Evaluation of Anti-Venom Potential of Areca catechu Seed Extract on Bungarus caeruleus Venom
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
2.1.2. Venom
2.1.3. Chemicals
2.2. Animals
2.3. Human and Animal Rights
2.4. Phytochemical Analysis of Seed Extracts
2.5. In Vitro Enzyme Assay and Neutralization Studies
2.6. Pharmacological Assays
2.7. Acute Toxicological In Vitro Studies Using Chick Embryo Model
2.7.1. Acute Toxicity Determination of Seed Extract
2.7.2. Lethal Toxicity (LD50) Quantification
2.7.3. Lethal Toxicity Neutralization by the Chick Embryo Model
2.8. Acute Toxicological In Vivo Studies Using Murine Model
2.8.1. Acute Oral Toxicity Investigation
2.8.2. Lethal Toxicity Neutralization and Evaluation
2.8.3. Neutralization of Myotoxicity
2.9. Statistical Analysis
3. Results
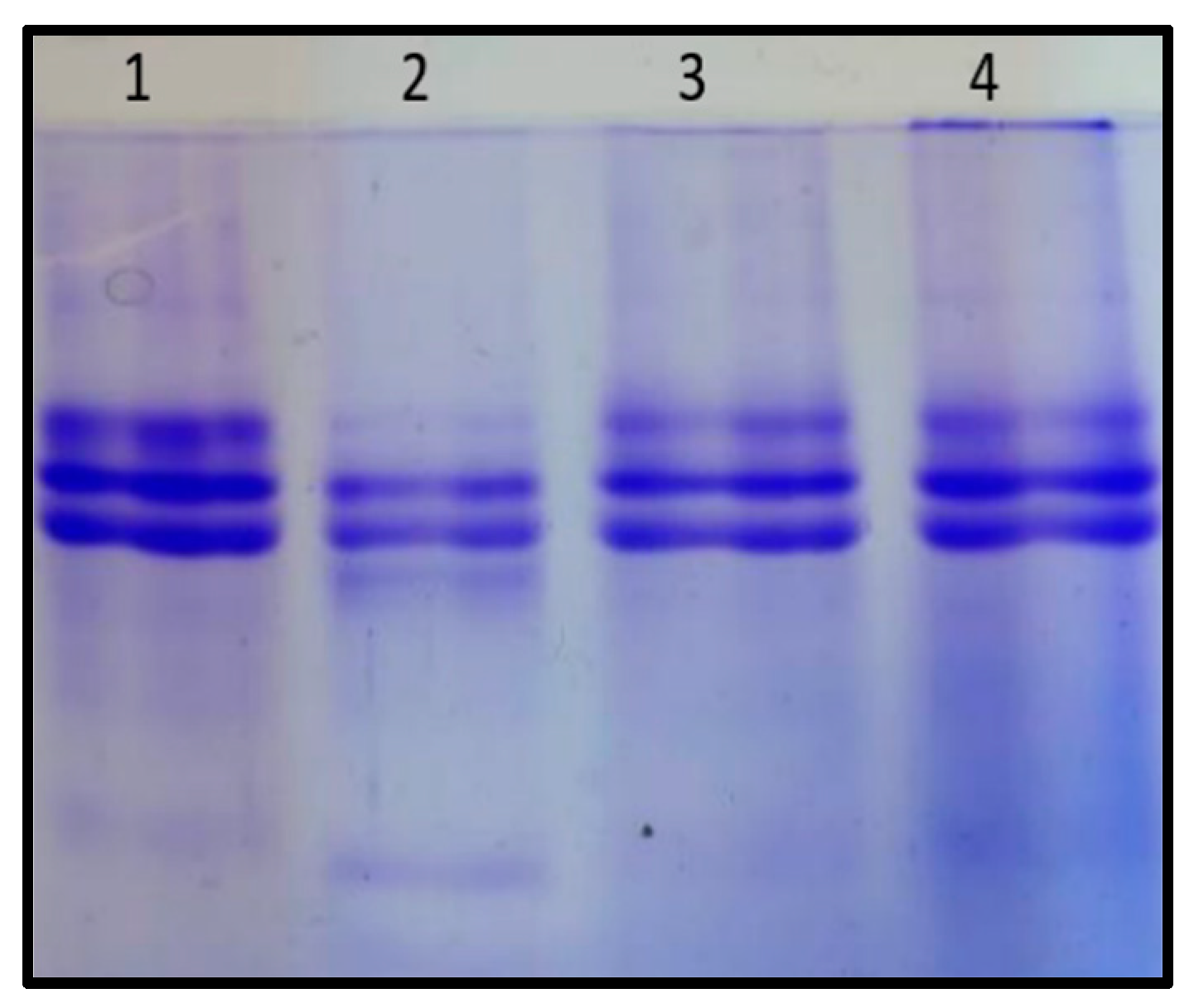
3.1. Analysis of Phytochemicals from A. catechu Seeds
3.2. In Vitro Enzyme Assay and Neutralization Studies
3.3. Inhibition of Pharmacological Activity
3.4. Acute Toxicological Studies Using Chick Embryo Model
3.5. Acute Toxicological Studies Using Murine Model
3.5.1. Acute Oral Toxicity of the A. catechu Extracts
3.5.2. Lethality Analysis and Neutralization
3.5.3. Neutralization of Myotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Snakeman, W.Z. The Story of a Naturalist; Penguin Books Ltd.: London, UK, 1990; p. 192. [Google Scholar]
- Gupta, Y.K.; Peshin, S.S. Snake bite in India: Current scenario of an old problem. J. Clin. Toxicol. 2014, 4, 182. [Google Scholar] [CrossRef]
- Alam, M.I. Inhibition of toxic effects of viper and cobra venom by Indian medicinal plants. Pharmacol. Pharm. 2014, 5, 828–837. [Google Scholar] [CrossRef]
- Girish, K.S.; Mohanakumari, H.P.; Nagaraju, S.; Vishwanath, B.S.; Kemparaju, K. Hyaluronidase and protease activities from Indian snake venoms: Neutralization by Mimosa pudica root extract. Fitoterapia 2004, 75, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Yingprasertchai, S.; Bunyasrisawat, S.; Ratanabanangkoon, K. Hyaluronidase inhibitors (sodium cromoglycate and sodium auro-thiomalate) reduce the local tissue damage and prolong the survival time of mice injected with Naja kaouthia and Calloselasma rhodostoma venoms. Toxicon 2003, 42, 635–646. [Google Scholar] [CrossRef]
- Melo, P.A.; Ownby, C.L. Ability of wedelolactone, heparin, and para-bromophenacyl bromide to antagonize the myotoxic effects of two crotaline venoms and their PLA2 myotoxins. Toxicon 1999, 37, 199–215. [Google Scholar] [CrossRef]
- Ushanandini, S.; Nagaraju, S.; Harish, K.; Vedavathi, M.; Machiah, D.K.; Kemparaju, K.; Vishwanath, B.S.; Gowda, T.V.; Girish, K.S. The anti-snake venom properties of Tamarindus indica (Leguminosae) seed extract. Phytother. Res. 2006, 20, 851–858. [Google Scholar] [CrossRef]
- Cannon, R.; Ruha, A.M.; Kashani, J. Acute hypersensitivity reactions associated with administration of crotalidae polyvalent immune Fab antivenom. Ann. Emerg. Med. 2008, 51, 407–411. [Google Scholar] [CrossRef]
- Theakston, R.D.; Phillips, R.E.; Warrell, D.A.; Galagedera, Y.; Abeysekera, D.T.; Dissanayaka, P.; de Silva, A.; Aloysius, D.J. Envenoming by the common krait (Bungaruscaeruleus) and Sri Lankan cobra (Najanajanaja): Efficacy and complications of therapy with Haffkineantivenom. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 301–308. [Google Scholar] [CrossRef]
- Alam, M.I.; Gomes, A. Snake venom neutralization by Indian medicinal plants (Vitexnegundo and Emblicaofficinalis) root extracts. J. Ethnopharmacol. 2003, 86, 75–80. [Google Scholar] [CrossRef]
- Soares, A.M.; Ticli, F.K.; Marcussi, S.; Lourenço, M.V.; Januário, A.H.; Sampaio, S.V.; Giglio, J.R.; Lomonte, B.; Pereira, P.S. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005, 12, 2625–2641. [Google Scholar] [CrossRef] [PubMed]
- Makhija, I.K.; Khamar, D. Anti-snake venom properties of medicinal plants. Der. Pharm. Lett. 2010, 2, 399–411. [Google Scholar]
- Geetha, R.M. Medicinal plants viz a viz indigenous knowledge among the tribals of Pachamalai hills. Indian J. Tradit. Knowl. 2010, 9, 209–215. [Google Scholar]
- Shwetha, V.; Veen, S.M.; Zameer, G.F.; Niyonzima, N.F.; More, S.S. In vitro neutralization of Najanaja venom enzymes by folk medicinal plant extracts. J. Biol. Act. Prod. Nat. 2019, 9, 278–288. [Google Scholar] [CrossRef]
- Meenatchisundaram, S.; Prajish, G.; Parameswari, S.T.; Michael, A. Studies on anti-venom activity of Andrographispaniculata and Aristolochiaindica plant extracts against Echiscarinatus venom. Internet J. Toxicol. 2008, 6, 1–7. [Google Scholar] [CrossRef]
- Umdor, M. Indigenous practice on protection of Areca catechu Linn. Seedlings—A case study in Meghalaya. Indian J. Tradit. Knowl. 2004, 3, 253–255. [Google Scholar]
- Amudhan, M.S.; Begum, V.H.; Hebbar, K.B. A review on phytochemical and pharmacological potential of Areca catechu L. seed. Int. J. Pharm. Sci. Res. 2012, 3, 4151–4157. [Google Scholar] [CrossRef]
- Badami, S.; Rai, S.R.; Suresh, B. In-vitro antioxidant properties of Indian traditional paan and its ingredients. Indian J. Tradit. Knowl. 2004, 3, 187–191. [Google Scholar]
- Bhat, S.K.; Sarpangala, M.; Ashwin, D. Antilipidemic activity of arecanut, Areca catechu L.: A valuable herbal medicine. Int. J. Herb. Med. 2017, 5, 35–38. [Google Scholar]
- Alcaraz, M.J.; Hoult, J.R. Actions of flavonoids and the novel anti-inflammatory flavone, hypolaetin-8-glucoside, on prostaglandin biosynthesis and inactivation. Biochem. Pharmacol. 1985, 15, 2477–2482. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenberg, W.J.; Farr, A.L.; Randell, R.J. Quantitation of protein using FolinCiocalteu reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis. In Phytochemical Methods; Chapman and Hall: London, UK, 1973; p. 132. [Google Scholar]
- Pukrittayakamee, S.; Warrell, D.A.; Desakorn, V.; McMichael, A.J.; White, N.J.; Bunnag, D. The hyaluronidase activities of some southeast Asian snake venoms. Toxicon 1988, 26, 629–637. [Google Scholar] [CrossRef]
- Marinetti, G.V. The action of phospholipase A on lipoproteins. Biochim. Biophys. Acta 1965, 98, 554–565. [Google Scholar] [CrossRef]
- Greenberg, D.M. Plant Proteolytic Enzymes. In Methods in Enzymology; Colowick, S.P., Kalpan, N.O., Eds.; Academic Press Inc.: New York, NY, USA, 1955; pp. 54–64. [Google Scholar]
- Gutierrez, J.; Avila, C.; Rojas, E.; Cerdas, L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 1988, 26, 411–413. [Google Scholar] [CrossRef]
- Ouyang, C.; Teng, C.M. Fibrinogenolytic enzymes of Trimeresurusmucrosquamatus venom. Biochim. Biophys. Acta 1976, 420, 298–308. [Google Scholar] [CrossRef]
- Sells, P.G.; Ioannou, P.; Theakston, R.D. A humane alternative to the measurement of the lethal effects (LD50) of non-neurotoxic venoms using hens’ eggs. Toxicon 1998, 36, 985–991. [Google Scholar] [CrossRef]
- van der Valk, T.; van der Meijden, A. Toxicity of scorpion venom in chick embryo and meal worm assay depending on the use of the soluble fraction versus the whole venom. Toxicon 2014, 88, 38–43. [Google Scholar] [CrossRef]
- Meier, J.; Theakston, R.D. Approximate LD50 determination of snake venoms using eight to ten experimental animals. Toxicon 1986, 24, 395–401. [Google Scholar] [CrossRef]
- Vineetha, M.S.; Bhavya, J.; Mirjakar, K.M.; More, S.S. In vitro evaluation of active phytochemicals from Tabernaemontanaalternifolia (Roxb) root against the Najanaja and Echiscarinatus Indian snake venom. J. Biol. Act. Prod. Nat. 2014, 4, 286–294. [Google Scholar] [CrossRef]
- Theakston, R.D.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venoms. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar]
- Krishnan, S.A.; Dileepkumar, R.; Nair, A.S.; Oommen, O.V. Studies on neutralizing effect of Ophiorrhizamungos root extract against Daboiarusselii venom. J. Ethnopharmacol. 2014, 151, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, B.; Shrikanth, V.M.; Mirajkar, K.K.; More, S.S. In vitro screening and evaluation of anti-venom phytochemicals from Azimatetracantha Lam. leaves against Bungaruscaeruleus and Viperarusselli. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, B.; Shrikanth, V.M.; More, V.S.; Melappa, G.; Zameer, F.; More, S.S. Bungaruscaeruleus venom neutralization activity of Azimatetracantha Lam. Extract. Heliyon 2019, 5, e02163. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, S.; More, S.S.; Annappa, G.S.; More, V.S. Anti-snake venom potential of Clerodendrumserratum extracts on Bungaruscaeruleus and Daboiarusselii venom. Bangladesh J. Pharmacol. 2018, 13, 187–191. [Google Scholar] [CrossRef]
- Baggai, B.I.; Yusuf, P.O.; Alloh, F.T. Inhibitory studies of Tamarindusindica seed extract and fractions on hematological activities of Bitisarietans venom. J. Adv. Biol. Biotechnol. 2020, 23, 48–57. [Google Scholar] [CrossRef]
- Meenatchisundaram, S.; Michael, A. Antitoxin activity of Mucunapruriens aqueous extracts against Cobra and krait venom by in vivo and in vitro methods. Int. J. Pharm. Tech. Res. 2010, 2, 870–874. [Google Scholar]
- Gopi, K.; Renu, K.; Jayaraman, G. Inhibition of Najanaja venom enzymes by the methanolic extract of Leucasaspera and its chemical profile by GC–MS. Toxicol. Rep. 2014, 1, 667–673. [Google Scholar] [CrossRef]
- Esmeraldino, L.E.; Souza, A.M.; Sampaio, S.V. Evaluation of the effect of aqueous extract of Croton urucurana Baillon (Euphorbiaceae) on the hemorrhagic activity induced by the venom of Bothropsjararaca, using new techniques to quantify hemorrhagic activity in rat skin. Phytomedicine 2005, 12, 570–576. [Google Scholar] [CrossRef]
- Pithayanukul, P.; Laovachirasuwan, S.; Bavovada, R.; Pakmanee, N.; Suttisri, R. Anti-venom potential of butanolic extract of Ecliptaprostrata against Malayan pit viper venom. J. Ethnopharmacol. 2004, 90, 347–352. [Google Scholar] [CrossRef]
- Sari, L.M.; Suyatna, F.D.; Utami, S.; Chairul, C.; Subita, G.P.; Auerkauri, E.I. Acute oral toxicity study of Areca catechu Linn. aqueous extract in sprague-dawley rats. Asian J. Pharm. Clin. Res. 2014, 7, 20–22. [Google Scholar]
- Vasudev, S.; More, V.S.; Ananthraju, K.S.; More, S.S. Potential of herbal cocktail of medicinal plant extracts against ‘big four’ snake venoms from India. J. Ayurveda Integr. Med. 2021, 12, 458–464. [Google Scholar] [CrossRef] [PubMed]




| Venom/Extract Concentration | Time of Death |
|---|---|
| Saline (Group 1) | 24 h |
| Venom alone (3 × LD50) (Group 2) | 2–4 h |
| Venom: extract (1:10 w/w) (Group 3) | 24 h |
| Venom: extract (1:20 w/w) (Group 4) | 24 h |
| Group | Venom in µg per /25–27 g of Murine Body Weight | Mice Dead Out of 5 | Mice Survived |
|---|---|---|---|
| Saline | - | 0 | 100% |
| Group I | 2 µg | 1 | 80% |
| Group II | 4 µg | 2 | 60% |
| Group III | 6 µg | 2 | 60% |
| Group IV | 8 µg | 3 | 40% |
| Group V | 10 µg | 5 | 0% |
| Venom/Extract Concentration | Time of Death |
|---|---|
| Saline (Group 1) | 24 h |
| Venom alone (3 × LD50) (Group 2) | 25 min |
| Venom: extract (1:10 w/w) (Group 3) | 30 min |
| Venom: extract (1:20 w/w) (Group 4) | 35 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, V.; Muhsinah, A.B.; Latha, G.S.; Alhazmi, A.Y.M.; Ibrahim, O.A.; S. Binshaya, A.; Mahnashi, M.H.; Almasoudi, H.H.; Gangadharappa, H.; Maruthi, S.N.; et al. Evaluation of Anti-Venom Potential of Areca catechu Seed Extract on Bungarus caeruleus Venom. Separations 2022, 9, 360. https://doi.org/10.3390/separations9110360
More V, Muhsinah AB, Latha GS, Alhazmi AYM, Ibrahim OA, S. Binshaya A, Mahnashi MH, Almasoudi HH, Gangadharappa H, Maruthi SN, et al. Evaluation of Anti-Venom Potential of Areca catechu Seed Extract on Bungarus caeruleus Venom. Separations. 2022; 9(11):360. https://doi.org/10.3390/separations9110360
Chicago/Turabian StyleMore, Veena, Abdullatif Bin Muhsinah, G. S. Latha, Abdulfattah Yahya M. Alhazmi, Osama Abdulaziz Ibrahim, Abdulkarim S. Binshaya, Mater H. Mahnashi, Hassan H. Almasoudi, Harshitha Gangadharappa, Sahana Nagappa Maruthi, and et al. 2022. "Evaluation of Anti-Venom Potential of Areca catechu Seed Extract on Bungarus caeruleus Venom" Separations 9, no. 11: 360. https://doi.org/10.3390/separations9110360
APA StyleMore, V., Muhsinah, A. B., Latha, G. S., Alhazmi, A. Y. M., Ibrahim, O. A., S. Binshaya, A., Mahnashi, M. H., Almasoudi, H. H., Gangadharappa, H., Maruthi, S. N., Rao, S., Janardhan, B., Khan, A. A., Muddapur, U. M., Shaikh, I. A., & More, S. S. (2022). Evaluation of Anti-Venom Potential of Areca catechu Seed Extract on Bungarus caeruleus Venom. Separations, 9(11), 360. https://doi.org/10.3390/separations9110360








