. Mol. Sci. 2017, 18, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Huang, W.; Guo, Y.; Xia, P.; Sun, X.; Pan, X.; Hu, W. Oxymatrine inhibits the proliferation of prostate cancer cells in vitro and in vivo. Mol. Med. Rep. 2015, 11, 4129–4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Cutsem, E.; Aerts, R.; Haustermans, K.; Topal, B.; Van Steenbergen, W.; Verslype, C. Systemic treatment of pancreatic cancer. Eur. J. Gastroenterol. Hepatol. 2004, 16, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Xu, X.; Wei, X.; Wang, W.; Zhou, B.; Wang, B.; Zheng, S. Oxymatrine induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome c. J. Exp. Clin. Cancer Res. 2011, 30, 66. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Song, Y.; Chen, H.; Pan, S.; Sun, X. Matrine inhibits proliferation and induces apoptosis of pancreatic cancer cells in vitro and in vivo. Biol. Pharm. Bull. 2010, 33, 1740–1745. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, X.; Bai, M.; Suo, Y.; Zhang, G.; Cao, X. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int. J. Clin. Exp. Pathol. 2015, 8, 14793. [Google Scholar]
- Shao, H.; Yang, B.; Hu, R.; Wang, Y. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-κB signaling pathway. Oncol. Lett. 2013, 6, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κ B signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef]
- Goto, Y.; Koyasu, S.; Kobayashi, M.; Harada, H. The emerging roles of the ubiquitination/deubiquitination system in tumor radioresistance regarding DNA damage responses, cell cycle regulation, hypoxic responses, and antioxidant properties: Insight into the development of novel radiosensitizing strategies. Mutat. Res. Fund. Mol. Mech. Mutagen. 2017, 803, 76–81. [Google Scholar]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor. Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, Y.; Deng, H.; Liang, L.; Peng, J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Med. 2014, 33, 1613–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.P.; Wang, P.Q.; Qiao, C.P.; Zhang, Q.; Zhang, J.P.; Chen, F.; Zhang, X.; Xie, W.F.; Yuan, Z.L.; Li, Z.S. Differentiation therapy of hepatocellular carcinoma by inhibiting the activity of AKT/GSK-3β/β-catenin axis and TGF-β induced EMT with sophocarpine. Cancer Lett. 2016, 376, 95–103. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Han, C.; Zhou, Y.; Li, F.; Li, D.; Zhang, X.; Yu, Z.; Duan, Z.; Kan, Q. Matrine induces cell cycle arrest and apoptosis with recovery of the expression of miR-126 in the A549 non-small cell lung cancer cell line. Mol. Med. Rep. 2016, 14, 4042–4048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.Q.; Li, X.L.; Wang, L.; Du, W.J.; Guo, R.; Liang, H.H.; Liu, X.; Liang, D.S.; Lu, Y.J.; Shan, H.L. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol. Biochem. 2012, 30, 631–641. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Liu, X.; Wu, H.; Lin, X.; Gu, J.; Wang, H.; Duan, Y. Matrine alters microRNA expression profiles in SGC-7901 human gastric cancer cells. Oncol. Rep. 2014, 32, 2118–2126. [Google Scholar] [CrossRef]
- Song, S.; Zhu, S.; Zhang, Z.; Mo, Z.; Ke, Q.; Luo, Z. A study on the inhibitory effect of matrine on gastric cancer SGC-7901 cells. Afr. J. Tradit. Complem. Altern. Med. 2013, 10, 435–438. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Chen, M.H.; May, B.H.; Liao, X.Z.; Liu, J.H.; Tao, L.T.; Sze, D.M.y.; Zhang, A.L.; Mo, S.L. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine 2018, 51, 214–225. [Google Scholar] [CrossRef]
- Chang, J.; Hu, S.; Wang, W.; Li, Y.; Zhi, W.; Lu, S.; Shi, Q.; Wang, Y.; Yang, Y. Matrine inhibits prostate cancer via activation of the unfolded protein response/endoplasmic reticulum stress signaling and reversal of epithelial to mesenchymal transition. Mol. Med. Rep. 2018, 18, 945–957. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lai, Y.; Wang, C.; Xu, G.; He, Z.; Shang, X.; Sun, Y.; Zhang, F.; Liu, L.; Huang, H. Matrine inhibits the proliferation, invasion and migration of castration-resistant prostate cancer cells through regulation of the NF-κB signaling pathway. Oncol. Rep. 2016, 35, 375–381. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Chong, T.; Ji, Z. Matrine inhibits proliferation and induces apoptosis of the androgen-independent prostate cancer cell line PC-3. Mol. Med. Rep. 2012, 5, 783–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, Y.; Liu, Q.; Zhao, Q.; Xu, L.; Huang, S.; Huang, S.; Wei, X. Oxymatrine inhibits proliferation of human bladder cancer T24 cells by inducing apoptosis and cell cycle arrest. Oncol. Lett. 2017, 13, 4453–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine attenuates tumor growth and deactivates STAT5 signaling in a lung cancer xenograft model. Cancers 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Bi, T.; Wang, Z.; Wu, G.; Qian, L.; Gao, Q.; Shen, G. Oxymatrine synergistically enhances antitumor activity of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway. Apoptosis 2016, 21, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Cai, Z.; Wang, Z.; Sun, S. Extraction and isolation of alkaloids of Sophora alopecuroides and their anti-tumor effects in H22 tumor-bearing mice. Afr. J. Tradit. Complem. Altern. Med. 2014, 11, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yang, S.; Zhou, H.; Sun, M.; Du, L.; Wei, M.; Luo, M.; Huang, J.; Deng, H.; Feng, Y. Aloperine executes antitumor effects against multiple myeloma through dual apoptotic mechanisms. J. Hematol. Oncol. 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Jiang, C.; Zhong, B.; Sheng, J.; Chen, T.; Chen, Q.; Li, J.; Zhao, H. Matrine ameliorates colorectal cancer in rats via inhibition of HMGB1 signaling and downregulation of IL-6, TNF-α, and HMGB1. J. Immunol. Res. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Zou, M.M.; Li, P.; Lin, X.J.; Jiang, Q.W.; Yang, Y.; Huang, J.R.; Yuan, M.L.; Xing, Z.H.; Wei, M.N. Oxymatrine and cisplatin synergistically enhance anti-tumor immunity of CD8+ T cells in non-small cell lung cancer. Front. Oncol. 2018, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Wang, Y.; Zheng, H. Oxymatrine induces apoptosis of ovarian cancer HO8910 cells. Acta Chin. Med. Pharmacol. 2004, 32, 10–11. [Google Scholar]
- Qian, X.; Li, J.; Luo, H.; Shen, G.; Tong, J. Study on Proliferation Inhibition by Oxymatrine on SMMC-7721 Cell In Vitro. J. Shanghai Second. Med. Univ. 2002, 22, 512–514. [Google Scholar]
- Luo, Q. Studies on Apoptosis of Human Hepatoma Cells Induced by Oxymatrine and Its Correlative Biological Mechanisms. Master’s Thesis, Chongqing University, Sichuan, China, 2005. [Google Scholar]
- Zhang, L.; Jiang, J.; Tam, J.; Zhang, Y.; Liu, X.; Xu, X.; Liu, B.; He, Y. Effects of matrine on proliferation and differentiation in K-562 cells. Leuk. Res. 2001, 25, 793–800. [Google Scholar] [CrossRef]
- Li, P. Comparative Study on Inhibiting Proliferation of Hepatocellular Carcinoma In Vitro and In Vivo by Matrine and Oxmatrine. Master’s Thesis, Third Military Medical University, Chongqing, China, 2004. [Google Scholar]
- Liu, B.; Jiang, J.; Zhang, Y.; Liu, X.; He, Y. Calcium signaling in K562 cells triggered by matrine. J. Clin. Lab. Sci. 2003, 38, 33–35. [Google Scholar]
- Liu, B.; Jiang, J.; He, Y.; Zhang, Y.; Liu, X. Change of the Activity and Expression of Phospholipase A2 in K562 Cells Induced by Matrine. Pract. J. Cancer 2002, 460–462. [Google Scholar]
- Liu, X.; Jiang, J.; Zhang, Y.; He, Y.; Ma, L. Effects of matrine on phosphorylation of intracellular tyrosine protein in human leukemia K562 cells. Chin. J. Pract. Tradit. Chin. West Med. 2004, 17, 3030–3031. [Google Scholar]
- Bharadwaj, S.; Thanawala, R.M.; Bon, G.; Falcioni, R.; Prasad, G.L. Resensitization of breast cancer cells to anoikis by Tropomyosin-1: Role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene 2005, 24, 8291–8303. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Keng, P.C. Studying the effects of actin cytoskeletal destabilization on cell cycle by cofilin overexpression. Mol. Biotechnol. 2005, 31, 1–10. [Google Scholar] [CrossRef]
- Stoicov, C.; Cai, X.; Li, H.; Klucevsek, K.; Carlson, J.; Saffari, R.; Houghton, J. Major histocompatibility complex class II inhibits Fas antigen-mediated gastric mucosal cell apoptosis through actin-dependent inhibition of receptor aggregation. Infect. Immun. 2005, 73, 6311–6321. [Google Scholar] [CrossRef] [Green Version]
- Huang-Fu, C.-S.; Geng, X.; Gu, J.L.; Liu, B. Change on Cytoskeleton of SMMC-7721 Cell Lines Differentiation Induced by Matrine. J. Henan. Univ. 2006, 25, 12–14. [Google Scholar]
- Zhang, B.H.; Yue, H.Y.; Li, X.M. Effect of matrine on expression of Bcl-2 in gastric carcinoma cells. Chin. J. Clin. Oncol. Rehabil. 2000, 7, 34–35. [Google Scholar]
- Si, W.; Gao, L.; Liu, B.; Chen, A.; Li, P.; Yao, J. Regulation of G1 Cell Cycle Regulation Factors in Differentiation and Apoptosis of Matrine-Inuced Hepatoma Cells. Chin. J. Cancer 2001, 20, 848–851. [Google Scholar]
- Zhang, L.; Jiang, J.; Joe, T. Expression of oncogene and protein regulating cell cycle progression and its significance in leukemia cell line by matrine. Chin. J. Oncol. 2001, 28, 347–350. [Google Scholar]
- Deng, H.; Luo, H.; Huang, F.; Li, X.; Gao, Q. Inhibition of proliferation and influence of proto-oncogenes expression by matrine in C6 cell. J. Chin. Med. Mater 2004, 27, 416–419. [Google Scholar]
- He, Y.; Jiang, J.; Ou, Y.; Liu, B.; Liu, X.; Zhang, Y.; Ma, L.; Tu, Z. Matrine affects early expression of proto-oncogenes in K562 cells. Chin. J. Cancer 2002, 21, 369–372. [Google Scholar]
- Hu, M.J.; Zeng, H.; Wu, Y.L.; Zhang, Y.P.; Zhang, S.; Qiao, M.M.; Fu, H. Synergistic effects of martine and 5-fluorouridine on tumor growth of the implanted gastric cancer in nude mouse. Chin. J. Dig. Dis. 2005, 6, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Huang, G.; Guo, Y. The expression of cathepsin D and Fas ligand on pct of JM cells induced by matrine. Chin. J. Cell Biol. 2003, 25, 238–241. [Google Scholar]
- Sun, F.; Wang, N.; Li, G.; Wang, X.; Li, X.; Yin, G. The effect of matrine on the expression of P170 LRP and TOPO II of obtained multi-drug resistance of mouse S180’s tumour cell. J. Chin. Med. Mater. 2004, 27, 838–840. [Google Scholar]
- Ma, L. Apoptosis of Human Heptoma Cell HepG-2 Induced by Sophoridine and Its Mechanisms of Regulatory Genes. Master’s Thesis, Lanzhou University, Lanzhou, China, 2006. [Google Scholar]
- Li, X.; Zhang, S.; Zheng, S. Cellular biological effects of matrine on K562 and K562/Vin cells. Chin. J. Pathophysiol. 2000, 18, 66–70. [Google Scholar]
- Yu, S.H.; Yan, L.N.; Zhou, S.B.; Zhang, Y.; Gou, X.H.; Han, L.; Chen, Y.B. Construction of microsphere encapusulating recombinant adenovirus with antisense multidrug resistance-associated protein gene and its effect on hepatocellular carcinoma. World Chin. J. Digestol. 2005, 13, 20–25. [Google Scholar] [CrossRef]
- Luo, H.; Yan, L.; Yang, J.; Liu, Z.; Lin, Q. Reversal of Multidrug Resistance Gene mdr1 of Drug-Resistant Human Hepatocellular Carcinoma Cells with Antisense Oligodeoxynucleotide in Vivo. Chin. J. Bases Clin. Gen. Surg. 2004, 11, 142–144. [Google Scholar]
- Wang, Y.; Peng, C.; Zhang, G.; Liu, Y.; Li, H.; Shan, J. Study on invasion and metastasis related factors in differentiation of SMMC-7721 cells induced by matrine. J. Chin. Med. Mater. 2003, 26, 566–569. [Google Scholar]
- Roy, M.; Reiland, J.; Murry, B.P.; Chouljenko, V.; Kousoulas, K.G.; Marchetti, D. Antisense-mediated suppression of heparanase gene inhibits melanoma cell invasion. Neoplasia 2005, 7, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Y. Inhibitory Effects of Matrine on Invasiveness and Metastasis of Human Malignant Melanoma Cell Line A375 In Vitro. Master’s Thesis, Zhejiang University, Zhejiang, China, 2006. [Google Scholar]
- Zhu, B.D.; Yuan, S.J.; Zhao, Q.C.; Li, X.; Li, Y.; Lu, Q.Y. Antitumor effect of Gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, combined with cytotoxic agent on murine hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Hao, Y.B.; Shi, Y.Q.; Han, S.; Jia, Y. Mechanism of matrine suppressing HT-29 cell line proliferation. Chin. J. Exp. Surg. 2005, 22, 75–76. [Google Scholar]
- Zuo, G.Q.; He, S.; Zhang, Y. Effects of matrine on telomerase activity and on proliferation of human HepG2 and QGY hepatoma cell lines. J. Oncol. 2005, 11, 126–128. [Google Scholar]
- Chen, W.; Lin, Y.; Xie, W.; Zhang, J.; Zhang, X.; Cheng, Z. Regulation of telomerase activity and cell cycle by matrine in hepatoma cells in vitro. J. Second Mil. Med. Univ. 2002, 23, 498–500. [Google Scholar]
- Xu, G.; Man, S.; Wang, Z.; Wang, L. Effect of oxidized bitter ginseng alkaloids on immune function in tumor-bearing mice. Chin. J. Clin. Oncol. Rehabil. 2001, 8, 9–10. [Google Scholar]
- Cheng, J.; Hu, J. WUH Effect of Immune Function in Advanced Cancer Patients Treated by Combined Yan-shu Injection. Chin. J. Inform. TCM 2004, 11, 478–479. [Google Scholar]
- Chen, G.H.; Li, S.R.; Yang, L. Therapeutic effects of complex kushen injection combined with double interventional therapy on treating primary hepatocarcinoma. Chin. J. Integr. Tradit. West Med. Digest. 2007, 15, 239–241. [Google Scholar]
- Luo, M.; He, P.; Wu, M.C.; Wei, L.X.; Li, L.F.; Guo, Y.J. The preventive effect of matrine on rathepatoma induced by diethylnitrosamine. Tumor 2001, 21, 239–241. [Google Scholar]
- Guo, H. Inhibitory Effect of Matrine on Lung Caner Activity and the Study of Clinical Application between Compound Kushen Injection and Chemotherapy in Treeating Lung Cancer. Master’s Thesis, Jinan University, Guangzhou, China, 2017. [Google Scholar]
- Du, R.; Zhang, Y.; Hu, Q. Effect of matrine injection on sensitization of chemotherapy for cervical cancer patients and on their survival quality and immune function. Chin. Arch. Tradit. Chin. Med. 2018, 36, 3051–3055. [Google Scholar]
- Lu, L.; Lan, G.; Zhu, X.; Ji, H. Effect of Matrine injection on Serum tumor markers in patients with Cervical Cancer after Neoadjuvant chemotherapy. Chin. J. Prev. Control. Chron. Dis. 2018, 26, 444–446. [Google Scholar]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Li, Y.; Wang, J.; Zhang, J.; Hou, T. ADME evaluation in drug discovery. 9. Prediction of oral bioavailability in humans based on molecular properties and structural fingerprints. Mol. Pharm. 2011, 8, 841–851. [Google Scholar] [PubMed]
- Pereira, C.; Araújo, F.; Barrias, C.C.; Granja, P.L.; Sarmento, B. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials 2015, 56, 36–45. [Google Scholar] [CrossRef]
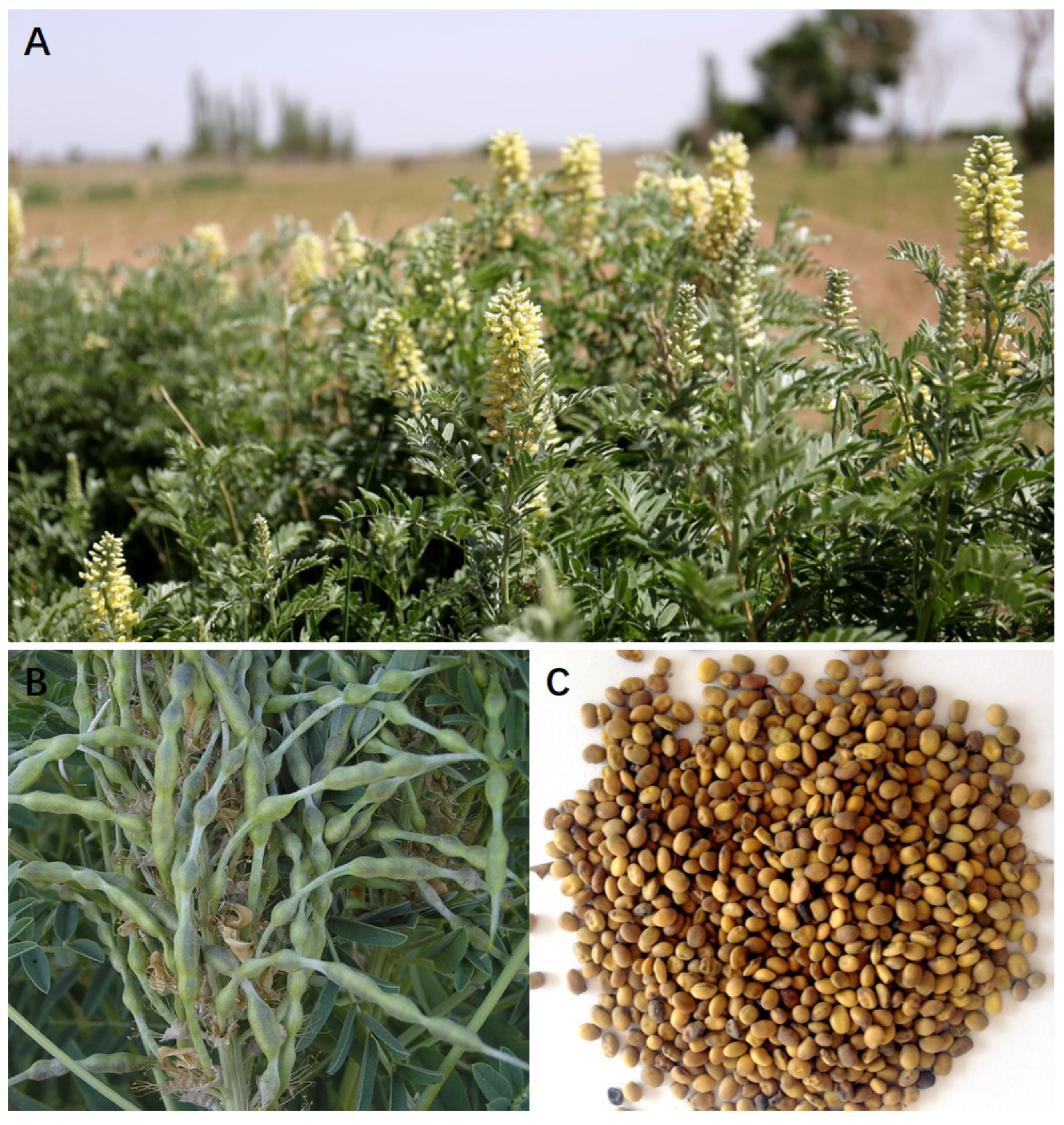
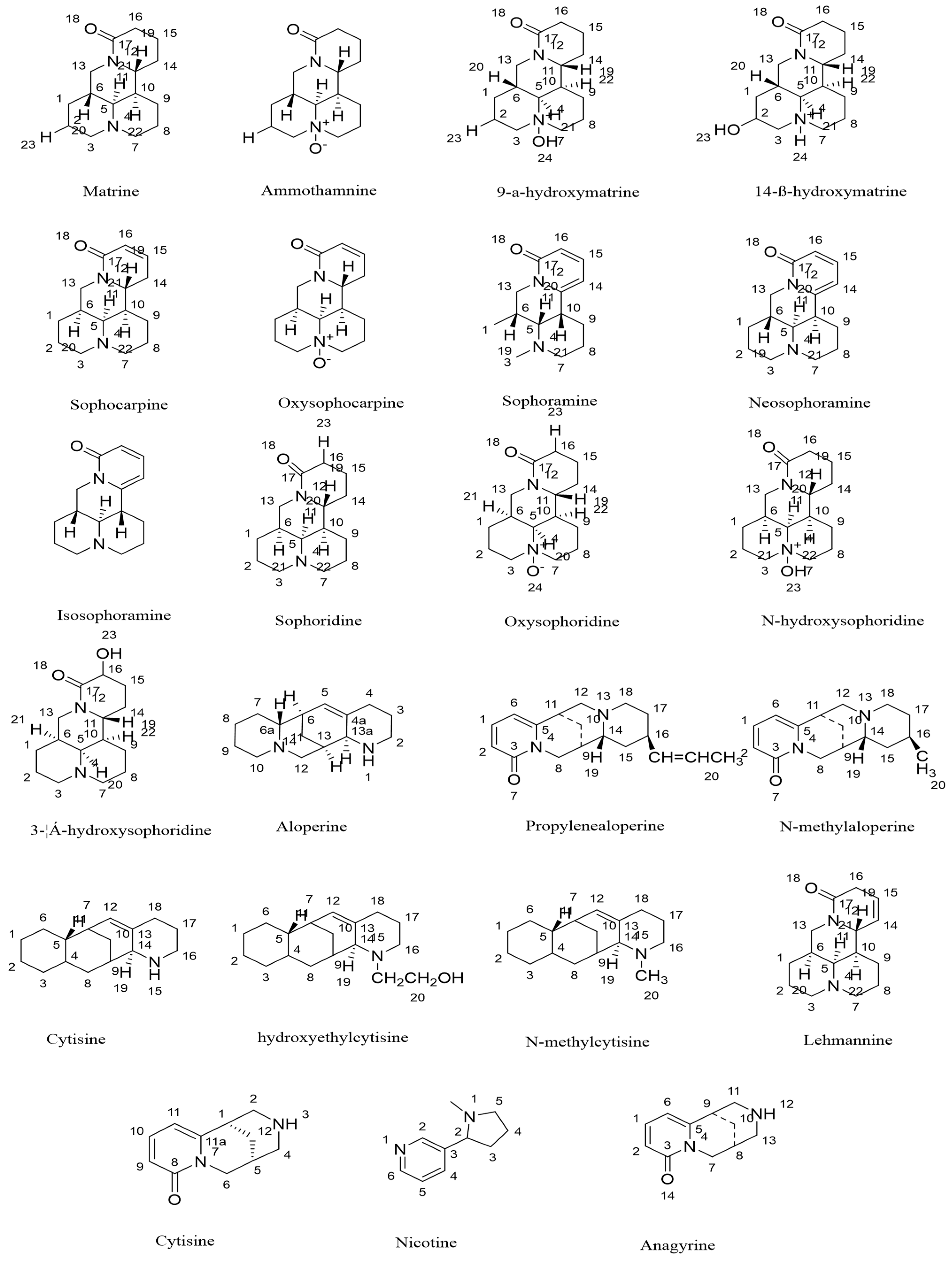

| No. | Compounds | CAS | References | Structure Type |
|---|---|---|---|---|
| 1 | Matrine | 519-02-8 | [1] | Matrine-tetracyclic |
| 2 | Sophoridine | 6882-68-4 | [1] | Matrine-tetracyclic |
| 3 | Sophocarpine | 6483-15-4 | [1] | Matrine-tetracyclic |
| 4 | N-oxide-1314-dehydro-sophoridine | 77077-09-9 | [2] | Matrine-tetracyclic |
| 5 | Neosophoramine | 52932-74-8 | [3] | Matrine-tetracyclic |
| 6 | Isosophoramine | 6838-34-2 | [3] | Matrine-tetracyclic |
| 7 | 7α-hydroxy-sophoramine | 259860-46-3 | [4] | Matrine-tetracyclic |
| 8 | 3α-hydroxy-sophoridine | 41645-69-6 | [4] | Matrine-tetracyclic |
| 9 | Lehmannine | 58480-54-9 | [4] | Matrine-tetracyclic |
| 10 | Sophoramine | 6882-66-2 | [5] | Matrine-tetracyclic |
| 11 | N-hydroxy-sophoridine | 32968-81-3 | [5] | Matrine-tetracyclic |
| 12 | 9α-hydroxy-matrine | 88509-92-6 | [4] | Matrine-tetracyclic |
| 13 | 14β-hydroxy-matrine | 183074-18-2 | [2] | Matrine-tetracyclic |
| 14 | Aloperine | 56293-29-9 | [1] | Chrysophylline-tetracyclic |
| 15 | N-methyl-aloperine | 63128-33-6 | [1] | Chrysophylline-tetracyclic |
| 16 | N-allyl-aloperine | 56595-96-1 | [1] | Chrysophylline-tetracyclic |
| 17 | Δ11-dehydroaloperine | 142808-31-9 | [5] | Chrysophylline-tetracyclic |
| 18 | Baptifoline | 732-50-3 | [4] | Chrysophylline-tetracyclic |
| 19 | Anagyrine | 486-89-5 | [3] | Chrysophylline-tetracyclic |
| 20 | Cytisine | 485-35-8 | [1] | Genistein-tricyclic |
| 21 | N-methyl-cytisine | 82438-76-4 | [3] | Genistein-tricyclic |
| 22 | N-2-hydroxyethyl-cytisine | 41645-70-9 | [1] | Genistein-tricyclic |
| 23 | Nicotine | 54-11-5 | [2] | Lupin-bicyclic |
| Compound ID | Molecular Weight | Compound Name | Oral Bioavailability (%) | Predicted Caco-2 Permeability |
|---|---|---|---|---|
| MOL003680 | 248.41 | Sophoridine | 60.07 | 1.13 |
| MOL003676 | 244.37 | Sophoramine | 42.16 | 1.43 |
| MOL003627 | 246.39 | Sophocarpine | 64.26 | 0.99 |
| MOL003680 | 264.41 | Oxymatrine | 60.07 | 1.13 |
| MOL003632 | 204.30 | N-methylcytisine | 76.70 | 1.06 |
| MOL005944 | 248.41 | Matrine | 63.77 | 1.39 |
| MOL005945 | 190.27 | Cytisine | 69.40 | 1.01 |
| MOL006566 | 246.39 | (+)-Lehmannine | 58.34 | 1.21 |
| Rank | Uniprot ID | Target Genes | Target Protein |
|---|---|---|---|
| 1 | Q16539 | MAPK14 | Mitogen-activated protein kinase 14 |
| 2 | P61812 | TGFB2 | Transforming growth factor beta-2 |
| 3 | P50579 | METAP2 | Methionine aminopeptidase 2 |
| 4 | P14061 | HSD17B1 | Estradiol 17-beta-dehydrogenase 1 |
| 5 | Q14994 | NR1I3 | Nuclear receptor subfamily 1 group I member 3 |
| 6 | P19793 | RXRA | Retinoic acid receptor RXR-alpha |
| 7 | Q02750 | MAP2K1 | Dual specificity mitogen-activated protein kinase 1 |
| 8 | P29373 | CRABP2 | Cellular retinoic acid-binding protein 2 |
| 9 | P00918 | CA2 | Carbonic anhydrase 2 |
| 10 | P27487 | DPP4 | Dipeptidyl peptidase 4 |
| 11 | P55263 | ADK | Adenosine kinase |
| 12 | P07900 | HSD90AA1 | Heat shock protein HSP 90-alpha |
| 13 | P18031 | PTPN1 | Tyrosine-protein phosphatase nonreceptor type 1 |
| 14 | P08263 | GSTA1 | Glutathione S-transferase A1 |
| 15 | P05413 | FABP3 | Fatty acid-binding protein, heart |
| 16 | O15540 | FABP7 | Fatty acid-binding protein, brain |
| 17 | Q02127 | DHODH | Dihydroorotate dehydrogenase (quinone), mitochondrial |
| 18 | P24941 | CDK2 | Cyclin-dependent kinase 2 |
| 19 | P15121 | AKR1B1 | Aldose reductase |
| 20 | P00519 | ABL1 | Tyrosine-protein kinase ABL1 |
| 21 | P28845 | HSD11B1 | Corticosteroid 11-beta-dehydrogenase isozyme 1 |
| 22 | P49354 | FNTA | Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha |
| 23 | P35968 | KDR | Vascular endothelial growth factor receptor 2 |
| 24 | P10275 | AR | Androgen receptor |
| 25 | Q96RI1 | NR1H4 | Bile acid receptor |
| 26 | P09960 | LTA4H | Leukotriene A-4 hydrolase |
| 27 | Q08499 | PDE4D | cAMP-specific 3′,5′-cyclic phosphodiesterase 4D |
| 28 | P10828 | THRB | Thyroid hormone receptor beta |
| 29 | P62942 | FKBP1A | Peptidyl-prolyl cis-trans isomerase FKBP1A |
| 30 | P00734 | F2 | Prothrombin |
| 31 | P16442 | ABO | Histo-blood group ABO system transferase |
| 32 | P00326 | ADH1C | Alcohol dehydrogenase 1C |
| 33 | P00517 | PRKACA | cAMP-dependent protein kinase catalytic subunit alpha |
| 34 | P14324 | FDPS | Farnesyl pyrophosphate synthase |
| 35 | P00390 | GSR | Glutathione reductase, mitochondrial |
| 36 | O15530 | PDPK1 | 3-phosphoinositide-dependent protein kinase 1 |
| 37 | P20248 | CCNA2 | Cyclin-A2 |
| 38 | P56817 | BACE1 | Beta-secretase 1 |
| 39 | P09211 | GSTP1 | Glutathione S-transferase P |
| 40 | P45452 | MMP13 | Collagenase 3 |
| 41 | P49638 | TTPA | Alpha-tocopherol transfer protein |
| 42 | P11712 | CYP2C9 | Cytochrome P450 2C9 |
| 43 | P27707 | DCK | Deoxycytidine kinase |
| 44 | P04150 | NR3C1 | Glucocorticoid receptor |
| 45 | P08581 | MET | Hepatocyte growth factor receptor |
| 46 | O75469 | NR1I2 | Nuclear receptor subfamily 1 group I member 2 |
| 47 | P13631 | RARG | Retinoic acid receptor gamma |
| 48 | Q9BY41 | HDAC8 | Histone deacetylase 8 |
| 49 | P49888 | SULT1E1 | Estrogen sulfotransferase |
| Rank | Uniprot ID | Target Gene | Target Protein |
|---|---|---|---|
| 1 | P19793 | RXRA | Retinoic acid receptor RXR-alpha |
| 2 | P10275 | AR | Androgen receptor |
| 3 | P35968 | KDR | Vascular endothelial growth factor receptor 2 |
| 4 | P08263 | GSTA1 | Glutathione S-transferase A1 |
| 5 | O15530 | PDPK1 | 3-phosphoinositide-dependent protein kinase 1 |
| 6 | P08581 | MET | Hepatocyte growth factor receptor |
| 7 | Q02750 | MAP2K1 | Dual specificity mitogen-activated protein kinase 1 |
| 8 | P09211 | GSTP1 | Glutathione S-transferase P |
| 9 | P24941 | CDK2 | Cyclin-dependent kinase 2 |
| 10 | Q16539 | MAPK14 | Mitogen-activated protein kinase 14 |
| 11 | P61812 | TGFB2 | Transforming growth factor beta-2 |
| 12 | P00519 | ABL1 | Tyrosine-protein kinase ABL1 |
| 13 | P28845 | HSD11B1 | Corticosteroid 11-beta-dehydrogenase isozyme 1 |
| 14 | P00326 | ADH1C | Alcohol dehydrogenase 1C |
| 15 | P00517 | PRKACA | cAMP-dependent protein kinase catalytic subunit alpha |
| 16 | P11712 | CYP2C9 | Cytochrome P450 2C9 |
| Rank | Pathway Name | Pathway ID | Count | Pathway Classifications |
|---|---|---|---|---|
| S1 | Pathways in cancer | hsa05200 | 9 | Human Diseases; Cancers |
| S2 | Proteoglycans in cancer | hsa05205 | 7 | Human Diseases; Cancers |
| S3 | Metabolism of xenobiotics by cytochrome P450 | hsa00980 | 5 | Metabolism; Xenobiotics Biodegradation and Metabolism |
| S4 | Chemical carcinogenesis | hsa05204 | 5 | Human Diseases; Cancers |
| S5 | FoxO signaling pathway | hsa04068 | 5 | Environmental Information Processing; Signal Transduction |
| S6 | Drug metabolism-cytochrome P450 | hsa00982 | 4 | Metabolism; Xenobiotics Biodegradation and Metabolism |
| S7 | PI3K-Akt signaling pathway | hsa04151 | 6 | Environmental Information Processing; Signal Transduction |
| S8 | Progesterone-mediated oocyte maturation | hsa04914 | 4 | Organismal Systems; Endocrine System |
| S9 | Prostate cancer | hsa05215 | 4 | Human Diseases; Cancers |
| S10 | Ras signaling pathway | hsa04014 | 5 | Environmental Information Processing; Signal Transduction |
| S11 | Oocyte meiosis 4 | hsa04114 | 4 | Cellular Processes; Cell Growth and Death |
| S12 | Thyroid hormone signaling pathway | hsa04919 | 4 | Organismal Systems; Endocrine System |
| S13 | Neurotrophin signaling pathway | hsa04722 | 4 | Organismal Systems; Nervous System |
| S14 | Nonsmall cell lung cancer | hsa05223 | 3 | Human Diseases; Cancers |
| S15 | VEGF signaling pathway | hsa04370 | 3 | Environmental Information Processing; Signal Transduction |
| S16 | Renal cell carcinoma | hsa05211 | 3 | Human Diseases; Cancers |
| S17 | Focal adhesion 4 | hsa04510 | 4 | Cellular Processes; Cellular Community-eukaryotes |
| S18 | Fc epsilon RI signaling pathway | hsa04664 | 3 | Organismal Systems; Immune System |
| S19 | Rap1 signaling pathway | hsa04015 | 4 | Environmental Information Processing; Signal Transduction |
| S20 | Chronic myeloid leukemia | hsa05220 | 3 | Human Diseases; Cancers |
| S21 | MAPK signaling pathway | hsa04010 | 4 | Environmental Information Processing; Signal Transduction |
| S22 | GnRH signaling pathway | hsa04912 | 3 | Organismal Systems; Endocrine System |
| S23 | T cell receptor signaling pathway | hsa04660 | 3 | Organismal Systems; Immune System |
| S24 | MicroRNAs in cancer | hsa05206 | 4 | Human Diseases; Cancers |
| S25 | Toxoplasmosis | hsa05145 | 3 | Human Diseases; Infectious Diseases: Parasitic |
| S26 | Serotonergic synapse | hsa04726 | 3 | Organismal Systems; Nervous System |
| S27 | Sphingolipid signaling pathway | hsa04071 | 3 | Environmental Information Processing; Signal Transduction |
| S28 | Cell cycle | hsa04110 | 3 | Cellular Processes; Cell Growth and Death |
| S29 | Osteoclast differentiation | hsa04380 | 3 | Organismal Systems; Development |
| S30 | Hepatitis C | hsa05160 | 3 | Human Diseases; Infectious Diseases: Viral |
| S31 | Insulin signaling pathway | hsa04910 | 3 | Organismal Systems; Endocrine System |
| S32 | Hepatitis B | hsa05161 | 3 | Human Diseases; Infectious Diseases: Viral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, R.; Zhao, S.; Chen, D.; Hao, F.; Wang, B.; Zhang, J.; Ma, Y.; Chen, X.; Gao, X.; et al. Extraction, Separation, Antitumor Effect, and Mechanism of Alkaloids in Sophora alopecuroides: A Review. Separations 2022, 9, 380. https://doi.org/10.3390/separations9110380
Zhang R, Wang R, Zhao S, Chen D, Hao F, Wang B, Zhang J, Ma Y, Chen X, Gao X, et al. Extraction, Separation, Antitumor Effect, and Mechanism of Alkaloids in Sophora alopecuroides: A Review. Separations. 2022; 9(11):380. https://doi.org/10.3390/separations9110380
Chicago/Turabian StyleZhang, Ruixia, Ruizhou Wang, Shipeng Zhao, Dan Chen, Fusheng Hao, Bo Wang, Jin Zhang, Yingying Ma, Xingyi Chen, Xiaojuan Gao, and et al. 2022. "Extraction, Separation, Antitumor Effect, and Mechanism of Alkaloids in Sophora alopecuroides: A Review" Separations 9, no. 11: 380. https://doi.org/10.3390/separations9110380
APA StyleZhang, R., Wang, R., Zhao, S., Chen, D., Hao, F., Wang, B., Zhang, J., Ma, Y., Chen, X., Gao, X., Han, L., & Bai, C. (2022). Extraction, Separation, Antitumor Effect, and Mechanism of Alkaloids in Sophora alopecuroides: A Review. Separations, 9(11), 380. https://doi.org/10.3390/separations9110380






