Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Quantification of Cyanotoxins
2.1.1. Sample Preparation and Extraction
2.1.2. UHPLC-MS/MS Parameters
2.1.3. Calculations of Toxin Concentration
2.2. Validation Parameters
2.2.1. Specificity
2.2.2. Ion Ratio
2.2.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
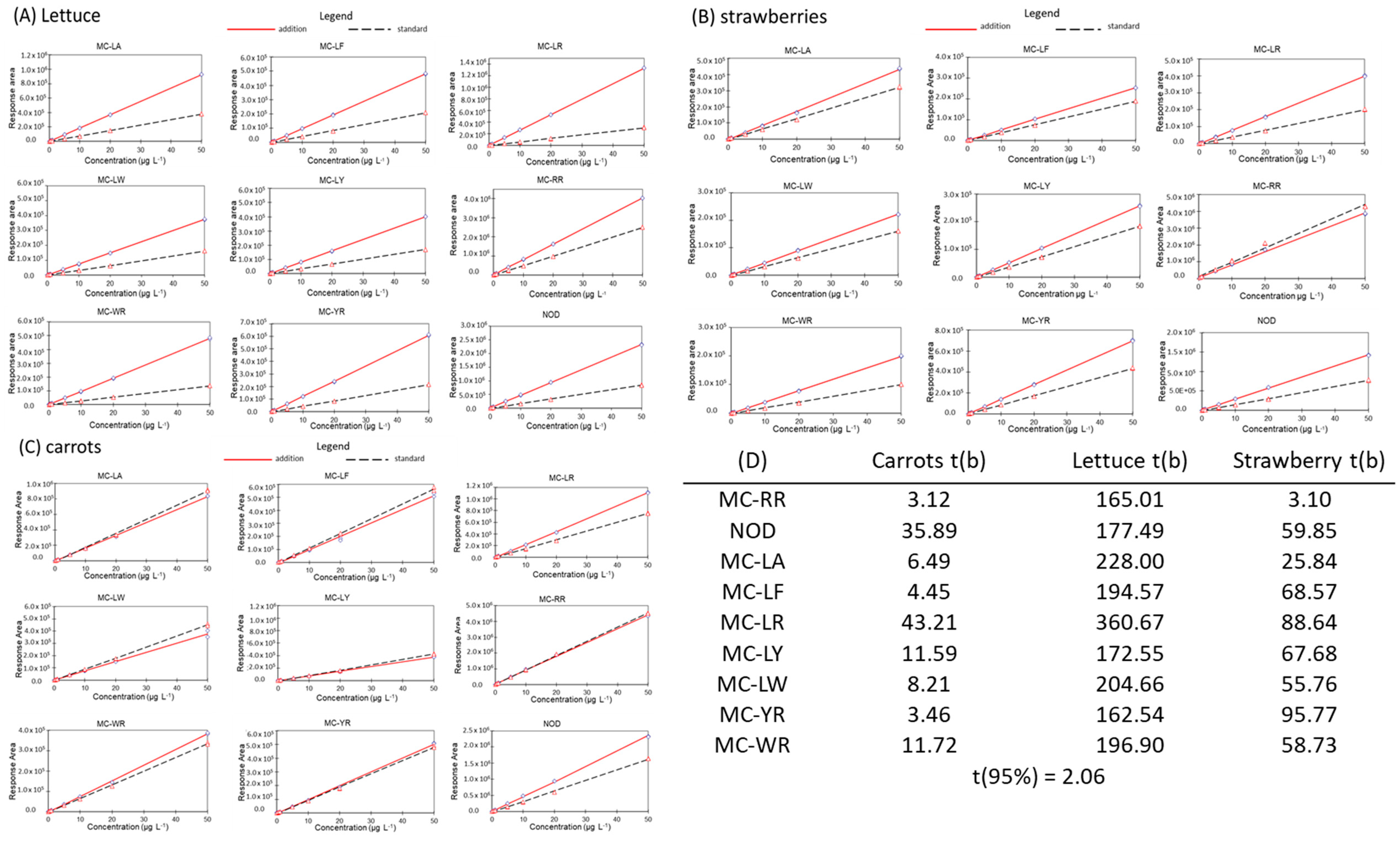
2.2.4. Linearity and Matrix Effect
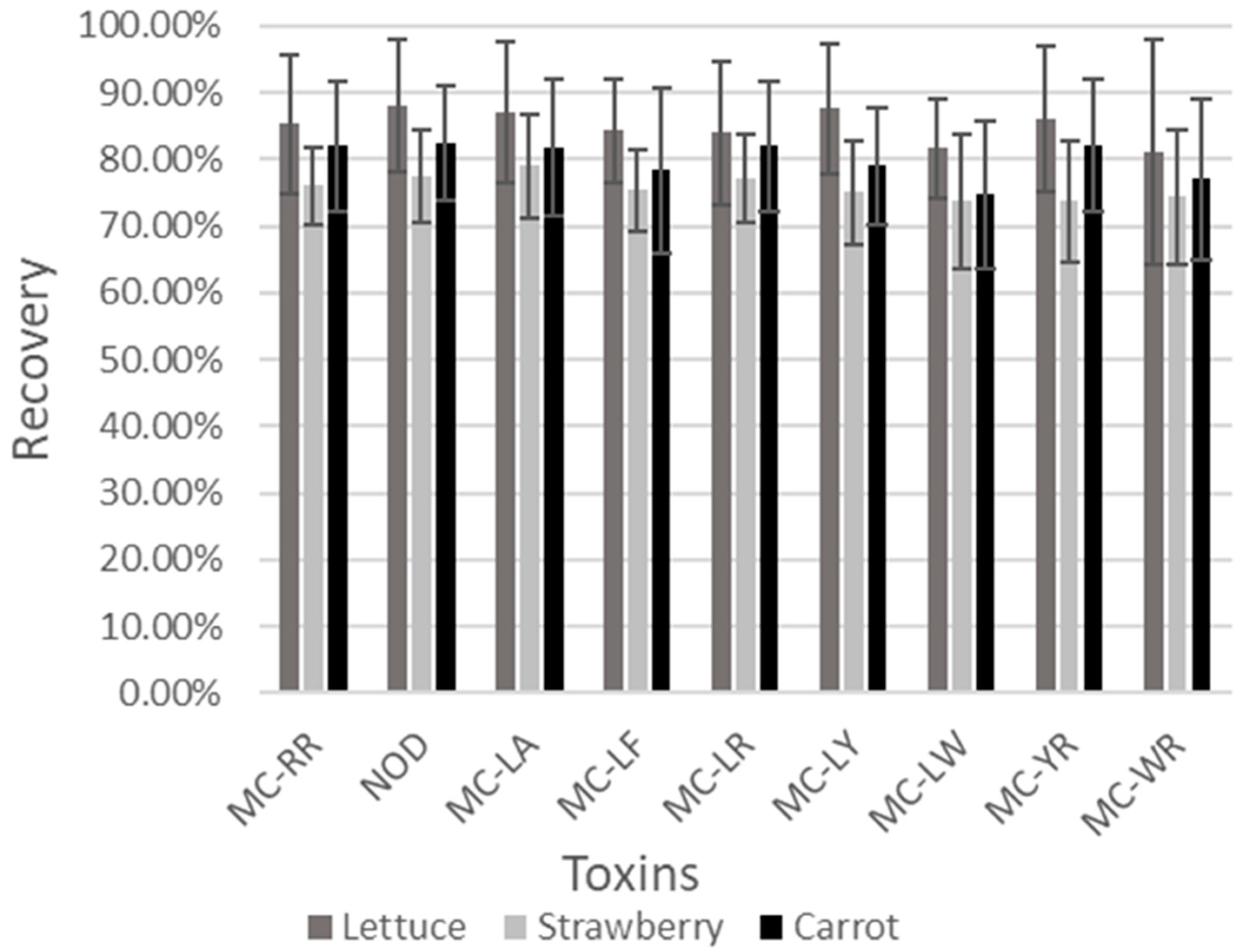
2.2.5. Recovery
2.2.6. Repeatability, Reproducibility and Measurement Uncertainty
2.3. Sampling from the Belgium Market
3. Results
3.1. Validation Results for the Different Matrices
3.2. Method Application on Different Vegetables and Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Falconer, I.R.; Buckley, T.; Runnegar, M.T.C. Biological Half-Life, Organ Distribution and Excretion of 125I-Labelled Toxic Peptide from the Blue-Green Alga Microcystis aeruginosa. Aust. J. Biol. Sci. 1986, 39, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.A.; Carmichael, W.W. Anatoxin-a(s), an Anticholinesterase from the Cyanobacterium Anabaena Flos-Aquae NRC-525-17. Toxicon 1987, 25, 1221–1227. [Google Scholar] [CrossRef]
- Mahmood, N.A.; Carmichael, W.W. Paralytic Shellfish Poisons Produced by the Freshwater Cyanobacterium Aphanizomenon Flos-Aquae NH-5. Toxicon 1986, 24, 175–186. [Google Scholar] [CrossRef]
- Namikoshi, M.; Rinehart, K.L.; Sakai, R.; Stotts, R.R.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W.; Evans, W.R. Identification of 12 Hepatotoxins from a Homer Lake Bloom of the Cyanobacteria Microcystis Aeruginosa, Microcystis Viridis, and Microcystis Wesenbergii: Nine New Microcystins. J. Org. Chem. 1992, 57, 866–872. [Google Scholar] [CrossRef]
- Welker, M.; Brunke, M.; Preussel, K.; Lippert, I.; von Döhren, H. Diversity and Distribution of Microcystis (Cyanobacteria) Oligopeptide Chemotypes from Natural Communities Studied by Single-Colony Mass Spectrometry. Microbiology (Reading) 2004, 150, 1785–1796. [Google Scholar] [CrossRef]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identification of Anatoxin-A in Benthic Cyanobacteria (Blue-Green Algae) and in Associated Dog Poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef]
- Harada, K.I.; Ohtani, I.; Iwamoto, K.; Suzuki, M.; Watanabe, M.F.; Watanabe, M.; Terao, K. Isolation of Cylindrospermopsin from a Cyanobacterium Umezakia Natans and Its Screening Method. Toxicon 1994, 32, 73–84. [Google Scholar] [CrossRef]
- Dahlmann, J.; Rühl, A.; Hummert, C.; Liebezeit, G.; Carlsson, P.; Granéli, E. Different Methods for Toxin Analysis in the Cyanobacterium Nodularia Spumigena (Cyanophyceae). Toxicon 2001, 39, 1183–1190. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Fessard, V.; Sialehaamoa, A. Review and Analysis of Occurrence, Exposure and Toxicity of Cyanobacteria Toxins in Food. EFSA Extern. Sci. Rep. 2016, 13, 998E. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Van Hassel, W.H.R.; Andjelkovic, M.; Wilmotte, A.; Rajkovic, A. Cyanotoxins and Food Contamination in Developing Countries: Review of Their Types, Toxicity, Analysis, Occurrence and Mitigation Strategies. Toxins 2021, 13, 786. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Wo´zniak, E. Temperature Effects Explain Continental Scale Distribution of Cyanobacterial Toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Marzec, H.; Sutryk, K.; Kobos, J.; Hebel, A.; Hohlfeld, N.; Błaszczyk, A.; Toruńska, A.; Kaczkowska, M.J.; Łysiak-Pastuszak, E.; Kraśniewski, W.; et al. Occurrence of Cyanobacteria and Cyanotoxin in the Southern Baltic Proper. Filamentous Cyanobacteria versus Single-Celled Picocyanobacteria. Hydrobiologia 2013, 701, 235–252. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial Microcystin-LR Is a Potent and Specific Inhibitor of Protein Phosphatases 1 and 2A from Both Mammals and Higher Plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Runnegar, M.T.; Kong, S.; Berndt, N. Protein Phosphatase Inhibition and in Vivo Hepatotoxicity of Microcystins. Am. J. Physiol. -Gastrointest. Liver Physiol. 1993, 265, G224–G230. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Hoeger, S.J.; Stemmer, K.; Feurstein, D.J.; Knobeloch, D.; Nussler, A.; Dietrich, D.R. The Role of Organic Anion Transporting Polypeptides (OATPs/SLCOs) in the Toxicity of Different Microcystin Congeners in Vitro: A Comparison of Primary Human Hepatocytes and OATP-Transfected HEK293 Cells. Toxicol. Appl. Pharmacol. 2010, 245, 9–20. [Google Scholar] [CrossRef]
- Feurstein, D.; Holst, K.; Fischer, A.; Dietrich, D.R. Oatp-Associated Uptake and Toxicity of Microcystins in Primary Murine Whole Brain Cells. Toxicol. Appl. Pharmacol. 2009, 234, 247–255. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Chandrasena, N.R.; Jones, G.J.; Humpage, A.R.; Falconer, I.R. Isolation and Toxicity of Cylindrospermopsis Raciborskii from an Ornamental Lake. Toxicon 1997, 35, 341–346. [Google Scholar] [CrossRef]
- Antunes, J.T.; Leão, P.N.; Vasconcelos, V.M. Cylindrospermopsis Raciborskii: Review of the Distribution, Phylogeography, and Ecophysiology of a Global Invasive Species. Front Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef]
- Merel, S.; Villarín, M.C.; Chung, K.; Snyder, S. Spatial and Thematic Distribution of Research on Cyanotoxins. Toxicon 2013, 76, 118–131. [Google Scholar] [CrossRef]
- Hoeger, S.J.; Shaw, G.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and Elimination of Cyanobacterial Toxins in Two Australian Drinking Water Treatment Plants. Toxicon 2004, 43, 639–649. [Google Scholar] [CrossRef]
- Clemente, Z.; Busato, R.H.; Oliveira Ribeiro, C.A.; Cestari, M.M.; Ramsdorf, W.A.; Magalhães, V.F.; Wosiack, A.C.; Silva de Assis, H.C. Analyses of Paralytic Shellfish Toxins and Biomarkers in a Southern Brazilian Reservoir. Toxicon 2010, 55, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Ballot, A.; Fastner, J.; Lentz, M.; Wiedner, C. First Report of Anatoxin-a-Producing Cyanobacterium Aphanizomenon Issatschenkoi in Northeastern Germany. Toxicon 2010, 56, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Metcalf, J.S.; Beattie, K.A. Retention of Microcystis Aeruginosa and Microcystin by Salad Lettuce (Lactuca Sativa) after Spray Irrigation with Water Containing Cyanobacteria. Toxicon 1999, 37, 1181–1185. [Google Scholar] [CrossRef]
- Chen, J.; Song, L.; Dai, J.; Gan, N.; Liu, Z. Effects of Microcystins on the Growth and the Activity of Superoxide Dismutase and Peroxidase of Rape (Brassica Napus L.) and Rice (Oryza Sativa L.). Toxicon 2004, 43, 393–400. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Beattie, K.A.; Krause, E.; Steinberg, C.E.W.; Codd, G.A. Uptake, Effects, and Metabolism of Cyanobacterial Toxins in the Emergent Reed Plant Phragmites Australis (CAV.) Trin. Ex Steud. Environ. Toxicol. Chem. 2001, 20, 846–852. [Google Scholar] [CrossRef]
- McElhiney, J.; Lawton, L.A.; Leifert, C. Investigations into the Inhibitory Effects of Microcystins on Plant Growth, and the Toxicity of Plant Tissues Following Exposure. Toxicon 2001, 39, 1411–1420. [Google Scholar] [CrossRef]
- Järvenpää, S.; Lundberg-Niinistö, C.; Spoof, L.; Sjövall, O.; Tyystjärvi, E.; Meriluoto, J. Effects of Microcystins on Broccoli and Mustard, and Analysis of Accumulated Toxin by Liquid Chromatography–Mass Spectrometry. Toxicon 2007, 49, 865–874. [Google Scholar] [CrossRef]
- Li, Y.-W.; Zhan, X.-J.; Xiang, L.; Deng, Z.-S.; Huang, B.-H.; Wen, H.-F.; Sun, T.-F.; Cai, Q.-Y.; Li, H.; Mo, C.-H. Analysis of Trace Microcystins in Vegetables Using Solid-Phase Extraction Followed by High Performance Liquid Chromatography Triple-Quadrupole Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 11831–11839. [Google Scholar] [CrossRef]
- Prieto, A.I.; Guzmán-Guillén, R.; Díez-Quijada, L.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Validation of a Method for Cylindrospermopsin Determination in Vegetables: Application to Real Samples Such as Lettuce (Lactuca Sativa L.). Toxins 2018, 10, 63. [Google Scholar] [CrossRef]
- Manubolu, M.; Lee, J.; Riedl, K.M.; Kua, Z.X.; Collart, L.P.; Ludsin, S.A. Optimization of Extraction Methods for Quantification of Microcystin-LR and Microcystin-RR in Fish, Vegetable, and Soil Matrices Using UPLC–MS/MS. Harmful Algae 2018, 76, 47–57. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Guzmán-Guillén, R.; Prieto Ortega, A.I.; Llana-Ruíz-Cabello, M.; Campos, A.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. New Method for Simultaneous Determination of Microcystins and Cylindrospermopsin in Vegetable Matrices by SPE-UPLC-MS/MS. Toxins 2018, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jiang, X.; Manubolu, M.; Riedl, K.; Ludsin, S.A.; Martin, J.F.; Lee, J. Fresh Produce and Their Soils Accumulate Cyanotoxins from Irrigation Water: Implications for Public Health and Food Security. Food Res. Int. 2017, 102, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Crush, J.R.; Briggs, L.R.; Sprosen, J.M.; Nichols, S.N. Effect of Irrigation with Lake Water Containing Microcystins on Microcystin Content and Growth of Ryegrass, Clover, Rape, and Lettuce. Environ. Toxicol. 2008, 23, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in Groundwater Wells and Their Accumulation in Vegetable Plants Irrigated with Contaminated Waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef]
- Hereman, T.C.; Bittencourt-Oliveira, M.d.C. Bioaccumulation of Microcystins in Lettuce. J. Phycol. 2012, 48, 1535–1537. [Google Scholar] [CrossRef]
- Levizou, E.; Statiris, G.; Papadimitriou, T.; Laspidou, C.S.; Kormas, K.A. Lettuce Facing Microcystins-Rich Irrigation Water at Different Developmental Stages: Effects on Plant Performance and Microcystins Bioaccumulation. Ecotoxicol. Environ. Saf. 2017, 143, 193–200. [Google Scholar] [CrossRef]
- do Bittencourt-Oliveira, M.C.; Cordeiro-Araújo, M.K.; Chia, M.A.; Arruda-Neto, J.D.d.T.; de Oliveira, Ê.T.; dos Santos, F. Lettuce Irrigated with Contaminated Water: Photosynthetic Effects, Antioxidative Response and Bioaccumulation of Microcystin Congeners. Ecotoxicol. Environ. Saf. 2016, 128, 83–90. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; de Arruda-Neto, J.D.T.; Tornisielo, V.L.; Vilca, F.Z.; do Bittencourt-Oliveira, M.C. Microcystin-LR Bioaccumulation and Depuration Kinetics in Lettuce and Arugula: Human Health Risk Assessment. Sci. Total Environ. 2016, 566–567, 1379–1386. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; Bittencourt-Oliveira, M.d.C. Potential Human Health Risk Assessment of Cylindrospermopsin Accumulation and Depuration in Lettuce and Arugula. Harmful Algae 2017, 68, 217–223. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Jos, A.; Cameán, A.; Oliveira, F.; Barreiro, A.; Machado, J.; Azevedo, J.; Pinto, E.; Almeida, A.; Campos, A.; et al. Analysis of the Use of Cylindrospermopsin and/or Microcystin-Contaminated Water in the Growth, Mineral Content, and Contamination of Spinacia Oleracea and Lactuca Sativa. Toxins 2019, 11, 624. [Google Scholar] [CrossRef]
- Machado, J.; Campos, A.; Vasconcelos, V.; Freitas, M. Effects of Microcystin-LR and Cylindrospermopsin on Plant-Soil Systems: A Review of Their Relevance for Agricultural Plant Quality and Public Health. Environ. Res. 2017, 153, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Papadimitriou, T.; Papavasileiou, E.; Papadimitriou, N.; Kormas, K.A. Root Vegetables Bioaccumulate Microcystins-LR in a Developmental Stage-Dependent Manner under Realistic Exposure Scenario: The Case of Carrot and Radish. Agric. Water Manag. 2020, 240, 106274. [Google Scholar] [CrossRef]
- Chen, J.; Han, F.X.; Wang, F.; Zhang, H.; Shi, Z. Accumulation and Phytotoxicity of Microcystin-LR in Rice (Oryza Sativa). Ecotoxicol. Environ. Saf. 2012, 76, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wijewickrama, M.M.; Manage, P.M. Accumulation of Microcystin-LR in Grains of Two Rice Varieties (Oryza Sativa L.) and a Leafy Vegetable, Ipomoea Aquatica. Toxins 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Corbel, S.; Mougin, C.; Nélieu, S.; Delarue, G.; Bouaïcha, N. Evaluation of the Transfer and the Accumulation of Microcystins in Tomato (Solanum Lycopersicum Cultivar MicroTom) Tissues Using a Cyanobacterial Extract Containing Microcystins and the Radiolabeled Microcystin-LR (14C-MC-LR). Sci. Total Environ. 2016, 541, 1052–1058. [Google Scholar] [CrossRef]
- Romero-Oliva, C.S.; Contardo-Jara, V.; Block, T.; Pflugmacher, S. Accumulation of Microcystin Congeners in Different Aquatic Plants and Crops – A Case Study from Lake Amatitlán, Guatemala. Ecotoxicol. Environ. Saf. 2014, 102, 121–128. [Google Scholar] [CrossRef]
- Peuthert, A.; Chakrabarti, S.; Pflugmacher, S. Uptake of Microcystins-LR and -LF (Cyanobacterial Toxins) in Seedlings of Several Important Agricultural Plant Species and the Correlation with Cellular Damage (Lipid Peroxidation). Environ. Toxicol. 2007, 22, 436–442. [Google Scholar] [CrossRef]
- Xiang, L.; Li, Y.-W.; Wang, Z.-R.; Liu, B.-L.; Zhao, H.-M.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Li, Q.X. Bioaccumulation and Phytotoxicity and Human Health Risk from Microcystin-LR under Various Treatments: A Pot Study. Toxins 2020, 12, 523. [Google Scholar] [CrossRef]
- Chen, J.; Dai, J.; Zhang, H.; Wang, C.; Zhou, G.; Han, Z.; Liu, Z. Bioaccumulation of Microcystin and Its Oxidative Stress in the Apple (Malus Pumila). Ecotoxicology 2010, 19, 796–803. [Google Scholar] [CrossRef]
- Corbel, S.; Bouaïcha, N.; Nélieu, S.; Mougin, C. Soil Irrigation with Water and Toxic Cyanobacterial Microcystins Accelerates Tomato Development. Env. Chem. Lett. 2015, 13, 447–452. [Google Scholar] [CrossRef]
- Haida, M.; El Khalloufi, F.; Mugani, R.; Redouane, E.M.; Campos, A.; Vasconcelos, V.; Oudra, B. Effects of Irrigation with Microcystin-Containing Water on Growth, Physiology, and Antioxidant Defense in Strawberry Fragaria Vulgaris under Hydroponic Culture. Toxins 2022, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Praena, D.; Campos, A.; Azevedo, J.; Neves, J.; Freitas, M.; Guzmán-Guillén, R.; Cameán, A.M.; Renaut, J.; Vasconcelos, V. Exposure of Lycopersicon Esculentum to Microcystin-LR: Effects in the Leaf Proteome and Toxin Translocation from Water to Leaves and Fruits. Toxins 2014, 6, 1837–1854. [Google Scholar] [CrossRef] [PubMed]
- Van Hassel, W.H.R.; Huybrechts, B.; Masquelier, J.; Wilmotte, A.; Andjelkovic, M. Development, Validation and Application of a Targeted LC-MS Method for Quantification of Microcystins and Nodularin: Towards a Better Characterization of Drinking Water. Water 2022, 14, 1195. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Ahn, A.-C.; Huybrechts, B.; Masquelier, J.; Wilmotte, A.; Andjelkovic, M. LC-MS/MS Validation and Quantification of Cyanotoxins in Algal Food Supplements from the Belgium Market and Their Molecular Origins. Toxins 2022, 14, 513. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Andjelkovic, M.; Durieu, B.; Marroquin, V.A.; Masquelier, J.; Huybrechts, B.; Wilmotte, A. A Summer of Cyanobacterial Blooms in Belgian Waterbodies: Microcystin Quantification and Molecular Characterizations. Toxins 2022, 14, 61. [Google Scholar] [CrossRef]
- P.O. of the E. Union, CELEX1, 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C(2002) 3044). Available online: http://op.europa.eu/en/publication-detail/-/publication/ed928116-a955-4a84-b10a-cf7a82bad858/language-en (accessed on 30 November 2020).
| Toxin | Precursor Ion (m/z) | Quantifier Ion (m/z) | Collision Energy (eV) | Cone Voltage (V) | Qualifier Ion (m/z) | Collision Energy (eV) | Cone Voltage (V) |
|---|---|---|---|---|---|---|---|
| MC-LR | 995.4 | 135.0 | 70 | 80 | 213.1 | 60 | 80 |
| MC-RR | 519.8 | 134.8 | 30 | 50 | 107.2 | 60 | 50 |
| MC-YR | 1045.5 | 135.3 | 80 | 60 | 212.9 | 60 | 60 |
| MC-WR | 1068.4 | 135.3 | 70 | 100 | 213.1 | 60 | 100 |
| MC-LY | 1002.4 | 135.3 | 60 | 50 | 213.0 | 50 | 50 |
| MC-LA | 910.3 | 135.1 | 60 | 50 | 107.1 | 80 | 50 |
| MC-LF | 986.3 | 135.0 | 60 | 70 | 213.1 | 60 | 70 |
| MC-LW | 1025.4 | 134.9 | 60 | 60 | 213.1 | 50 | 60 |
| NOD | 825.25 | 134.9 | 50 | 80 | 102.7 | 90 | 80 |
| Sample Matrix | Consumption Rank * | Eaten Raw | Contact with Water | Harvested during Bloom Period | Scientific Relevance | Cultural Relevance |
|---|---|---|---|---|---|---|
| Carrots | 4 | X | X | |||
| Onions | 5 | X | X | |||
| Potato | 5 | X | X | X | ||
| Chicory | 3 | X | X | X | X | X |
| Radish | 2 | X | X | X | X | |
| Strawberries | 4 | X | X | X | X | |
| Tomato | 4 | X | X | X | ||
| Cherry Tomato | 4 | X | X | X | ||
| Lettuce | 4 | X | X | X |
| Carrots | Lettuce | Strawberries | ||||
|---|---|---|---|---|---|---|
| Average Ion Ratio | Standard Deviation Ion Ratio | Average Ion Ratio | Standard Deviation Ion Ratio | Average Ion Ratio | Standard Deviation Ion Ratio | |
| MC-RR | 15.28 | 5.43 | 16.15 | 5.82 | 13.13 | 2.63 |
| NOD | 42.66 | 4.69 | 41.24 | 1.65 | 46.58 | 2.29 |
| MC-LA | 47.36 | 7.07 | 51.46 | 6.07 | 40.28 | 1.78 |
| MC-LF | 40.45 | 1.77 | 41.14 | 2.14 | 39.31 | 0.89 |
| MC-LR | 31.98 | 1.20 | 32.57 | 2.81 | 31.75 | 1.68 |
| MC-LY | 50.01 | 3.87 | 49.58 | 6.26 | 51.80 | 5.08 |
| MC-LW | 45.36 | 2.05 | 44.85 | 2.32 | 45.19 | 2.00 |
| MC-YR | 36.45 | 3.98 | 35.84 | 4.49 | 38.88 | 4.65 |
| MC-WR | 43.05 | 10.74 | 41.22 | 4.03 | 38.93 | 2.21 |
| Sample Type | MC-RR | NOD | MC-LA | MC-LF | MC-LR | MC-LY | MC-LW | MC-YR | MC-WR |
|---|---|---|---|---|---|---|---|---|---|
| Chicory | 98.53 | 97.07 | 95.87 | 96.93 | 99.93 | 105.20 | 110.27 | 89.47 | 94.80 |
| Onion | 84.05 | 87.50 | 89.85 | 83.70 | 87.40 | 83.60 | 75.45 | 87.65 | 80.60 |
| Cherry Tomato | 87.38 | 85.78 | 90.38 | 79.33 | 86.70 | 88.58 | 70.15 | 83.68 | 80.38 |
| Raspberry | 80.10 | 79.50 | 95.10 | 81.20 | 82.10 | 90.50 | 72.80 | 86.50 | 86.30 |
| Tomato | 91.40 | 99.20 | 106.80 | 100.30 | 89.50 | 98.30 | 104.40 | 108.20 | 109.50 |
| Carrot | 81.58 | 86.02 | 91.05 | 103.15 | 96.28 | 84.70 | 101.65 | 81.90 | 80.15 |
| Potato | 79.38 | 88.05 | 89.58 | 97.60 | 95.48 | 85.53 | 96.75 | 87.87 | 80.57 |
| Strawberries | 79.93 | 91.13 | 84.47 | 75.07 | 87.73 | 82.13 | 84.47 | 80.00 | 93.07 |
| Lettuce | 70.0% | 82.67 | 89.07 | 98.47 | 105.67 | 77.07 | 83.13 | 76.00 | 70.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hassel, W.H.R.; Masquelier, J.; Andjelkovic, M.; Rajkovic, A. Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products. Separations 2022, 9, 319. https://doi.org/10.3390/separations9100319
Van Hassel WHR, Masquelier J, Andjelkovic M, Rajkovic A. Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products. Separations. 2022; 9(10):319. https://doi.org/10.3390/separations9100319
Chicago/Turabian StyleVan Hassel, Wannes Hugo R., Julien Masquelier, Mirjana Andjelkovic, and Andreja Rajkovic. 2022. "Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products" Separations 9, no. 10: 319. https://doi.org/10.3390/separations9100319
APA StyleVan Hassel, W. H. R., Masquelier, J., Andjelkovic, M., & Rajkovic, A. (2022). Towards a Better Quantification of Cyanotoxins in Fruits and Vegetables: Validation and Application of an UHPLC-MS/MS-Based Method on Belgian Products. Separations, 9(10), 319. https://doi.org/10.3390/separations9100319







