A New Partially Phosphorylated Polyvinyl Phosphate-PPVP Composite: Synthesis and Its Potentiality for Zr (IV) Extraction from an Acidic Medium
Abstract
1. Introduction
2. Experimental
2.1. Instrumentation
2.2. Reagents and Chemicals
2.3. Experimental Procedures
2.4. The Extraction Activities
- Co is the initial concentration (mg/L) of Zr(IV);
- Ce is the equilibrium concentration (mg/L) of Zr(IV);
- v is the working volume of the solution with Zr(IV) in liters;
- m is the dry weight of the prepared composite (g).
3. Results and Discussion
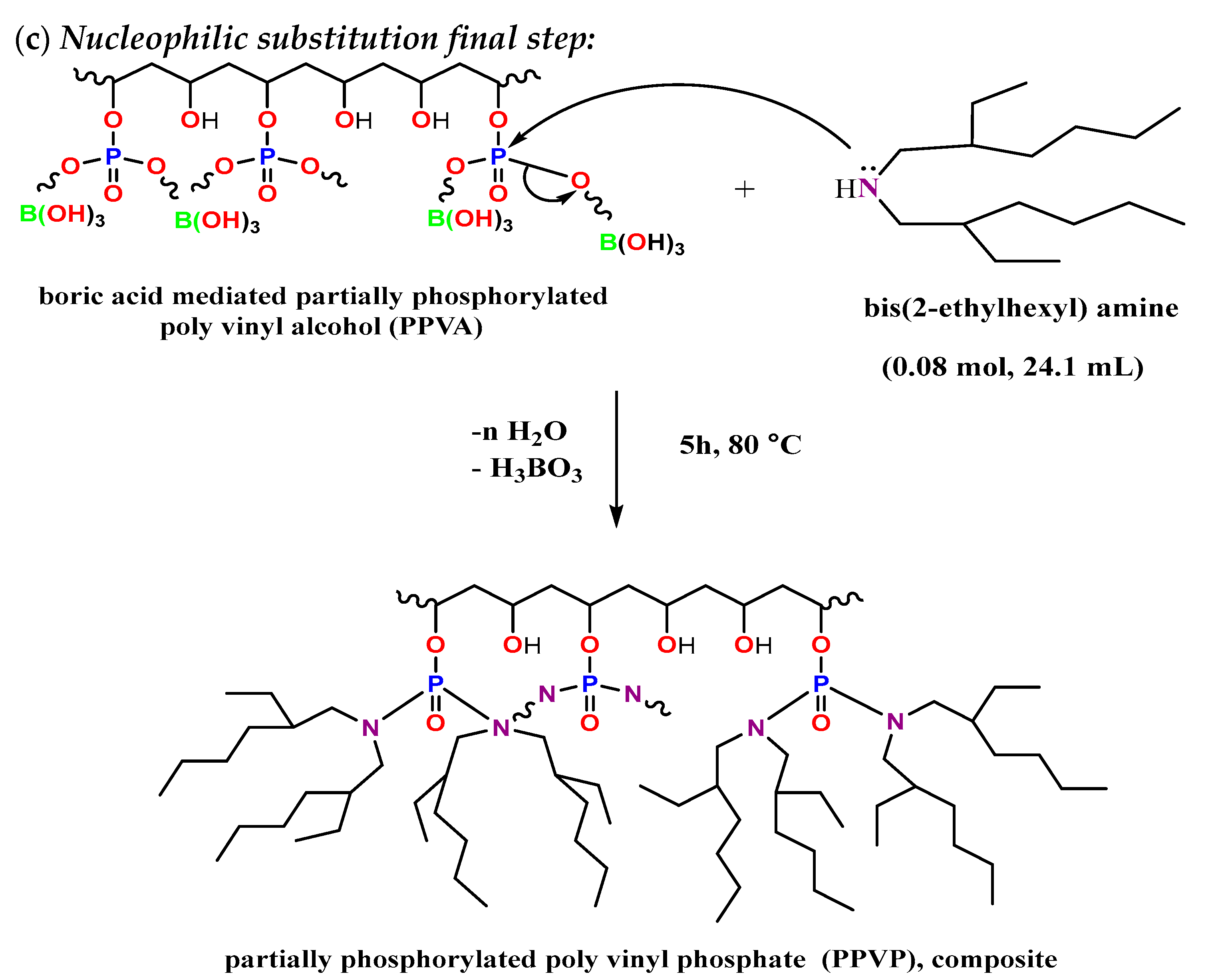
3.1. Preparation of Partially Phosphorylated Polyvinyl Phosphate Derivative (PPVP) Composite
3.2. Characterization of Partially Phosphorylated Polyvinyl Phosphate (PPVP) Composite
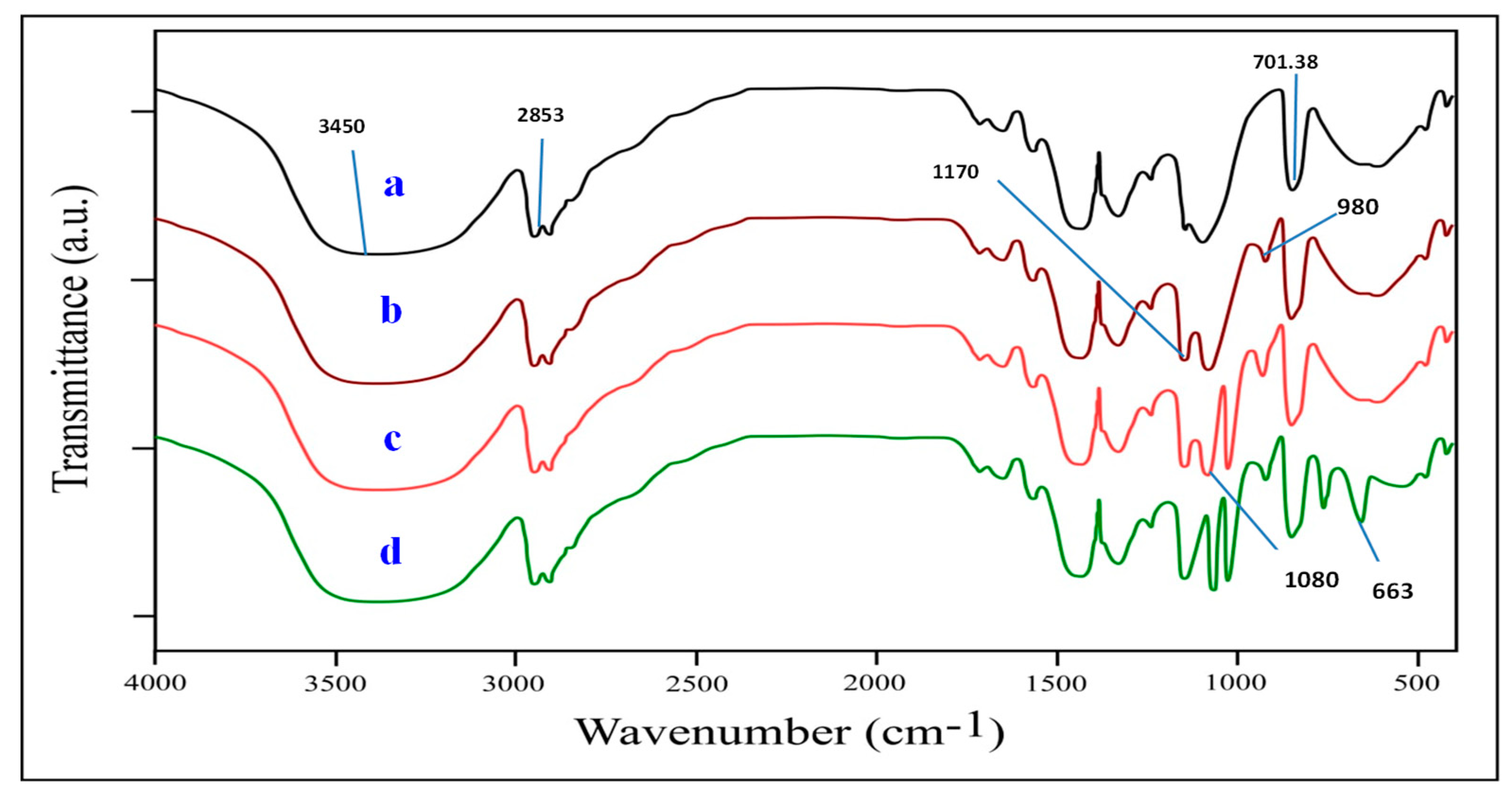
3.2.1. FT-IR Analysis
3.2.2. XPS Interpretation
3.2.3. BET (Brunauer–Emmett–Teller) Interpretation
3.2.4. SEM-EDX Interpretation
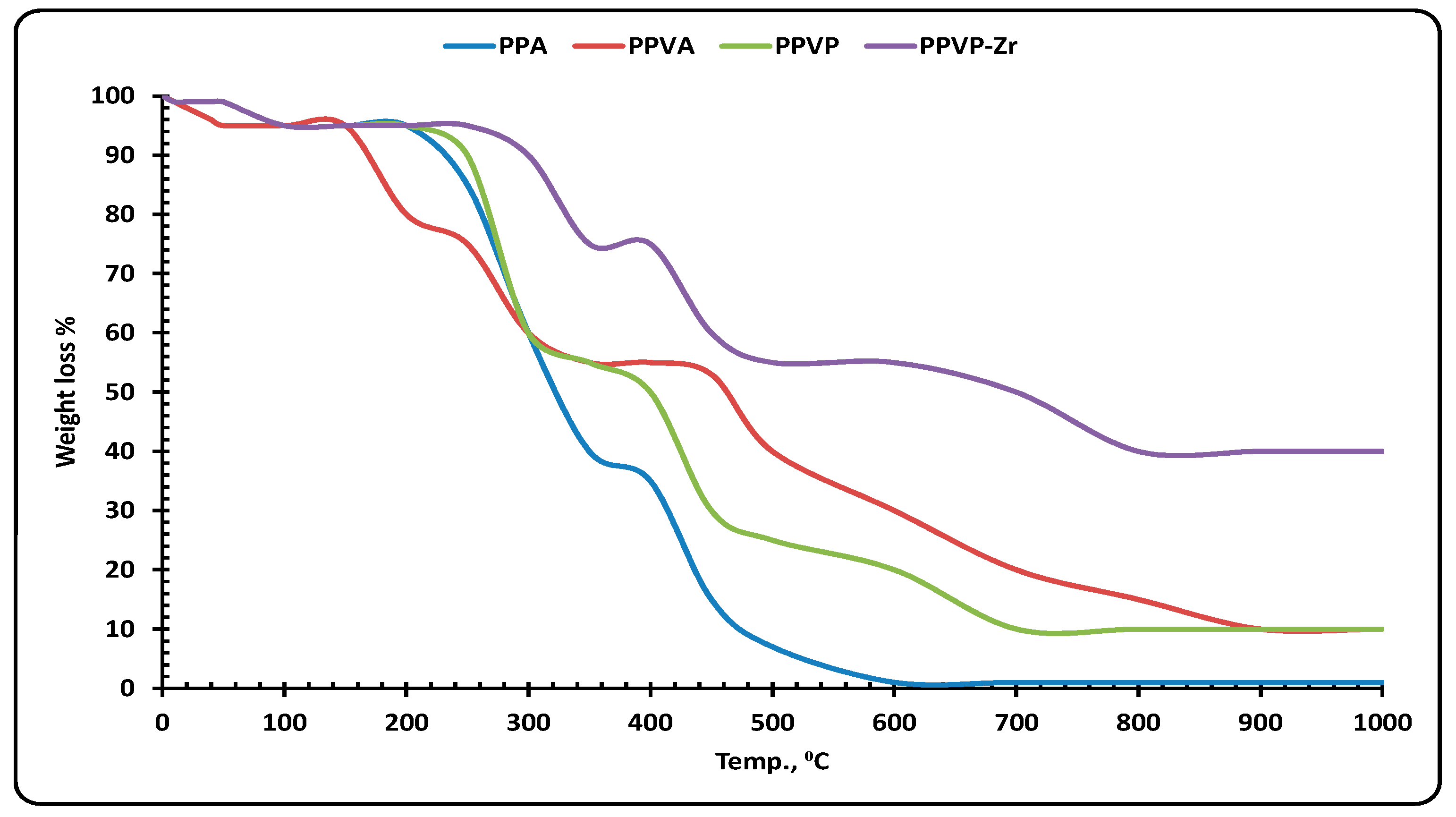
3.2.5. TGA Interpretation
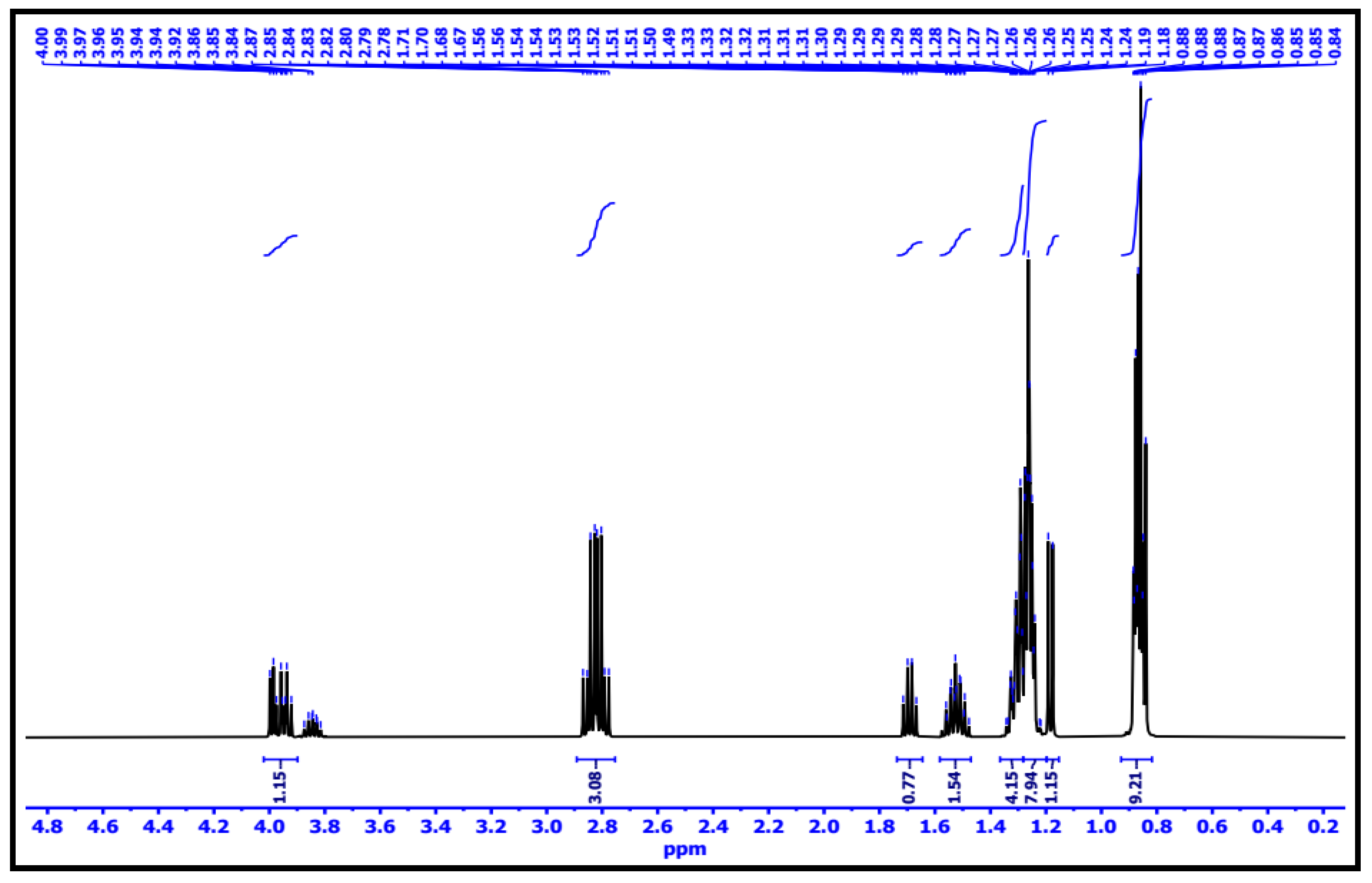
3.2.6. 1H-NMR Interpretation
3.2.7. 13C-NMR Interpretation
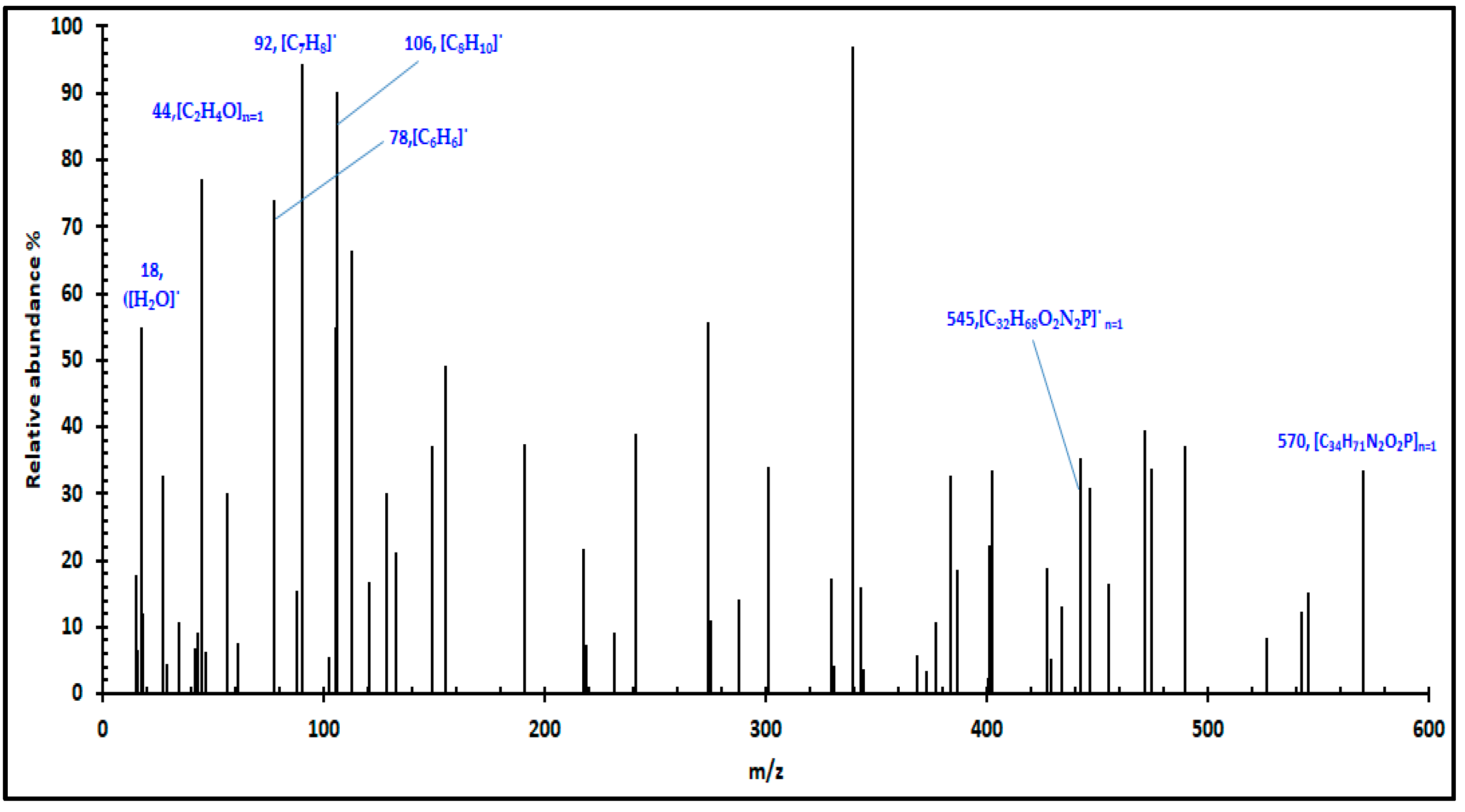
3.2.8. Mass Interpretation
3.3. Factors Controlling the Extraction Process
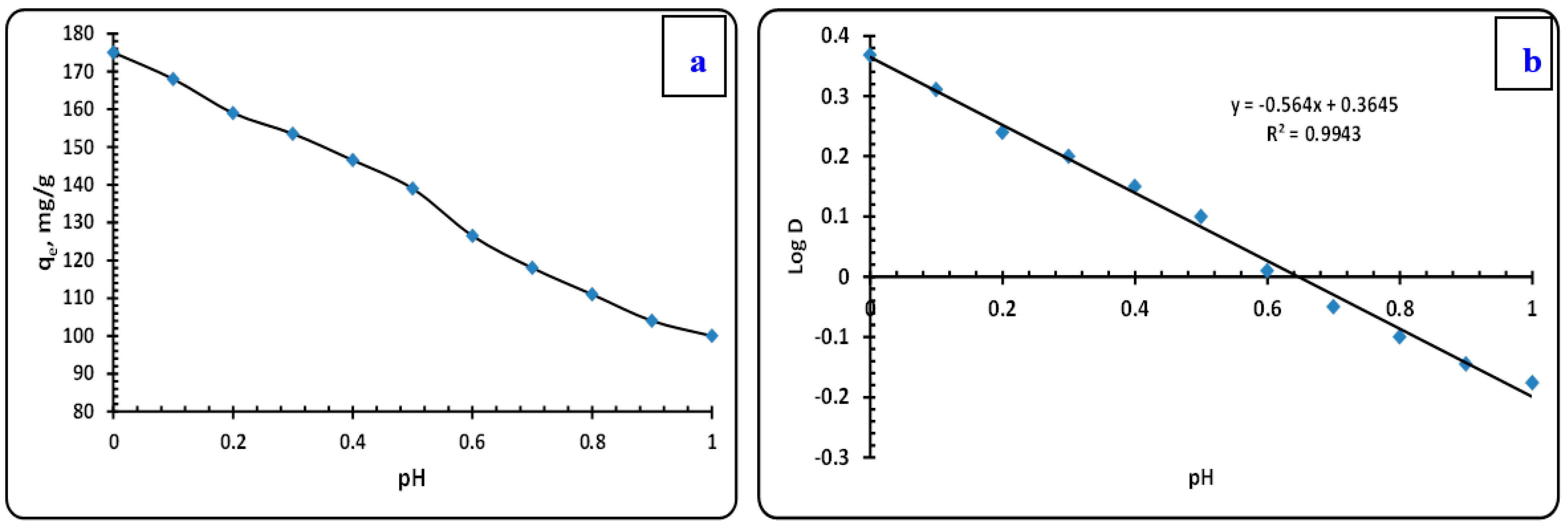
3.3.1. Effect of pH
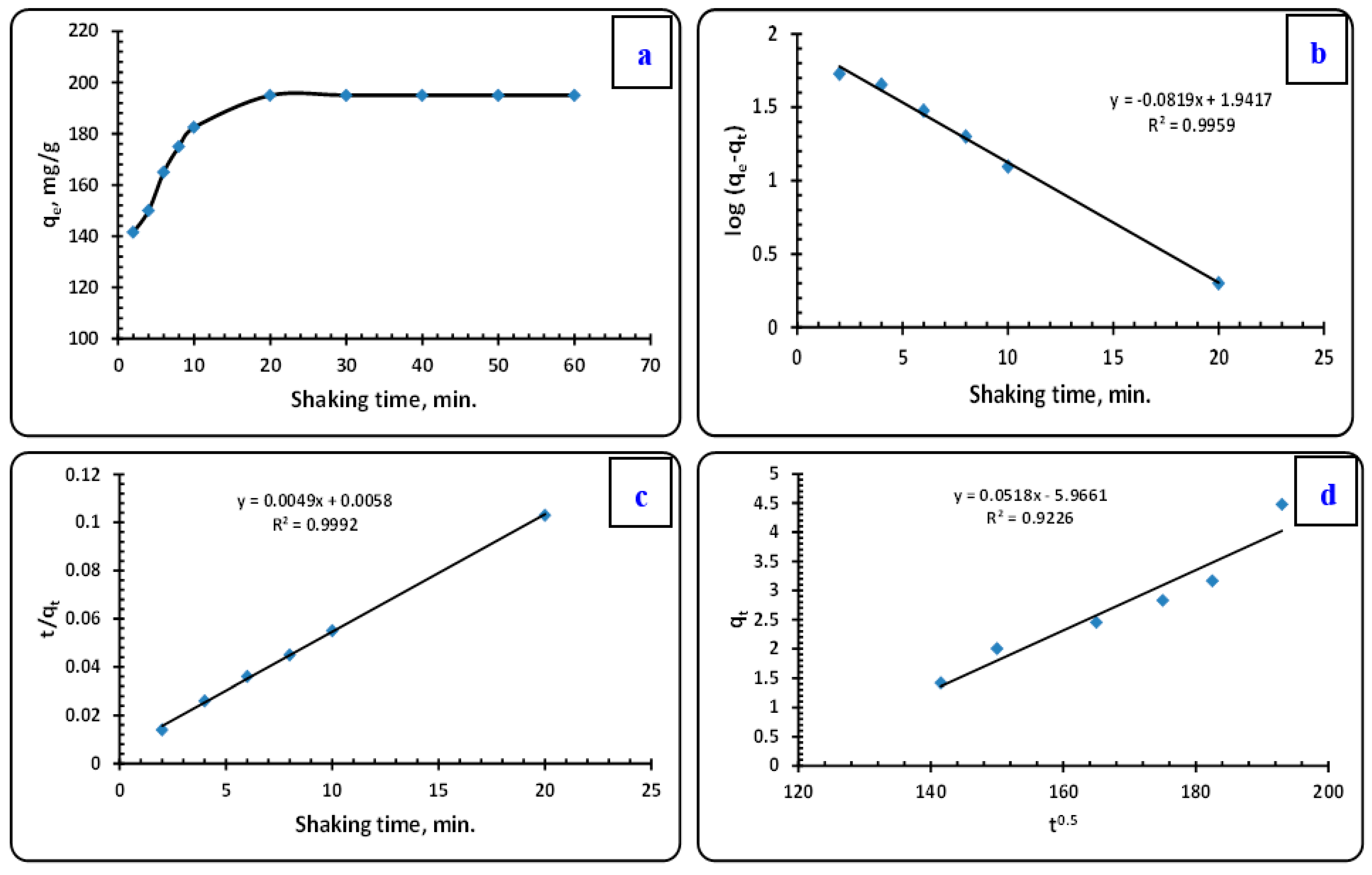
3.3.2. The Effect of Shaking Time
Kinetic Prospects
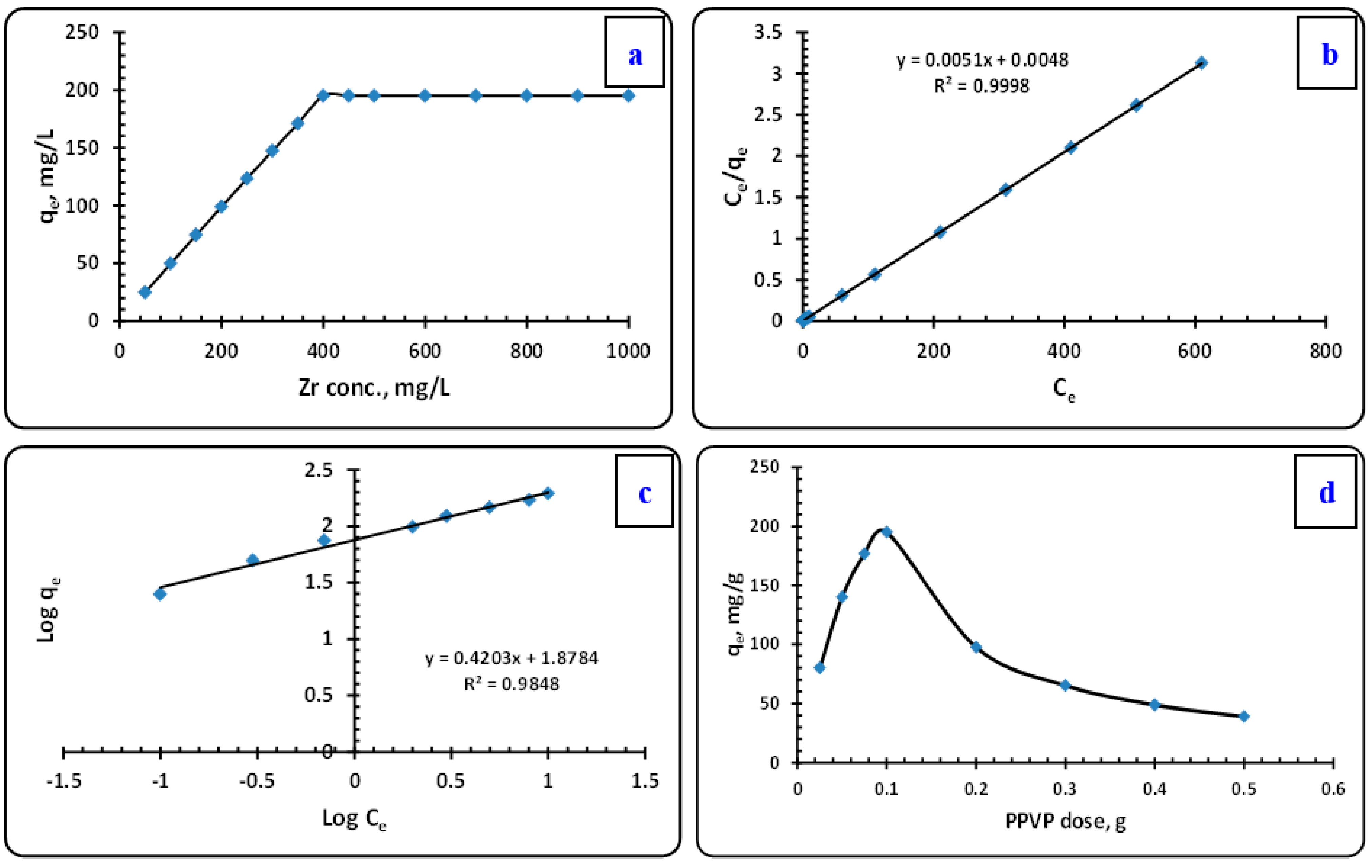
3.3.3. The Influence of Initial Zirconium Ions Concentration
Extraction-Distribution Isotherm Modeling
3.3.4. The Influence of PPVP Dose
3.3.5. The Influence of Nitrate Concentration
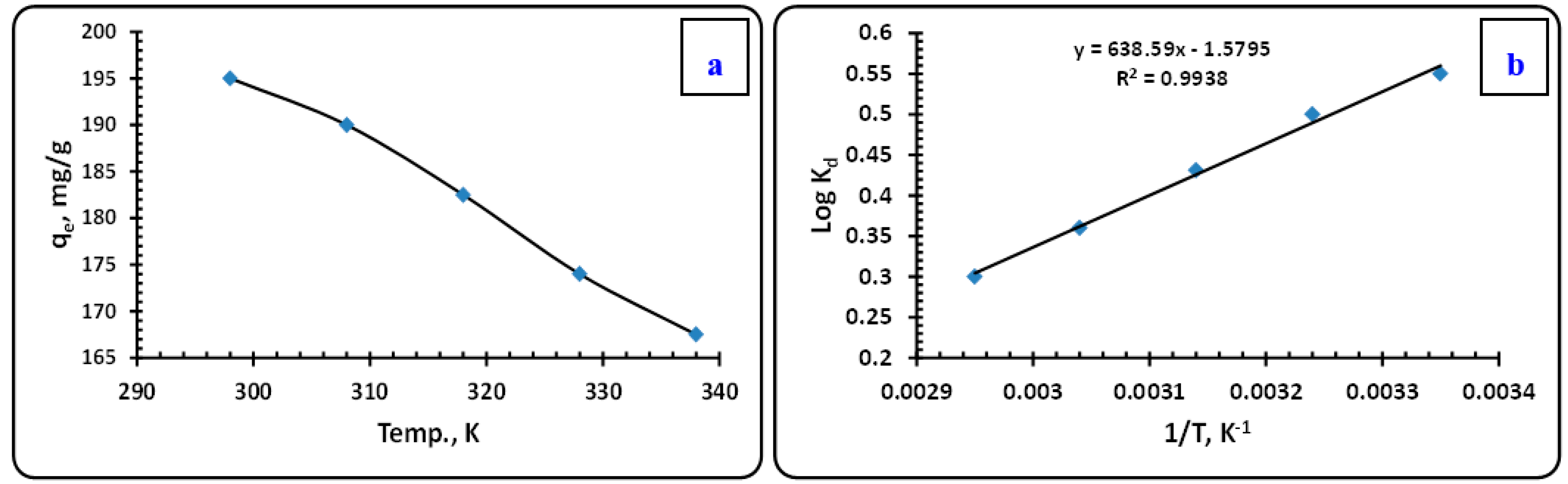
3.3.6. Thermodynamic Prospects
- Kd is the partition coefficient,
- Ea represents the extraction activation energy (kJ/mol),
- R means the molar gas constant (8.314 J/mol·K),
- T is the temperature in Kelvin (K),
- A signifies the pre-exponential factor that is independent of temperature.
3.3.7. The Effect of Co-Ions
3.3.8. Zirconium Elution and Precipitation
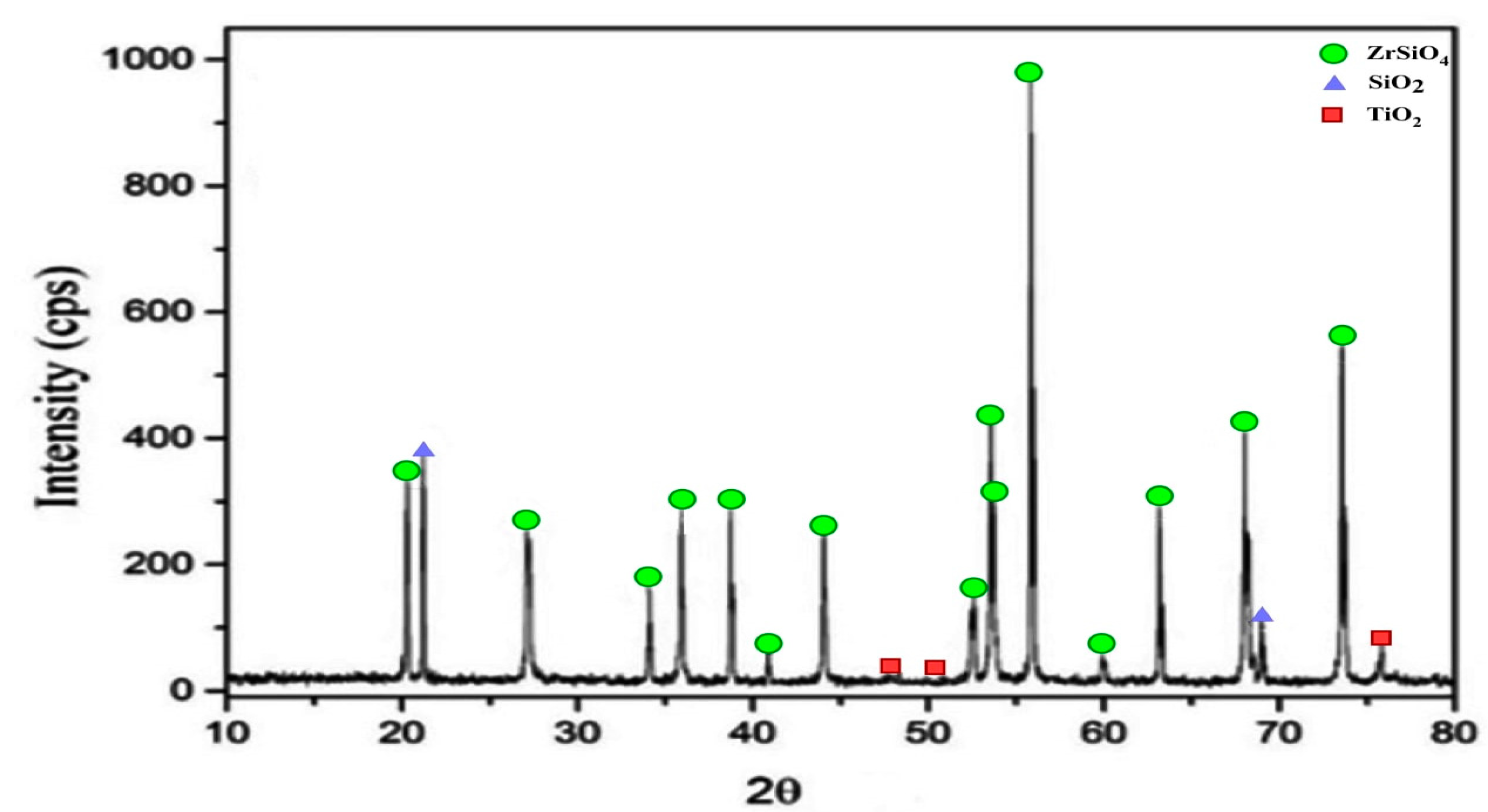
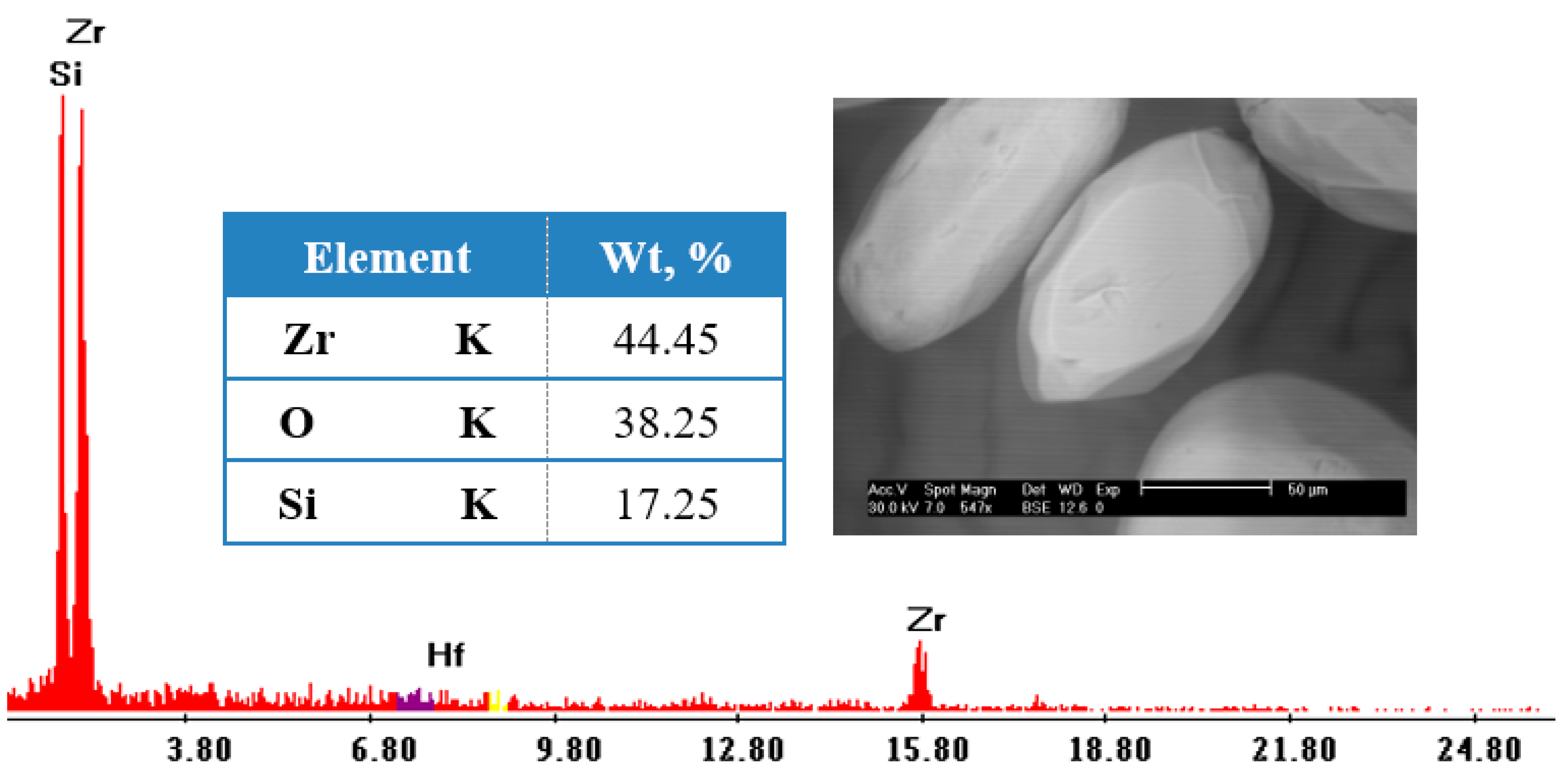
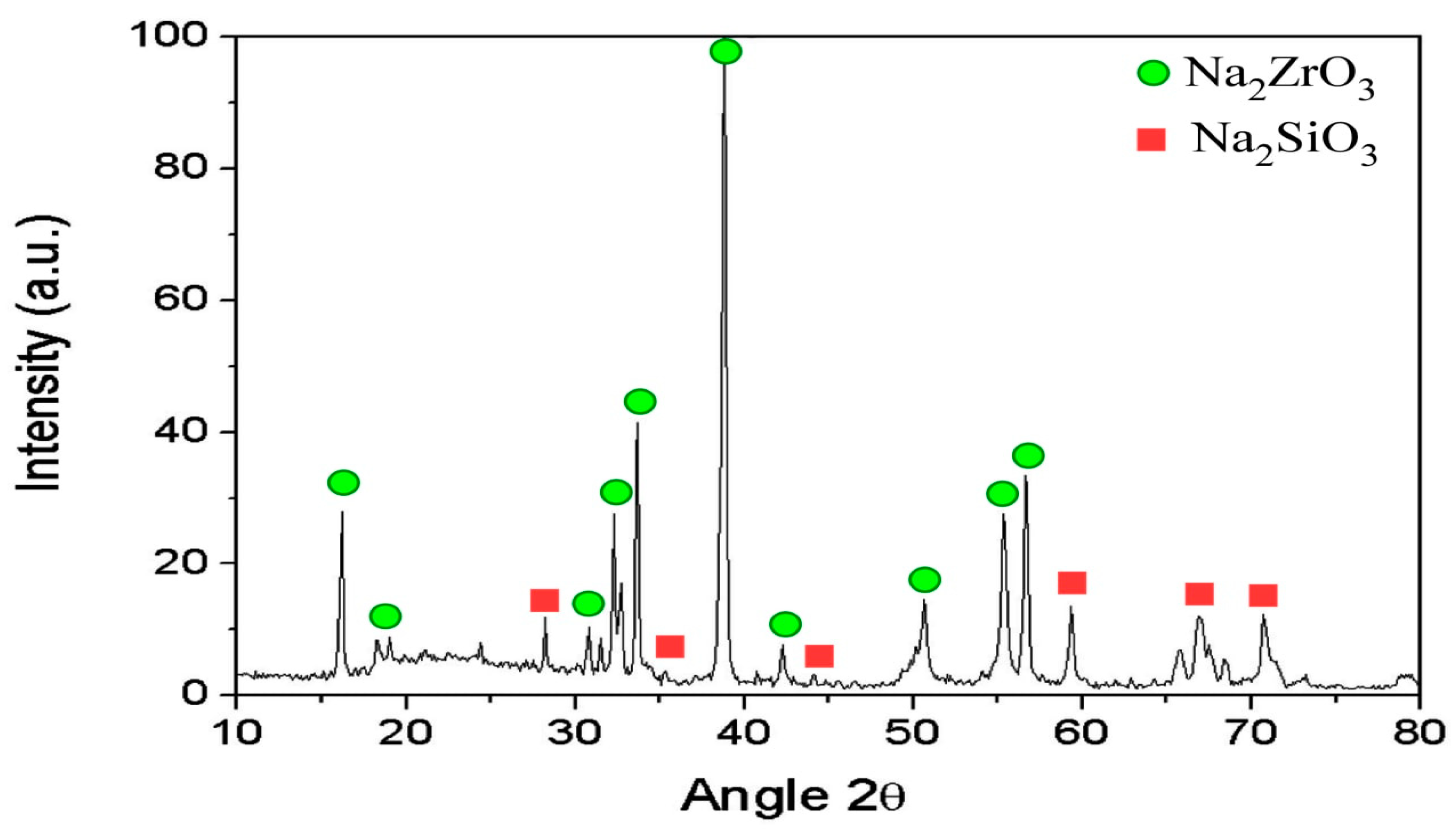
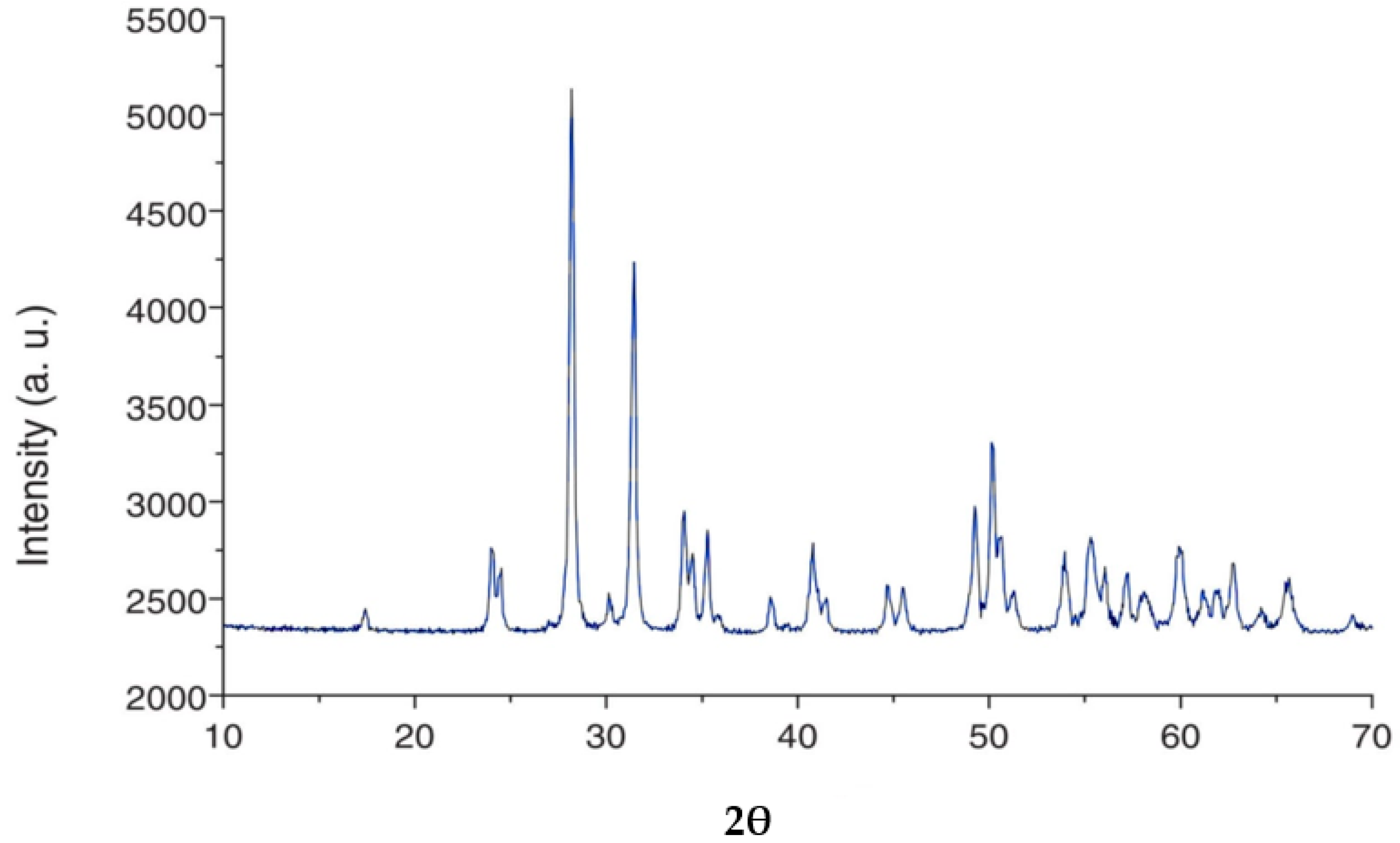
3.3.9. Characterization and Mineralogy of Zircon Concentrate Sample
3.3.10. Dissolution of Zircon Sand
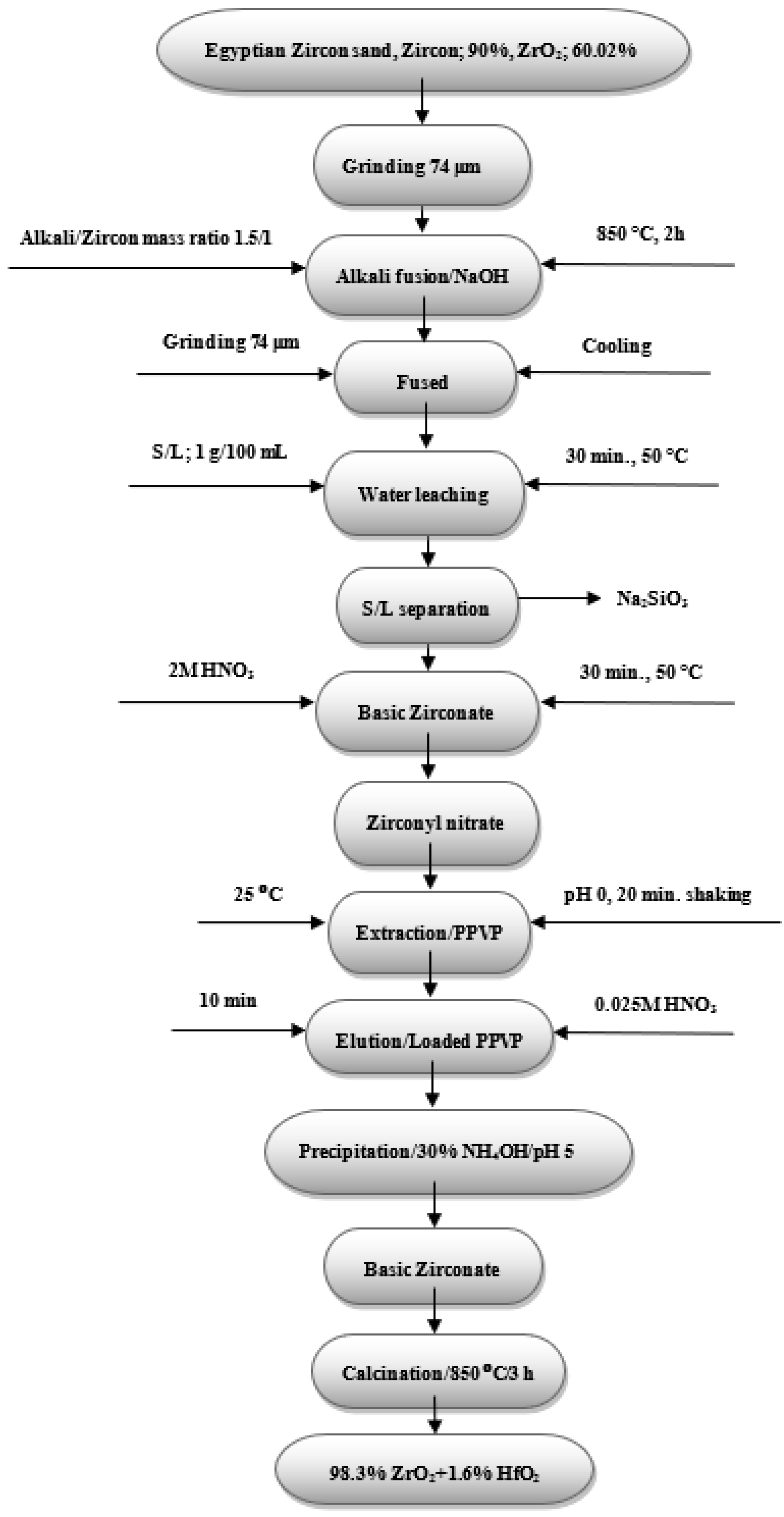
3.3.11. Application: Zirconium Recovery from Egyptian Zircon sand Using PPVP Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill Companies, Inc.: New York, NY, USA, 2003; p. 1086. ISBN 0-07-049439-8. [Google Scholar]
- Taftazani, A.; Rozana, K.; Djati, F.K. Synthesis and characterization of TENORM-free nano zirconia from zircon sand. J. Indian Chem. Soc. 2022, 99, 100678. [Google Scholar] [CrossRef]
- Samin, H.P.; Kharistya, R.S. The processing and validation of zircon sand concentrate process into zircon opacifier. AIP Conf. Proc. 2020, 2296, 020073. [Google Scholar] [CrossRef]
- Nur, S.A.; Axl, B.G.V.; Nur, A.F.; Suminar, P. Synthesis of a high-purity zircon powder with variations of HCI concentration and heating temperature. AIP Conf. Proc. 2020, 2296, 020124. [Google Scholar] [CrossRef]
- Ali, A.H. Production of highly purified zirconium oxide from zircon mineral leach liquor using bis-(2-ethylhexyl) phosphate as a promising extractant. Chem. Pap. 2022, 76, 6723–6734. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Ghasemzadeh, R.; Ashrafizadeh, S.N.; Saberyan, K.; Ghannadi, M.M. Selective zirconium stripping of a loaded CYANEX-272 using Taguchi orthogonal array design. Miner. Eng. 2007, 20, 1401–1403. [Google Scholar] [CrossRef]
- Song, J.; Fan, J.F.; Liu, J.C.; Liu, R.; Qu, J.K.; Qi, T. A two-step zircon decomposition method to produce zirconium oxychloride: Alkali fusion and water leaching. Rare Met. 2020, 39, 448–454. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, Y.; Sandwijk, A.V.; Xu, Q.; Yang, Y. Production of nuclear grade zirconium: A review. J. Nucl. Mater. 2015, 466, 21–28. [Google Scholar] [CrossRef]
- Bidaye, A.C.; Venkatachalam, S.; Gupta, C.K. Kinetic studies on the chlorination of Zircon, High Temp. Mater. Process. 2002, 21, 1–10. [Google Scholar] [CrossRef]
- Bidaye, A.C.; Venkatachalam, S.; Gupta, C.K. Studies on the chlorination of Zircon: Part I static bed investigation, Metall. Mater. Trans. B 1999, 30, 205–213. [Google Scholar] [CrossRef]
- Donald, V. Zirconium Chlorination Behavior: A Literature Review. In EPD Congress 2011; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.M. A new technique for extracting Zirconium form Egyptian zircon concentrate. Int. J. Miner. Process. 2005, 76, 234–243. [Google Scholar] [CrossRef]
- Biswas, R.K.; Habib, M.A.; Karmakar, A.K.; Islam, M.R. A novel method for processing of Bangladeshi Zircon: Part I: Baking, and fusion with NaOH. Hydrometallurgy 2010, 103, 124–129. [Google Scholar] [CrossRef]
- Da Silva, R.J.F.; Dutra, A.J.B.; Afonso, J.C. Alkali fusion followed by a two-step leaching of brazilian zircon concentrate. Hydrometallurgy 2012, 117, 93–100. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Daher, A.; El-Kashef, E. Novel decomposition method for zircon. J. Alloys Compd. 2008, 460, 577–580. [Google Scholar] [CrossRef]
- Ozel, E.; Turan, S. Production of colored zircon pigments from Zircon. J. Eur. Ceram. Soc. 2007, 27, 1751–1757. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Jiang, D. Decomposition process of zircon sand concentrate with CaO-NaOH. Rare Met. 2012, 31, 410–414. [Google Scholar] [CrossRef]
- El-Barawy, K.A.; El-Tawil, S.Z.; Francis, A.A. Production of zirconia from Zircon by thermal reaction with calcium oxide. J. Ceram. Soc. Jpn. 1999, 107, 97–102. [Google Scholar] [CrossRef][Green Version]
- El-Tawil, S.Z.; El-Barawy, K.A.; Francis, A.A. Cubic zirconia from zircon sand by firing with CaO/MgO mixture. J. Ceram. Soc. Jpn. 1999, 107, 193–198. [Google Scholar] [CrossRef]
- Welham, N.J. New route for the extraction of crude zirconia from Zircon. J. Am Ceram. Soc. 2002, 85, 2217–2221. [Google Scholar] [CrossRef]
- Kostic, Z.G.; Stefanovic, P.L.; Pavlovic, P.B.; Pavlovic, Z.N.; Živkovic, N.V. Optimal thermal plasma processes for zirconium carbide powder production from Zircon concentrates. Ceram Int. 2001, 27, 547–557. [Google Scholar] [CrossRef]
- Yuhelda, D.A.; Enggan, P.N. Processing zirconia through zircon sand smelting with NaOH as a flux. Indones. Min. J. 2016, 19, 39–49. [Google Scholar] [CrossRef]
- Aziz, I.A.; Jalhom, M.G.; Kheriallah, M.A. Extraction of Zirconium From Iraqi Bauxite Ore. Eng. Technol. J. 2020, 38, 1421–1429. [Google Scholar] [CrossRef]
- Yustanti, E.; Ode, F.A.F.; Sulistiyono, E. Primary study of KOH alkali fusion—Nitric acid leaching process in extraction of West Kalimantan zircon concentrate. IOP Conf. Ser. Mater. Sci. Eng. 2020, 763, 012065. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Wang, X.; Forsberg, K.; Friedrich, B. Basic Sulfate Precipitation of Zirconium from Sulfuric Acid Leach Solution. Metals 2020, 10, 1099. [Google Scholar] [CrossRef]
- Abd El-Hamid, A.M.; Zahran, M.A.; Khalid, F.M.; Mahmoud, A.H. Leaching of hafnium, Zirconium, uranium and other nuclear economic elements from petroleum ash. RSC Adv. 2014, 4, 12506–12513. [Google Scholar] [CrossRef]
- Atia, B.M.; Sakr, A.K.; Gado, M.A.; El-Gendy, H.S.; Abdelazeem, N.M.; El-Sheikh, E.M.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Synthesis of a New Chelating Iminophosphorane Derivative (Phosphazene) for U(VI) Recovery. Polymers 2022, 14, 1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Lee, M.S. A review on the aqueous chemistry of Zr(IV) and Hf(IV) and their separation by solvent extraction. J. Ind. Eng. Chem. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Banda, R.; Lee, M.S. Solvent Extraction for the Separation of Zr and Hf from Aqueous Solutions. Sep. Purif. Rev. 2015, 44, 199–215. [Google Scholar] [CrossRef]
- Janúbia, C.B.S.; Amaral, C.A.M. Equilibrium of Zirconium and hafnium in the process of extraction with TBP in nitric medium—Influence in the Zr/Hf separation. Miner. Eng. 2020, 146, 106138. [Google Scholar] [CrossRef]
- Amaral, J.C.; Souza, A.L.; Morais, C.A. Liquid–liquid separation of Zirconium and hafnium from nitric liquor in order to obtain nuclear zirconium oxide using TBP as extractant. Chem. Eng. Commun. 2020, 207, 73–83. [Google Scholar] [CrossRef]
- Amaral, J.C.B.; de Morais, C.A. Study of Zirconium and Hafnium Separation by Solvent Extraction Technique from Nitric and Hydrochloric Solutions with Acid, Basic and Neutral Extractants. World J. Eng. Technol. 2016, 4, 138–150. [Google Scholar] [CrossRef]
- Sadigzadeh, A.; Chehrenama, S.; Shokri, H. Solvent extraction of Zirconium from nitric-hydrochloric acid solutions using TBP. J. Theor. Appl. Phys. 2009, 3, 20–26. [Google Scholar]
- Taghizadeh, M.; Ghanadi, M.; Zolfonoun, E. Separation of Zirconium and hafnium by solvent extraction using mixture of TBP and cyanex 923. J. Nucl. Mater. 2011, 412, 334–337. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Ghasemzadeh, R.; Ashrafizadeh, S.N.; Saberyan, K.; Maragheh, M.G. Determination of optimum process conditions for the extraction and separation of Zirconium and hafnium by solvent extraction. Hydrometallurgy 2008, 90, 115–120. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, H.Y.; Lee, M.S. Solvent extraction of Zirconium and hafnium from hydrochloric acid solutions using acidic organophosphorus extractants and their mixtures with TOPO. Mater. Trans. 2013, 54, 1460–1466. [Google Scholar] [CrossRef]
- Pandey, G.; Mukhopadhyay, S.; Renjith, A.U.; Joshi, J.M.; Shenoy, K.T. Recovery of Hf and Zr from slurry waste of zirconium purification plant using solvent extraction. Hydrometallurgy 2016, 163, 61–68. [Google Scholar] [CrossRef]
- Yadollahi, A.; Saberyan, K.; Torab-Mostaedi, M.; Charkhi, A.; Pourjavid, M.R. Solvent extraction separation of Zirconium and hafnium from nitric acid solutions using mixture of Cyanex-272 and TBP. Radiochim. Acta 2018, 106, 675–684. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Wu, M.; Xu, Y.; Chi, R.; Li, P.; Zhao, J. Separation of Zirconium and hafnium by solvent extraction using mixture of DIBK and P204. Hydrometallurgy 2015, 165, 275–281. [Google Scholar] [CrossRef]
- Biswas, R.K.; Hayat, M.A. Solvent extraction of Zirconium (IV) from chloride media by D2EHPA in kerosene. Hydrometallurgy 2002, 63, 149–158. [Google Scholar] [CrossRef]
- Juang, R.S.; Lo, R.H. Equilibrium studies of the extraction of Zirconium(IV) from sulfuric acid solutions with di(2-ethylhexyl)phosphoric acid. J. Chem. Technol. Biotechnol. 1993, 58, 261–269. [Google Scholar] [CrossRef]
- Reddy, B.R.; Kumar, J.R.; Raja, K.P.; Reddy, A.V. Solvent extraction of Hf(IV) from acidic chloride solutions using Cyanex 302. Miner. Eng. 2004, 17, 939–942. [Google Scholar] [CrossRef]
- Kumar, J.R.; Reddy, B.R.; Reddy, K.R.; Reddy, A.V. Liquid-liquid extraction of tetravalent hafnium from acidic chloride solutions using bis(2,4,-trimethylpentyl) dithiophosphonic acid (Cyanex 301). Sep. Sci. Technol. 2007, 42, 865–877. [Google Scholar] [CrossRef]
- Reddy, B.R.; Kumar, J.R.; Reddy, A.V. Solvent extraction of Zirconium (IV) from acidic chloride solutions using the thio-substituted organo-phosphorous acids Cyanex 301 and 302. J. Chem. Technol. Biotechnol. 2004, 79, 1301–1307. [Google Scholar] [CrossRef]
- Reddy, B.R.; Kumar, J.R.; Reddy, A.V. Solvent extraction of Zr(IV) from acid chloride solutions using LIX 84-IC. Hydrometallurgy 2004, 74, 173–177. [Google Scholar] [CrossRef]
- Reddy, B.R.; Kumar, J.R. Studies on liquid-liquid extraction of tetravalent hafnium from weakly hydrochloric acid solutions by LIX 84-IC. Sep. Purif. Technol. 2005, 42, 169–174. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Separation of Zirconium and hafnium from nitric acid solutions with LIX 63, PC 88A and their mixture by solvent extraction. Hydrometallurgy 2014, 150, 153–160. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.G.; Oh, J.K. Stoichiometric relation for extraction of Zirconium and hafnium from acidic chloride solutions with Versatic acid 10. Hydrometallurgy 2004, 73, 91–97. [Google Scholar] [CrossRef]
- El-Hefny, N.E.; El-Nadi, Y.A.; Daoud, J.A. Effect of diluents on the extraction of Zirconium from nitrate medium by thenoyltrifluoroacetone. Solvent Extr. Ion Exch. 2006, 24, 703–717. [Google Scholar] [CrossRef]
- Katsuta, S.; Yanagihara, H. Extraction of Zirconium(IV) and Hafnium(IV) with acetylacetone in the presence of 3,5-dichlorophenol: Remarkable hydrogen-bond acceptor ability of tetrakis (acetylacetanato) chelates. Solvent Extr. Ion Exch. 1997, 15, 577–589. [Google Scholar] [CrossRef]
- Saleh, A.S.H. Solvent extraction of Zr(IV) and Hf(IV) with N,N,N′,N′-tertraoctyldiglycolamide. J. Radioanal. Nucl. Chem. 2012, 292, 1109–1114. [Google Scholar] [CrossRef]
- Prathibha, T.; Suneesh, A.S.; Chandra, S.; Sriram, S.; Ramanathan, N. Selective removal of Zr (IV) from simulated High-Level liquid waste of metallic fuel reprocessing using hydroxyacetamide Extractant: Insights from solvent extraction and density functional theory computations. Polyhedron 2021, 208, 115410. [Google Scholar] [CrossRef]
- Rajmane, M.M.; Sargar, B.M.; Mahamuni, S.V.; Anuse, M.A. Solvent extraction separation of zirconium(IV) from succinate media with N-n-octylaniline. J. Serb. Chem. Soc. 2006, 71, 223–234. [Google Scholar] [CrossRef]
- Banda, R.; Lee, H.W.; Lee, M.S. Separation of Zr from Hf in hydrochloric acid solution using amine based extractants. Ind. Eng. Chem. Res. 2012, 51, 9652–9660. [Google Scholar] [CrossRef]
- Karve, M.A.; Khopkar, S.M. Application of liquid anion exchangers for the separation of Zirconium and Hafnium. Anal. Sci. 1992, 8, 237–241. [Google Scholar] [CrossRef][Green Version]
- Vibhute, C.P.; Khopkar, S.M. Liquid/liquid extraction of separation of hafnium with Amberlite LA-1 from Zirconium and other elements in citric acid solutions. Anal. Chim. Acta 1987, 193, 387–392. [Google Scholar] [CrossRef]
- Mishra, P.K.; Chakravortty, V.; Dash, K.C.; Das, N.R.; Bhattacharyya, S.N. Extraction of Zirconium(IV) from HCl solutions by mixture Aliquat-336 and Alamine 336 with TBP. J. Radioanal. Nucl. Chem. 1989, 134, 259–264. [Google Scholar] [CrossRef]
- Mishra, P.K.; Chakravortty, V.; Dash, K.C.; Das, N.R.; Bhattacharyya, S.N. Solvent extractions of Zirconium and niobium from HCl solutions by Aliquat 336 and DOSO mixtures. J. Radioanal. Nucl. Chem. 1989, 131, 281–287. [Google Scholar] [CrossRef]
- Mishra, P.K.; Chakravortty, V.; Dash, K.C.; Das, N.R.; Bhattacharyya, S.N. Extraction and separation of Zr, Nb and Hf by Aliquat 336 and its mixture with TOPO from acidic thiocyanate media. J. Radioanal. Nucl. Chem. 1992, 162, 289–298. [Google Scholar] [CrossRef]
- Biswas, S.; Basu, S. Extraction of Zirconium(IV) from hydrochloric acid solutions by tri-octylamine and neutral donors. J. Radioanal. Nucl. Chem. 1999, 242, 253–258. [Google Scholar] [CrossRef]
- El-Yamani, I.S.; Farah, M.Y.; Abd El-Aleim, F.A. Co-extraction and separation of Zirconium and hafnium by long-chain amines from sulfate media. Talanta 1978, 25, 523–525. [Google Scholar] [CrossRef]
- Banda, R.; Lee, H.W.; Lee, M.S. Separation of Zr and Hf from strong hydrochloric acid solution by solvent extraction with TEHA. J. Radioanal. Nucl. Chem. 2013, 295, 1537–1543. [Google Scholar] [CrossRef]
- El-Yamani, I.S.; Farah, M.Y.; Abd El-Aleim, F.A. Studies on extraction of Zirconium by quaternary ammonium halides from hydrochloric acid media and its separation from hafnium. Talanta 1978, 25, 714–716. [Google Scholar] [CrossRef]
- Awada, H.; Daneault, C. Chemical Modification of Poly(Vinyl Alcohol) in Water. Appl. Sci. 2015, 5, 840–850. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Rescignanoa, N.; Fortunati, E.; Montesano, S.; Emiliani, C.; Kenny, J.M.; Martino, S.; Armentano, I. PVA bio-nanocomposites: A new take-off using cellulose nanocrystals and PLGA nanoparticles. Carbohydr. Polym. 2014, 99, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gaina, C.; Ursache, O.; Gaina, V.; Ionita, D. Study on the Chemical Modification of Poly(Vinyl Alcohol) with 4-Maleimidophenyl Isocyanate. Polym. Plast. Technol. Eng. 2012, 51, 65–70. [Google Scholar] [CrossRef]
- Saat, A.M.; Johan, M.R. Effect of Phosphoric Acid Concentration on the Optical Properties of Partially Phosphorylated PVA Complexes. Int. J. Polym. Sci. 2014, 2014, 495875. [Google Scholar] [CrossRef]
- Saat, A.M.; Johan, M.R. Enhanced thermal and structural properties of partially phosphorylated polyvinyl alcohol—Aluminum phosphate (PPVA-AlpO4) nano-composites with aluminium nitrate source, Advanced Materials for Sustainability and Growth. AIP Conf. Proc. 2017, 1901, 130011-1–130011-8. [Google Scholar] [CrossRef]
- Muller, J.; Prelot, B.; Zajac, J.; Monge, S. Synthesis and study of sorption properties of polyvinyl alcohol (PVA)-based hybrid materials. React. Funct. Polym. 2019, 144, 104364. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chiu, Y.C. Novel approach to the chemical modification of poly(vinyl alcohol): Phosphorylation. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1107–1113. [Google Scholar] [CrossRef]
- Crispim, E.G.; Piai, J.F.; Schüquel, I.T.A.; Rubira, A.F.; Muniz, E.C. Functionalization of poly(vinyl alcohol) by addition of methacryloyl groups: Characterization by FTIR and NMR and optimization of reaction conditions by RSM. e-Polymers 2006, 6, 1–18. [Google Scholar] [CrossRef]
- Ferreira, F.B.N.; Rocha, A.M.; Zille, A.; Marques, A.D.; Fangueiro, R. Polyester-Polyvinylalcohol Hydrophilization Strategies. Mater. Sci. Forum 2022, 1063, 173–180. [Google Scholar] [CrossRef]
- Xiao, M.; Chery, J.; Frey, M.W. Functionalization of Electrospun Poly(vinyl alcohol) (PVA) Nanofiber Membranes for Selective Chemical Capture. ACS Appl. Nano Mater. 2018, 1, 722–729. [Google Scholar] [CrossRef]
- Joseph, P.; McNally, S.T. Reactive modifications of some chain- and step-growth polymers with phosphorus-containing compounds: Effects on flame retardance—A review. Polym. Adv. Technol. 2011, 22, 395–406. [Google Scholar] [CrossRef]
- Ren, Y.; Tian, T.; Jiang, L.; Liu, X.; Han, Z. Polyvinyl Alcohol Reinforced Flame-Retardant Polyacrylonitrile Composite Fiber Prepared by Boric Acid Cross-Linking and Phosphorylation. Materials 2018, 11, 2391. [Google Scholar] [CrossRef]
- Prajapati, G.K.; Roshan, R.; Gupta, P.N. Effect of plasticizer on ionic transport and dielectric properties of PVA–H3PO4 proton conducting polymeric electrolytes. J. Phys. Chem. Solids 2010, 71, 1717–1723. [Google Scholar] [CrossRef]
- Sheha, E.; Nasr, M.; El-Mansy, M.K. Characterization of poly (vinyl alcohol)/poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) polymer blend: Structure, optical absorption, electrical and dielectric properties. Phys. Scr. 2013, 88, 035701. [Google Scholar] [CrossRef]
- An, Y.; Ushida, T.; Suzuki, M.; Koyama, T.; Hanabusa, K.; Shirai, H. Complex-Formation of Partially Phosphorylated Poly(Vinyl Alcohol), with Metal-Ions in Aqueous-Solution. Polymer 1996, 37, 3097–3100. [Google Scholar] [CrossRef]
- Chen, X.; Huang, R.; Pelton, R. The Reinforcement of Calcium Carbonate Filled Papers with Phosphorus-Containing Polymers. Ind. Eng. Chem. Res. 2005, 44, 2078–2085. [Google Scholar] [CrossRef]
- Somani, P.R.; Viswanath, A.K.; Aiyer, R.C.; Radhakrishnan, S. Novel dye+solid polymer electrolyte material for optical humidity sensing. Org. Electron. 2001, 2, 83–88. [Google Scholar] [CrossRef]
- Yu, Q.; Carlsen, P. Synthesis of Polynucleotide Analogs Containing a Polyvinyl Alcohol Backbone. Molecules 2008, 13, 701–715. [Google Scholar] [CrossRef]
- Susilawati, S.; Prayogi, S.; Arif, M.F.; Ismail, N.M.; Bilad, M.R.; Asy’ari, M. Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays. Polymers 2021, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Pramanik, N.; Ghosh, S.K.; Pramanik, P. Synthesis and characterization of ultrafine poly(vinylalcohol phosphate) coated magnetite nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.L. Analytical applications of xylenol orange-I: Determination of traces of Zirconium. Talanta 1959, 2, 61–66. [Google Scholar] [CrossRef]
- Okai, T. Determination of Zirconium in Carbonate Rock Reference Materials with Xylenol Orange Spectrophotometric Method. Geostand. Newsl. 1997, 21, 97–99. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Awwad, N.S.; Gado, M.A.; Hassanin, M.A.; Nayl, A.A.; Atia, B.M. Physico-Chemical Aspects on Uranium and Molybdenum Extraction from Aqueous Solution by Synthesized Phosphinimine Derivative Chelating Agent. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3640–3657. [Google Scholar] [CrossRef]
- Tang, P. Boric acid catalyzed amide formation from carboxylic acids and amines: N-Benzyl-4-phenylbutyramide. Org. Synth. 2005, 81, 262–272. [Google Scholar] [CrossRef]
- Das, R.S.; Warkhade, S.K.; Kumar, A.; Wankhade, A.V. Graphene oxide-based zirconium oxide nanocomposite for enhanced visible light-driven photocatalytic activity. Res. Chem. Intermed. 2019, 45, 1689–1705. [Google Scholar] [CrossRef]
- Ren, Y.L.; Huo, T.G.; Qin, Y.W.; Liu, X.H. Preparation of flame retardant polyacry-lonitrile fabric based on sol-gel and layer-by-layer assembly. Materials 2018, 11, 483. [Google Scholar] [CrossRef]
- Gillani, Q.F.; Ahmad, F.; Abdul Mutalib, M.I.; Megat-Yusoff, P.S.M.; Ullah, S.; Messet, P.J.; Zia-ul-Mustafa, M. Thermal degradation and pyrolysis analysis of zinc borate reinforced intumescent fire retardant coatings. Prog. Org. Coat. 2018, 123, 82–98. [Google Scholar] [CrossRef]
- Ren, Y.L.; Zhang, Y.; Zhao, J.Y.; Wang, X.L.; Zeng, Q.; Gu, Y.T. Phosphorus-doped organic-inorganic hybrid silicon coating for improving fire retardancy of polyacrylonitrile fabric. J. Sol. Gel. Sci. Technol. 2017, 82, 280–288. [Google Scholar] [CrossRef]
- Ren, Y.L.; Zhang, Y.; Gu, Y.T.; Zeng, Q. Flame retardant polyacrylonitrile fabrics prepared by organicinorganic hybrid silica coating via sol-gel technique. Prog. Org. Coat. 2017, 112, 225–233. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, S.; Huang, J.; Zhao, G.; Liu, Y. Attaching ZrO2 nanoparticles onto the surface of graphene oxide via electrostatic self-assembly for enhanced mechanical and tribological performance of phenolic resin composites. J. Mater. Sci. 2019, 54, 8247–8261. [Google Scholar] [CrossRef]
- Adair, J.H.; Krarup, H.G.; Venigalla, S.; Tsukada, T. A Review of the Aqueous Chemistry of the Zirconium—Water System to 200 °C. MRS Online Proc. Libr. 1996, 432, 101–112. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Atia, B.M.; Awwad, N.S.; Nayl, A.A.; Radwan, H.A.; Gado, M.A. Efficient preparation of phosphazene chitosan derivatives and its applications for the adsorption of molybdenum from spent hydrodesulfurization catalyst. J. Dispers. Sci. Technol. 2022, 1–16. [Google Scholar] [CrossRef]
- Ibrahium, H.A.; Gado, M.A.; Ali, H.E.; Fathy, W.M.; Atia, B.M.; Awwad, N.S. Synthesis of chelating N-hydroxyl amine derivative and its application for vanadium separation from Abu Zeneima ferruginous siltstone ore, Southwestern Sinai, Egypt. Int. J. Environ. Anal. Chem. 2021, 1–23. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. Am. Chem. Soc. 1918, 40, 1361–1368. [Google Scholar] [CrossRef]
- Atia, B.M.; Khawassek, Y.M.; Hussein, G.M.; Gado, M.A.; El-Sheify, M.A.; Cheira, M.F. One-pot synthesis of pyridine dicarboxamide derivative and its application for uranium separation from acidic medium. J. Environ. Chem. Eng. 2021, 9, 105726. [Google Scholar] [CrossRef]
- Freundlich, H. Adsorption in solution. Phys. Chem. Soc. 1906, 40, 1361–1368. [Google Scholar]
- Gado, M.A.; Atia, B.M.; Cheira, M.F.; Elawady, M.E.; Demerdash, M. Highly efficient adsorption of uranyl ions using hydroxamic acid-functionalized graphene oxide. Radiochim. Acta 2021, 109, 743–757. [Google Scholar] [CrossRef]
- Atia, B.M.; Gado, M.A.; Abd El-Magied, M.O.; Elshehy, E.A. Highly efficient extraction of uranyl ions from aqueous solutions using multi-chelators functionalized graphene oxide. Sep. Sci. Technol. 2020, 55, 2746–2757. [Google Scholar] [CrossRef]

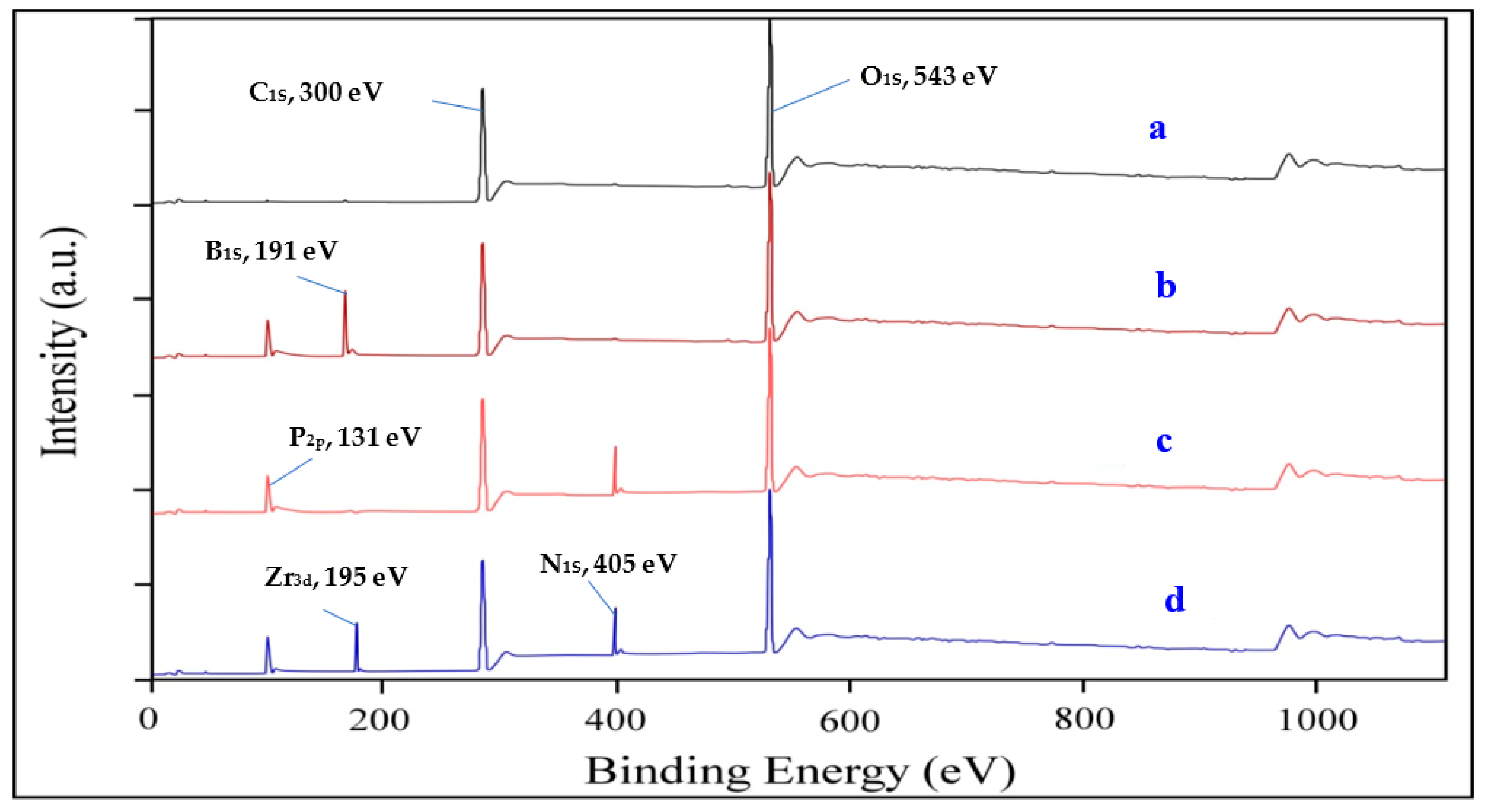



| Sample | C1S, % | O1S, % | N1S, % | P2P, % | B1S, % | Zr3d, % |
|---|---|---|---|---|---|---|
| PVA | 54.5 | 36.35 | - | - | - | - |
| PPVA | 19.3 | 47.46 | - | 23 | 1.5 | - |
| PPVP | 71.38 | 5.6 | 5 | 5.42 | - | - |
| PPVP-Zr | 61.75 | 4.83 | 4.22 | 4.68 | - | 10.77 |
| Sample | Pore Volume, cc⋅g−1 | SBET, m2⋅g−1 | Pore Size, nm |
|---|---|---|---|
| PVA | 0.03 | 9.25 | 9.1 |
| PPVA | 0.05 | 12.03 | 11.33 |
| PPVP | 0.06 | 17.48 | 16.01 |
| Co-Ions | Feed Soln.mg/L | Raffinate, mg/L | S.F * | Co-Ions | Feed Soln.mg/L | Raffinate, mg/L | S.F * |
|---|---|---|---|---|---|---|---|
| Zr4+ | 500 | 110 | - | Fe3+ | 1000 | 750 | 10.64 |
| Na+ | 1000 | 1000 | 3545 | Ti4+ | 1000 | 890 | 28.82 |
| K+ | 1000 | 1000 | 3545 | Ca2+ | 1000 | 930 | 47.26 |
| Si4+ | 1000 | 1000 | 3545 | Mg2+ | 1000 | 950 | 68.17 |
| Al3+ | 1000 | 1000 | 3545 | V5+ | 1000 | 960 | 86.46 |
| Mn2+ | 1000 | 850 | 20.14 | Hf4+ | 1000 | 100 | 2.54 |
| U6+ | 1000 | 950 | 68.17 | Th4+ | 1000 | 890 | 28.82 |
| Acid Conc., (M) | Elution Efficiency, (%) | ||
|---|---|---|---|
| HNO3 | HCl | H2SO4 | |
| 0.025 | 99 | 99 | 99 |
| 0.05 | 95.6 | 95 | 97 |
| 0.1 | 86.6 | 88 | 85 |
| 0.5 | 72 | 72 | 69 |
| 1 | 63 | 62 | 60 |
| 2 | 49 | 55 | 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ene, A.; Zakaly, H.M.H.; Salem, A.R.; Gouda, A.A.; Althumayri, K.; Alshammari, K.F.; Awad, H.A.; Issa, S.A.M.; Alluhaybi, A.A.; Zaki, S.A.; et al. A New Partially Phosphorylated Polyvinyl Phosphate-PPVP Composite: Synthesis and Its Potentiality for Zr (IV) Extraction from an Acidic Medium. Separations 2022, 9, 382. https://doi.org/10.3390/separations9110382
Ene A, Zakaly HMH, Salem AR, Gouda AA, Althumayri K, Alshammari KF, Awad HA, Issa SAM, Alluhaybi AA, Zaki SA, et al. A New Partially Phosphorylated Polyvinyl Phosphate-PPVP Composite: Synthesis and Its Potentiality for Zr (IV) Extraction from an Acidic Medium. Separations. 2022; 9(11):382. https://doi.org/10.3390/separations9110382
Chicago/Turabian StyleEne, Antoaneta, Hesham M. H. Zakaly, Amany R. Salem, Ayman A. Gouda, Khalid Althumayri, Khaled F. Alshammari, Hamdi A. Awad, Shams A. M. Issa, Ahmad A. Alluhaybi, Salah A. Zaki, and et al. 2022. "A New Partially Phosphorylated Polyvinyl Phosphate-PPVP Composite: Synthesis and Its Potentiality for Zr (IV) Extraction from an Acidic Medium" Separations 9, no. 11: 382. https://doi.org/10.3390/separations9110382
APA StyleEne, A., Zakaly, H. M. H., Salem, A. R., Gouda, A. A., Althumayri, K., Alshammari, K. F., Awad, H. A., Issa, S. A. M., Alluhaybi, A. A., Zaki, S. A., Ibrahim, H. A., Gado, M. A., El-Sheikh, E. M., & Atia, B. M. (2022). A New Partially Phosphorylated Polyvinyl Phosphate-PPVP Composite: Synthesis and Its Potentiality for Zr (IV) Extraction from an Acidic Medium. Separations, 9(11), 382. https://doi.org/10.3390/separations9110382










