Interaction of Manganese Ions with Scheelite Surfaces and Its Effect on Collector Adsorption and Flotation
Abstract
:1. Introduction
2. Experimental
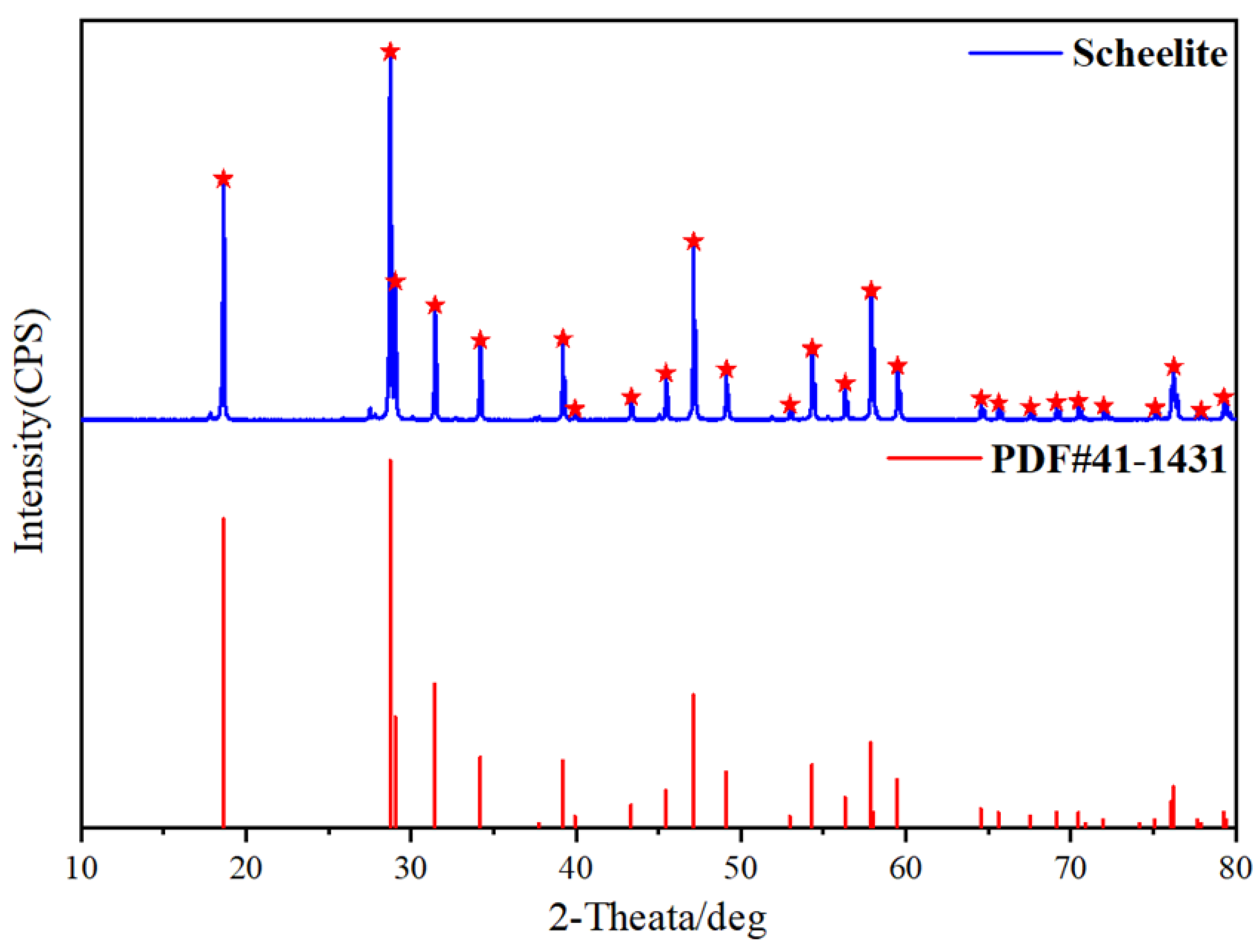
2.1. Materials and Reagents
2.2. Micro–Flotation Tests
2.3. UV–Vis Spectrophotometer Measurements
2.4. Fourier Infrared Spectroscopy Analysis
2.5. Zeta Potential Measurements
2.6. XPS Analysis
3. Results and Discussions
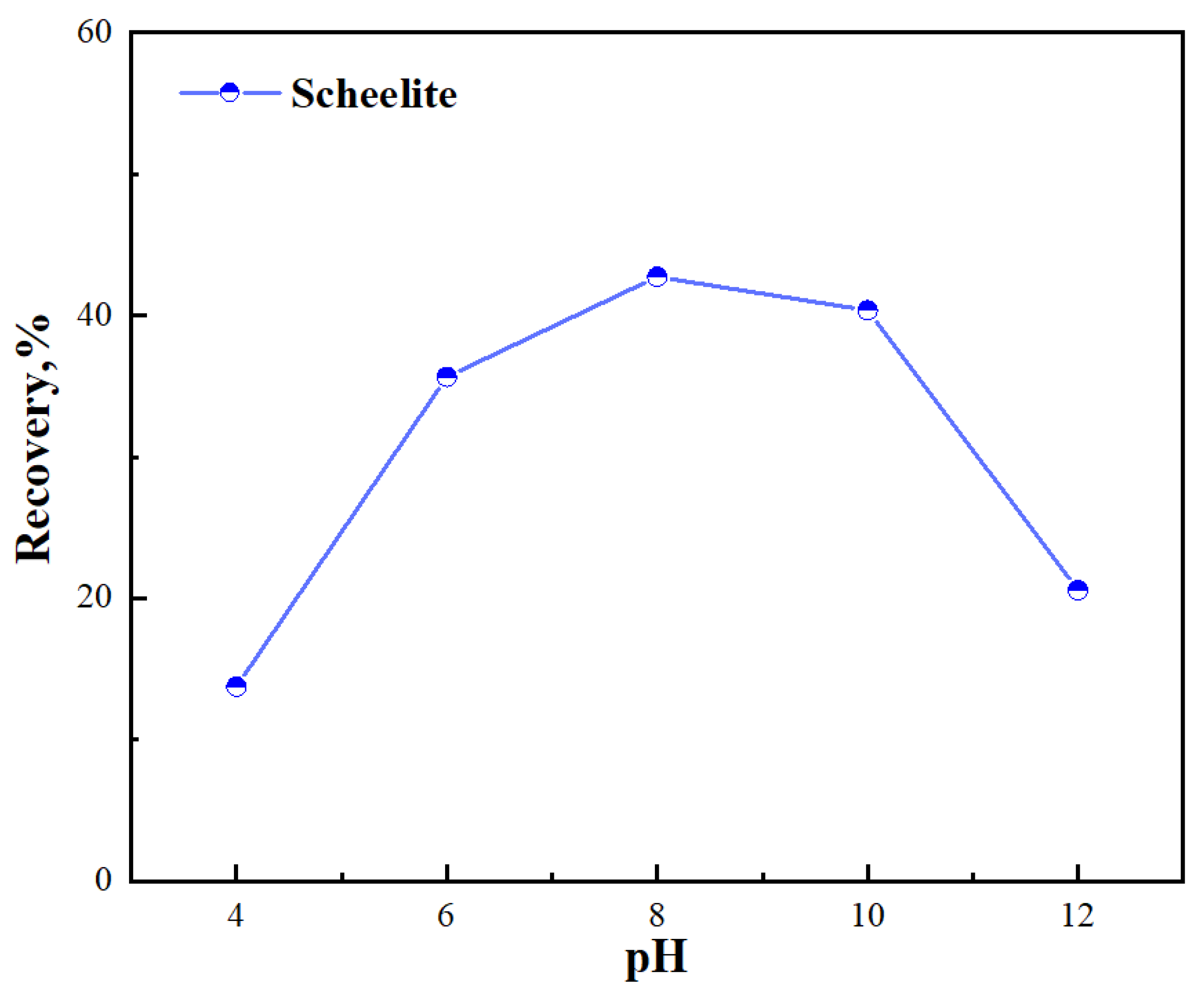
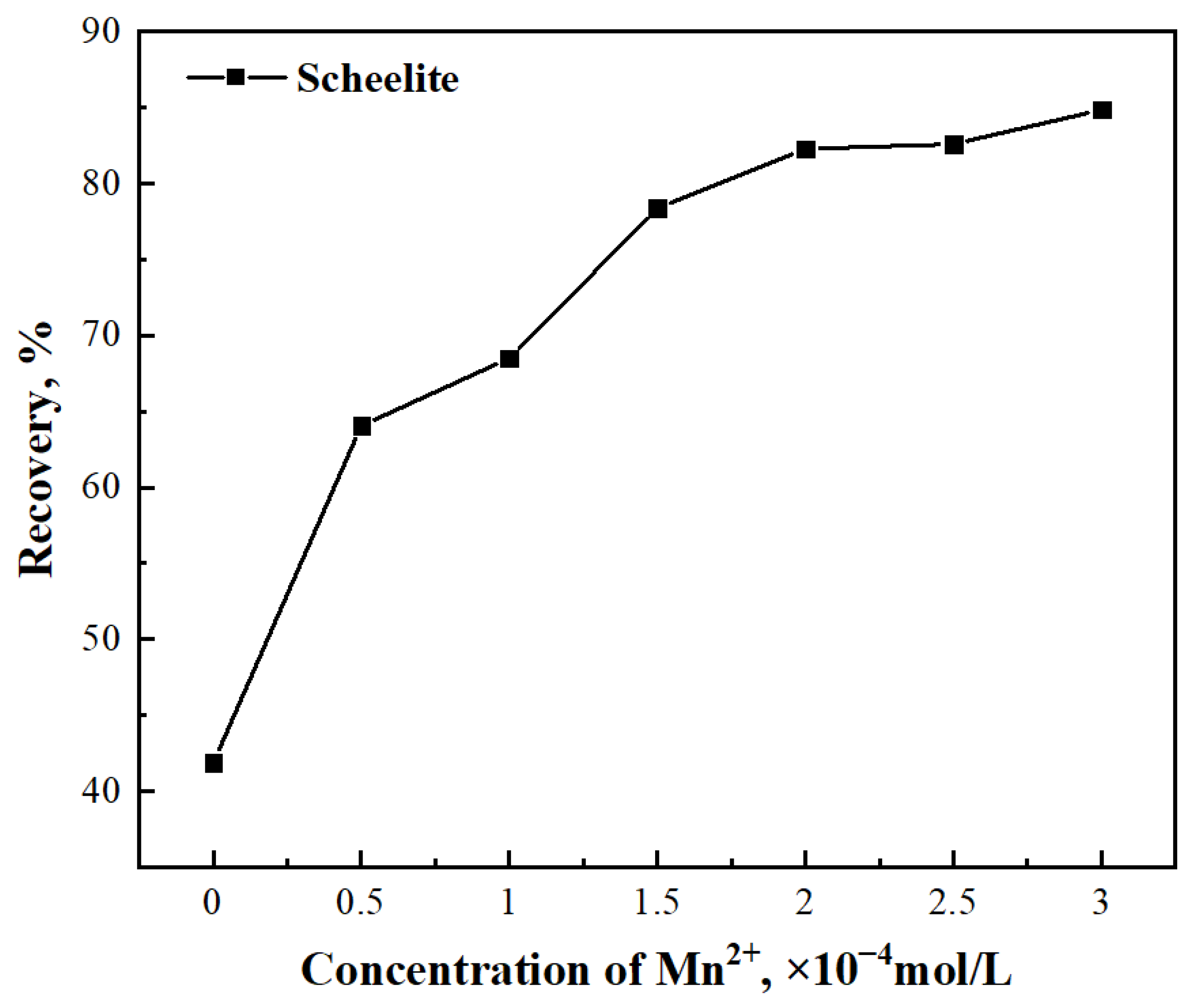
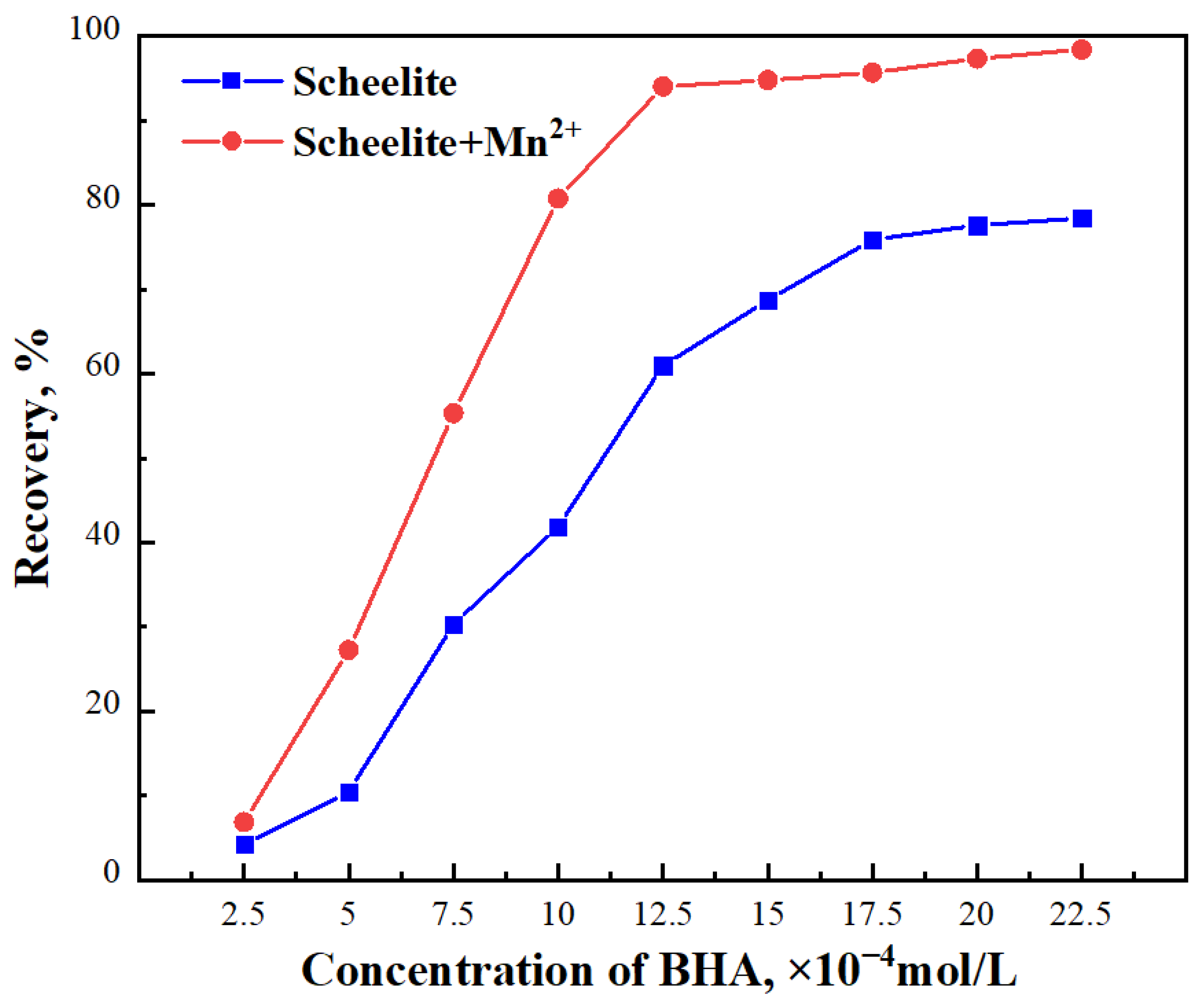
3.1. Micro–Flotation Tests
3.2. UV–Vis Analysists
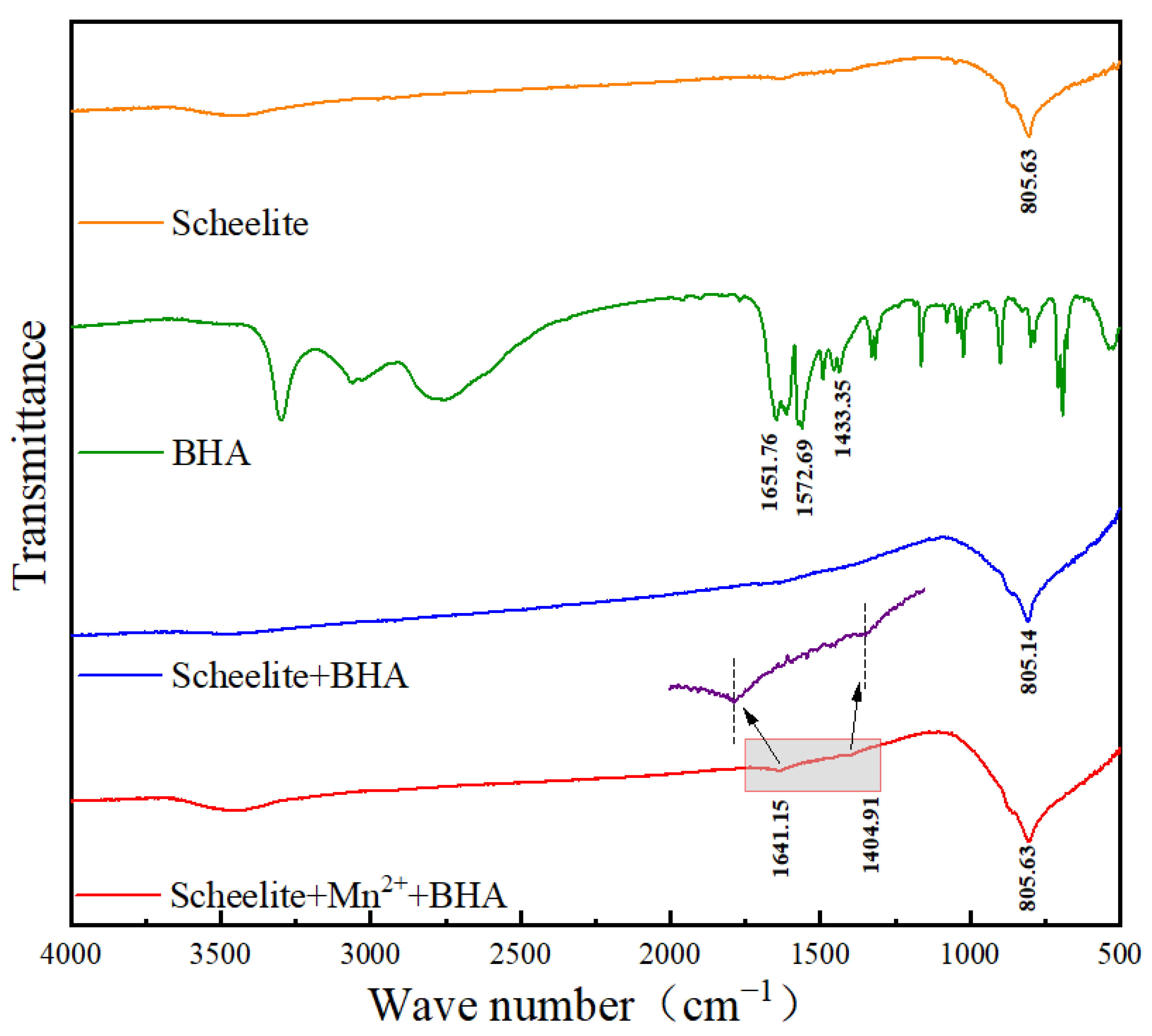
3.3. Infrared Spectral Analysis
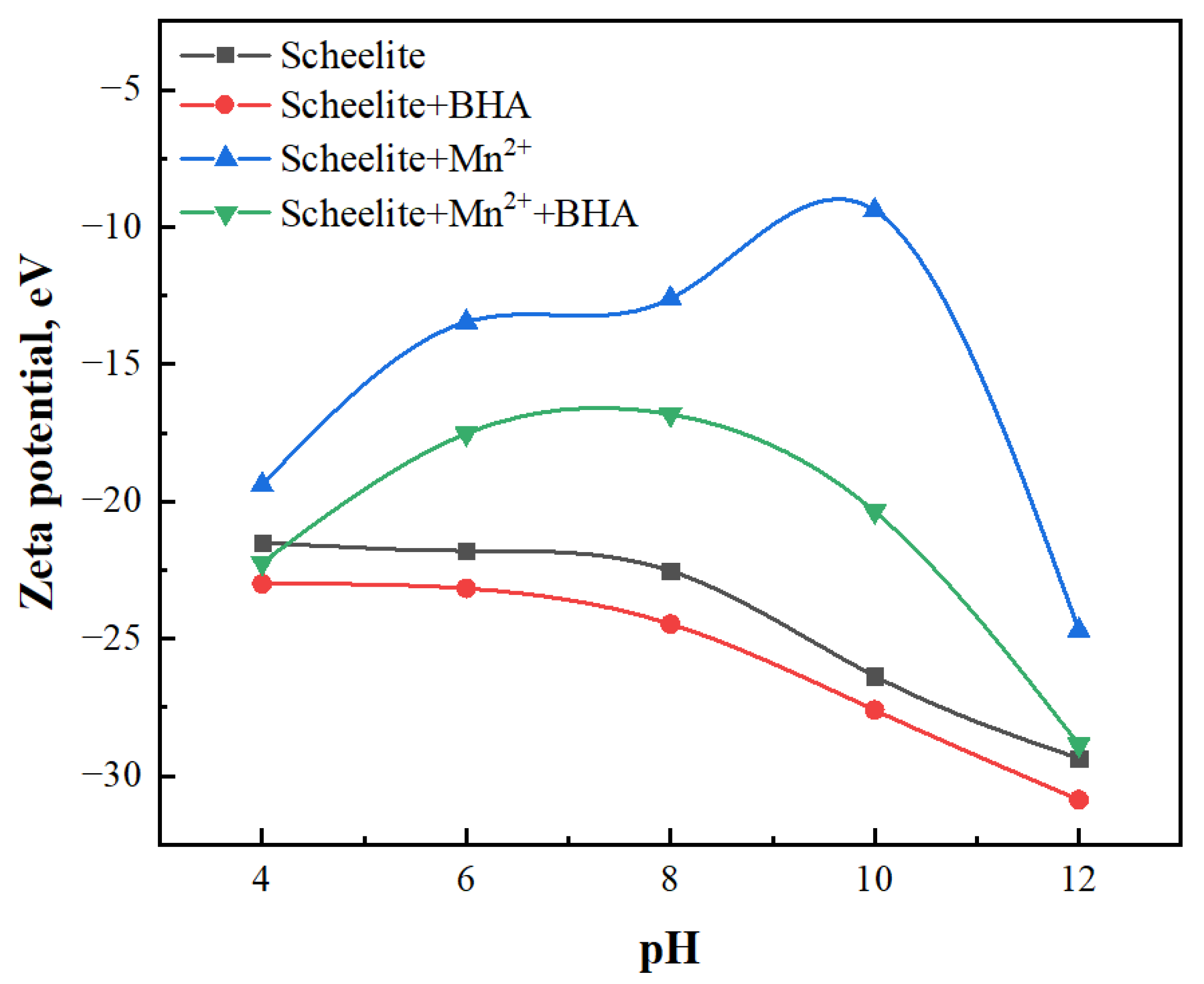
3.4. Zeta Potential Measurements
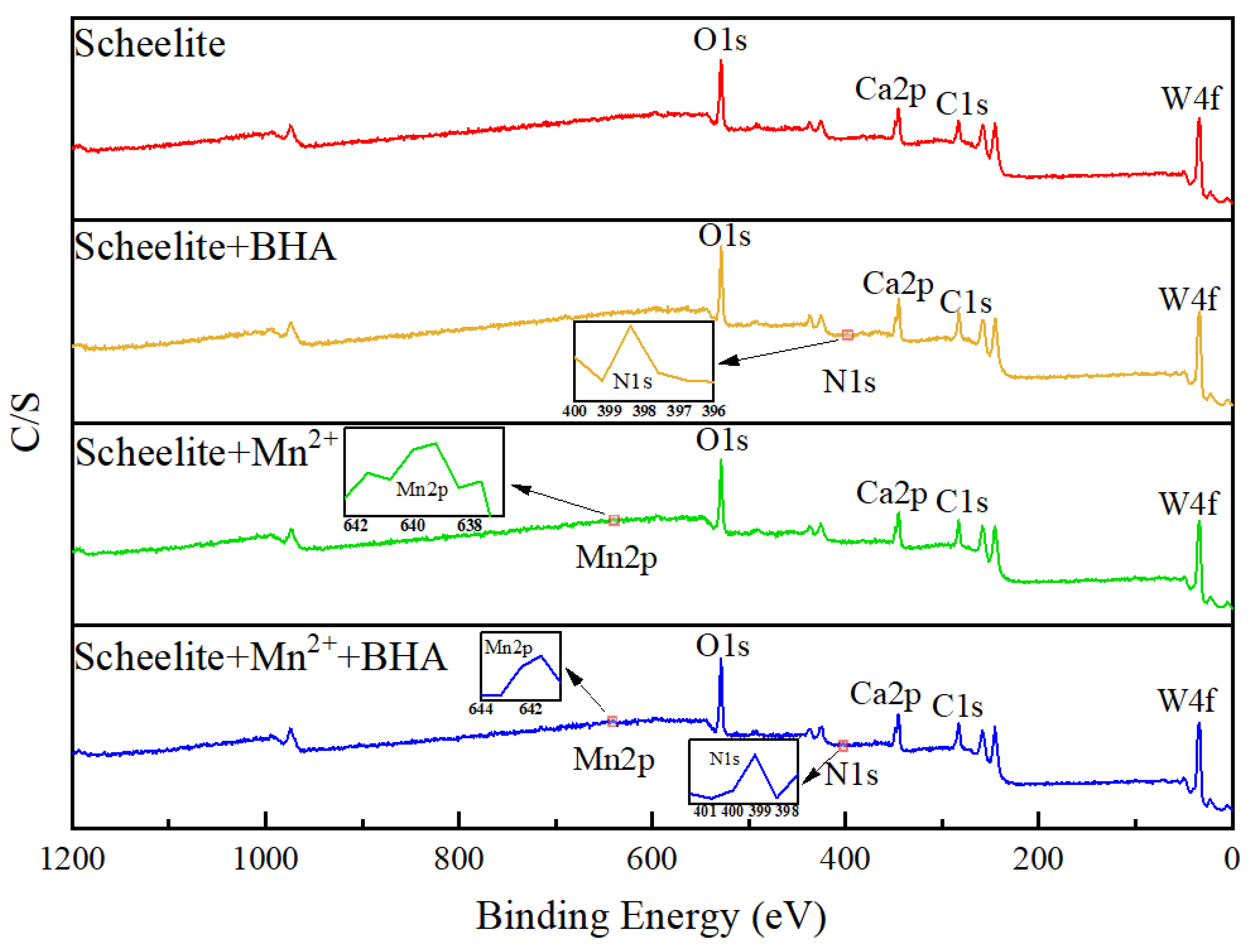
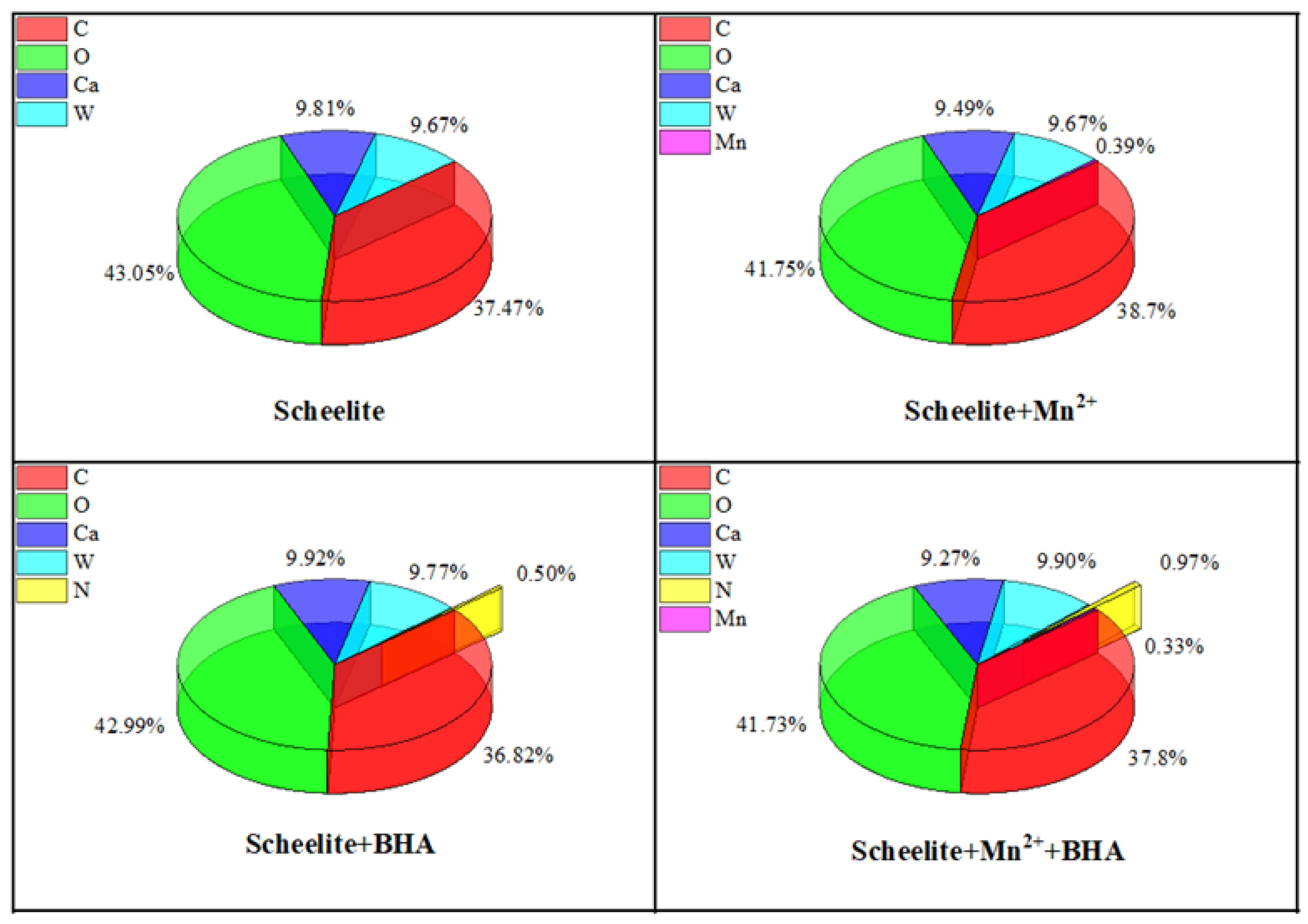
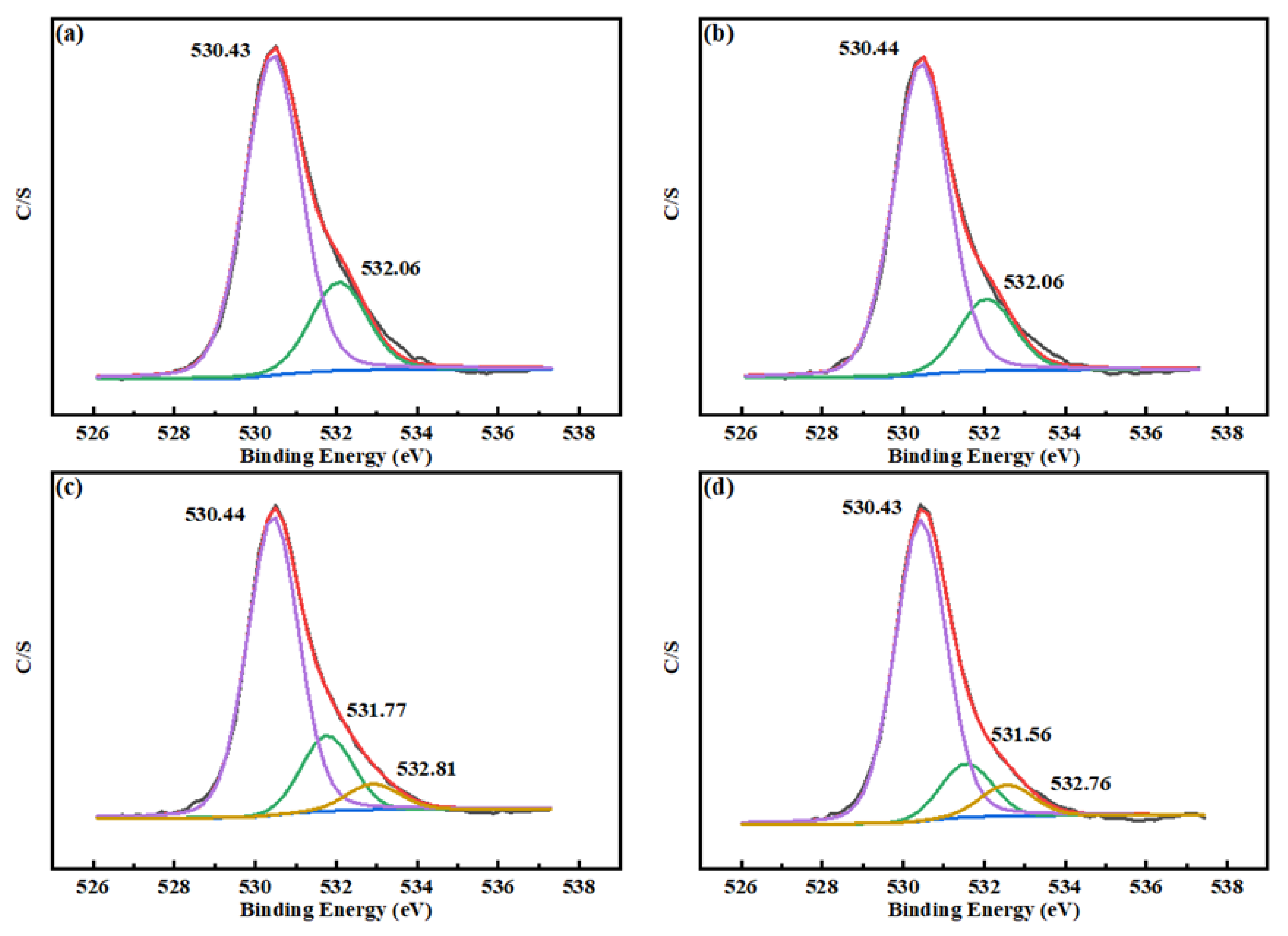
3.5. XPS Analysis
4. Conclusions
- (1)
- Mn2+ had a significant activation effect on the flotation of scheelite. The micro–flotation test and the adsorption test showed that the addition of Mn2+ was beneficial to the flotation of scheelite. The addition of Mn2+ increased the adsorption strength of BHA on the scheelite surface and can achieve the best flotation recovery (98.40%).
- (2)
- In order to further study the interaction mechanism between Mn2+ and scheelite surface, adsorption tests and infrared spectral analysis were carried out. These results showed that BHA could form Mn-BHA adsorption on scheelite surface after the addition of Mn2+, and this adsorption effect was stronger than that of BHA alone. And they implied that Mn2+ can increase the number of active sites on the scheelite surface, so that the more BHA was adsorbed, given rise to the higher strength of its characteristic peaks.
- (3)
- XPS analysis showed that when Mn2+ was added, the adsorption of BHA on scheelite surface increased, relative to the study in which only BHA were added, and therefore the amount of observed C–N species was also increased. After the addition of Mn2+, Mn–O chemical bonds were formed on the surface of the scheelite and promote the BHA to combine with scheelite surface to form a new hydrophobic component. The binding energy of Ca–O was shifted by 0.21 eV, which indicated that the newly generated Mn-BHA can also interact with scheelite surface, and the adsorption capacity of Mn-BHA on scheelite surface was more than that of BHA alone. The XPS results, combined with zeta potential analysis, indicated that adding Mn2+ can enhance BHA adsorption on the mineral surface and improve its adsorption stability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Feng, B.; Zhang, L.; Chen, Y.; Gao, Z. Flotation separation of scheelite and apatite by polysaccharide depressant xanthan gum. Miner. Eng. 2021, 170, 107045. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Yang, C.; He, R.; Jiao, F.; Qin, W.; Cui, Y.; Zhang, Z.; Li, W.; Song, H. Innovative application of sodium tripolyphosphate for the flotation separation of scheelite from calcite. Miner. Eng. 2021, 170, 106981. [Google Scholar] [CrossRef]
- Liu, C.; Ni, C.; Yao, J.; Chang, Z.; Wang, Z.; Zeng, G.; Luo, X.; Yang, L.; Ren, Z.; Shao, P.; et al. Hydroxypropyl amine surfactant: A novel flotation collector for efficient separation of scheelite from calcite. Miner. Eng. 2021, 167, 106898. [Google Scholar] [CrossRef]
- Dong, L.; Wei, Q.; Qin, W.; Jiao, F. Effect of iron ions as assistant depressant of citric acid on the flotation separation of scheelite from calcite. Chem. Eng. Sci. 2021, 241, 116720. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, G.; Liu, S.; Chen, W.; Liu, G. Strengthening flotation enrichment of Pb(Ⅱ)-activated scheelite with N-[(3-hydroxyamino)-propoxy]-N-hexyl dithiocarbamate. J. Ind. Eng. Chem. 2022, 114, 338–346. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effects of Pb2+ ions on the flotation behavior of scheelite, calcite, and fluorite in the presence of water glass. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127826. [Google Scholar] [CrossRef]
- Kuang, J.; Zou, Z.; Huang, Z.; Liu, P.; Yuan, W.; Zhu, L. Surface dissolution of scheelite under different regulators and its effect on flotation behavior. Miner. Eng. 2021, 164, 106811. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Zhu, H.; Liu, R. A novel green depressant for flotation separation of scheelite from calcite. Trans. Nonferrous Met. Soc. China 2021, 31, 2493–2500. [Google Scholar] [CrossRef]
- Foucaud, Y.; Collet, A.; Filippova, I.V.; Badawi, M.; Filippov, L.O. Synergistic effects between fatty acids and non-ionic reagents for the selective flotation of scheelite from a complex tungsten skarn ore. Miner. Eng. 2022, 182, 107566. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, C.; Yin, W.; Luo, B. An investigation of the mechanism of using iron chelate as a collector during scheelite flotation. Miner. Eng. 2019, 131, 146–153. [Google Scholar]
- Wang, J.; Gao, Z.; Han, H.; Sun, W.; Gao, Y.; Ren, S. Impact of NaOL as an accelerator on the selective separation of scheelite from fluorite using a novel self-assembled Pb-BHA-NaOL collector system. Appl. Surf. Sci. 2021, 537, 147778. [Google Scholar] [CrossRef]
- Wang, C.; Ren, S.; Sun, W.; Wu, S.; Tao, L.; Duan, Y.; Wang, J.; Gao, Z. Selective flotation of scheelite from calcite using a novel self-assembled collector. Miner. Eng. 2021, 171, 107120. [Google Scholar] [CrossRef]
- Guan, Z.; Lu, K.; Zhang, Y.; Yang, H.; Li, X. Mechanism of manganese ion interaction with the surface of scheelite and calcite and its effect on flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129397. [Google Scholar] [CrossRef]
- He, J.; Sun, W.; Zeng, H.; Fan, R.; Hu, W.; Gao, Z. Unraveling roles of lead ions in selective flotation of scheelite and fluorite from atomic force microscopy and first-principles calculations. Miner. Eng. 2022, 179, 107424. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Gao, Y. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Liu, J.; Ejtemaei, M.; Nguyen, A.V.; Wen, S.; Zeng, Y. Surface chemistry of Pb-activated sphalerite. Miner. Eng. 2020, 145, 106058. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Xiao, W.; Shao, Y.; Yu, J.; Zhang, B.; Shu, H.; Zhang, Y. Activation of ilmenite flotation by Al3+ in the benzohydroxamic acid (BHA) system. Sep. Purif. Technol. 2022, 299, 121770. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Cao, J.; Gui, X.; Xing, Y.; Zhang, C.; Zou, J. Enhanced electronic effect improves the collecting efficiency of benzohydroxamic acid for scheelite flotation. Miner. Eng. 2020, 152, 106308. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Sulfidization regulation of cuprite by pre-oxidation using sodium hypochlorite as an oxidant. Int. J. Min. Sci. Technol. 2021, 31, 1117–1128. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Yang, B.; Feng, Q.; Liu, D. Enhanced sulfidization flotation mechanism of smithsonite in the synergistic activation system of copper–ammonium species. Miner. Eng. 2022, 187, 107796. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Chen, K.; Zhu, Y.; Anh, V.N.; Tian, M.; Wang, L.; Yue, T.; et al. Novel catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Sun, W.; Lan, L.; Zeng, H.; Zhou, J.; Ahmed, K.S.; Wang, L. Study on the flotation separation mechanism of diaspore from kaolinite using mixed NaOL/BHA collector. Miner. Eng. 2022, 186, 107719. [Google Scholar] [CrossRef]
- Eileen, R.L.; Espiritu, S.N.; Kristian, E.W. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254–265. [Google Scholar]
- Al-Saadi, A.A. Conformational analysis and vibrational assignments of benzohydroxamic acid and benzohydrazide. J. Mol. Struct. 2012, 1023, 115–122. [Google Scholar] [CrossRef]
- Fu, J.; Han, H.; Wei, Z.; Liu, R.; Li, W.; Xu, T.; Ji, D. Selective separation of scheelite from calcite using tartaric acid and Pb-BHA complexes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126657. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Decoupling the effects of solid and liquid phases in a Pb-water glass mixture on the selective flotation separation of scheelite from calcite. Miner. Eng. 2020, 154, 106423. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Luo, L.; Wu, H.; Xu, L.; Meng, J.; Lu, J.; Zhou, H.; Huo, X.; Huang, L. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. Int. J. Min. Sci. Technol. 2021, 31, 689–697. [Google Scholar] [CrossRef]
- Meng, Q.; Xu, Y.; Yuan, Z.; Zhao, X.; Du, Y. Separation mechanism of ilmenite from titanaugite with mixed BHA/NaOL collector. Miner. Eng. 2022, 176, 107363. [Google Scholar] [CrossRef]
- Huang, Z.; Kuang, J.; Yuan, W.; Yu, M.; Wang, X. Regulation mechanism of ultrasonication on surface hydrophobicity of scheelite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127412. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Z.; Han, H.; Sun, W.; Hu, Y.; Wang, J.; Wang, L.; Liu, H.; Yang, Y.; Zhang, C.; et al. Fluorite particles as a novel calcite recovery depressant in scheelite flotation using Pb-BHA complexes as collectors. Miner. Eng. 2019, 132, 84–91. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Z.; Meng, Q.; Zhao, X.; Du, Y. Study on the flotation behavior and interaction mechanism of ilmenite with mixed BHA/NaOL collector. Miner. Eng. 2021, 170, 107034. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, L.; Gao, Z.; Sun, W.; Cao, X. Activation mechanism of zinc ions in cassiterite flotation with benzohydroxamic acid as a collector. Miner. Eng. 2020, 156, 106523. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Peng, L.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z. In situ adsorption of mixed collectors BHA/DDA in spodumene-feldspar flotation system. Sep. Purif. Technol. 2020, 251, 117325. [Google Scholar] [CrossRef]
- Meng, Q.; Feng, Q.; Shi, Q.; Ou, L. Studies on interaction mechanism of fine wolframite with octyl hydroxamic acid. Miner. Eng. 2015, 79, 133–138. [Google Scholar] [CrossRef]


| Composition | WO3 | CaO | SiO2 | Fe | Mg |
| Content | 78.54 | 10.12 | <0.50 | 0.28 | <0.005 |
| Sample | Species | Atomic Concentration, % |
|---|---|---|
| c | C–H | 33.13 |
| C–O | 2.63 | |
| C–N | 1.06 | |
| d | C–H | 33.9 |
| C–O | 2.26 | |
| C–N | 1.64 |
| Sample | Species | O 1 s Binding Energy, eV | Species Distribution, % |
|---|---|---|---|
| a | W–O | 530.43 | 78.33 |
| Ca–O | 532.06 | 21.67 | |
| OH | - | - | |
| b | W–O | 530.43 | 81.22 |
| Ca–O | 532.06 | 18.78 | |
| OH | - | - | |
| c | W–O | 530.44 | 75.76 |
| Ca–O | 531.77 | 17.82 | |
| OH | 532.81 | 6.42 | |
| d | W–O | 530.43 | 78.84 |
| Ca–O | 531.56 | 13.13 | |
| OH | 532.76 | 8.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wen, S.; Zuo, Q.; Liao, R.; Meng, S.; Tao, Y.; Shen, Z.; Feng, Q. Interaction of Manganese Ions with Scheelite Surfaces and Its Effect on Collector Adsorption and Flotation. Separations 2022, 9, 365. https://doi.org/10.3390/separations9110365
Wang X, Wen S, Zuo Q, Liao R, Meng S, Tao Y, Shen Z, Feng Q. Interaction of Manganese Ions with Scheelite Surfaces and Its Effect on Collector Adsorption and Flotation. Separations. 2022; 9(11):365. https://doi.org/10.3390/separations9110365
Chicago/Turabian StyleWang, Xiao, Shuming Wen, Qi Zuo, Runpeng Liao, Shengbing Meng, Yuanyuan Tao, Zhihao Shen, and Qicheng Feng. 2022. "Interaction of Manganese Ions with Scheelite Surfaces and Its Effect on Collector Adsorption and Flotation" Separations 9, no. 11: 365. https://doi.org/10.3390/separations9110365
APA StyleWang, X., Wen, S., Zuo, Q., Liao, R., Meng, S., Tao, Y., Shen, Z., & Feng, Q. (2022). Interaction of Manganese Ions with Scheelite Surfaces and Its Effect on Collector Adsorption and Flotation. Separations, 9(11), 365. https://doi.org/10.3390/separations9110365







