Other study [
4] tested an MDC system with a six-tray cascading crystallizer integrated with meshed surfaces that act as nucleation and crystal growth sites. The system was tested on three artificial feed solutions containing NaCl, KCl, and NaNO
3, respectively, which the system was able to recover. A DCMD module with polytetrafluoroethylene membranes was used to operate at 50 °C. The system works as an MDC loop using an ultrasonic bath which dissolves any crystals that may have formed in the feed to avoid scaling in the MD module. Half of the crystallizer trays are fitted with cooling water to induce cooling crystallization for KCl and NaNO
3, which have positive T–S behavior. The average salt productions were 0.437, 0.931, and 1.141 kg/m²/h for KCl, NaCl, and NaNO
3, respectively, per unit area of the membrane. NaNO
3 had the highest production because of its strong positive T–S behavior. The authors of [
6] designed a ceramic membrane-promoted crystallization (MPC) system capable of forming needle-like NaCl crystals on the membrane surface for harvesting. The system is based on the salt excretion mechanisms of mangrove leaves using capillary action using hydrophilic mesoporous silica membranes. Capillary action through the pores creates a suction of the brine from the inner membrane surface to the outer membrane surface and water evaporation which leads to heterogeneous crystallization on the outer membrane surface. The salt is then manually harvested with a brush. The system operating at 50 °C was tested on 200,000 ppm NaCl brines and was able to recover 194.6 g/m² of NaCl per unit area of the membrane as well as recover 790.3 g/m² of water. The authors of [
7] tested a similar MPC system to recover KCl salts from brines. The system can generate needle-shaped KCl crystals. The system operated at 60 °C and was tested on 150,000 ppm KCl brines and was able to recover 134.3 g/m² of KCl and 738.7 g/m² of water. This technology can operate stably after several cycles and is thought to be more economical than other KCl crystallization techniques. The authors of [
8] modeled an innovative crystallizer with ion exchange membranes to recover magnesium from brines using Ca(OH)
2 as a cheap alkaline reactant. The system consists of an anion exchange membrane that separates the saline feed from the alkaline solution. The membrane exchanges Cl
− ions from the brine with OH
− ions from the alkaline solution. Hydroxide ions react with Mg
2+ ions to produce magnesium hydroxide Mg(OH)
2, which quickly reaches supersaturation due to its low solubility. The authors of [
9] studied the application of MDC for NaCl recovery using flat–sheet polyvinylidene fluoride membranes modified with lithium chloride or acetone to improve water and salt recovery. The system was tested at 60–70 °C and was able to produce the maximum recovery at 70 °C. The water and NaCl recovery ranged from 1–1.8 kg/m²/h and 0.5–0.7 kg/m²/h depending on the temperature and membrane modification. The acetone modification yielded the maximum recovery, followed by the LiCl modification and the membrane without modification. The authors of [
10] studied the application of vacuum-assisted membrane distillation crystallization (VMDC) to recover CaSO
4 and NaCl from sub-soil brines using a poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) membrane. The system was able to crystallize all the CaSO
4 and NaCl crystals onto the membrane surface with a maximum vapor flux of 14.40 kg/m²/h. The authors of [
11] studied the application of F-SMDC to recover sodium sulfate (Na
2SO
4) from SWRO brines. The system was tested on artificial SWRO brines at 73,050 ppm and first resulted in membrane scaling from CaSO
4 deposition, which prevented Na
2SO
4 supersaturation. However, the removal of calcium ions and addition of (NH
4)
2SO
4 into the crystallizer allowed creating a sulfate-rich environment allowing fast Na
2SO
4 crystallization at the bottom of the crystallizer, recovering 223.73 g of Na
2SO
4 and 72% of water. The presence of NaCl negatively impacted crystallization due to its low T–S sensitivity. The authors also suggested a novel ZLD approach to recover NH
3, Na
2SO
4, Mg
2+, K
+, and sodium hypochlorite (NaOCl) from SWRO brines using submerged VMD (S-VMD). The authors of [
12] studied the extraction of lithium from salt lake brines at 255,300 ppm containing 2.5 g/L of Li
+ with a high Mg/Li ratio > 20 using a novel crystallization-precipitation method. The removal of Mg is achieved in two stages: first, solvent evaporation at 40 °C combined with KCl addition precipitates 53.1% of Mg into carnallite (KMgCl
3·6(H
2O)), which is extracted by filtration and floatation along with KCl. Then the remaining Mg is precipitated into MgHPO
4 by adding Na
2HPO
4 and then vacuum filtered to produce a solution with an Mg/Li ratio of 0.16, which can be further treated in industries to produce lithium carbonate by reaction with sodium carbonate. The filter cake goes through a melamine complex process to recycle Na
2HPO
4. This method was able to remove 99.6% of Mg and recover 93.2% of lithium and can recycle reactants which reduces operating costs. The authors of [
13] studied the application of graphene oxide composite pervaporation membrane distillation crystallization (GOCP-MDC) on the recovery of lithium from salt lake brines. The system is powered by a solar collector combined with TES. A layer of graphene oxide is added onto the hydrophobic polypropylene hollow fibre membrane to prevent membrane wetting and scaling. The system was tested on salt lake brines at 200,000 ppm with 300 ppm of Li
+ at a feed temperature of 70 °C. This set-up can produce a water flux of 11 L/m²/h and increase Li
+ concentration to 1270 ppm. The crystallizer is used to precipitate LiOH and other salts. An economic analysis was conducted and compared with using a traditional solar evaporation pond. The selling price of lithium is taken at 20 USD/kg and that of water between 0.015–0.05 USD/L. To treat 10 m
3/day of brines, results indicate a levelized cost of water (LCOW) and levelized cost of lithium (LCOL) of 36.6 USD/m
3 and 36.6 USD/kg LiOH respectively as opposed to 9.5 USD/kg LiOH for a traditional evaporation pond. The payback period is estimated at 3.6–27 years for the GOCP-MDC system and 4.5 years for the evaporation pond. However, the GOPC-MDC can recover water and only requires a footprint area of 1010 m² as opposed to the evaporation pond which requires about 10,000 m². The authors of [
14] designed a pilot plant to selectively recover Mg(OH)
2 and Ca(OH)
2 from brines using crystallization. The system is used to treat brines from ion-exchange processes which contain high levels of Na, Cl, Mg, and Ca. Brines are first treated with NF to produce a retentate rich in bivalent ions (Mg
2+ and Ca
2+) on one side and a permeate rich in monovalent ions (Na
+ and Cl
−) on the other side. Then the retentate is fed into the selective crystallization process. Sodium hydroxide is used to precipitate (reaction crystallization) Mg and Ca at increasing specific pH. Vacuum drum filters are used to separate the crystals from the remaining brine (still containing Na
+ and Cl
−), which is then mixed with the NF permeate and post-treated in a MED unit for further water recovery. Mg(OH)
2 precipitates first at pH between 9.8–10.4, and then Ca(OH)
2 precipitates at pH between 11.75–12.4. This system is able to recover 100% of magnesium and 97% of calcium with 90% and 96% purity, respectively. The authors of [
15] studied the application of vacuum MDC to recover salts from tannery wastewater at 18,436 ppm utilizing a modified PVDF-HFP nanocomposite flat sheet membrane coated with TiO
2, which improves hydrophobicity, porosity, salt rejection, and water flux and decreases scaling risks. Crystallization of NaCl and Na
2SO
4 is achieved by evaporation of the solvent in the VMD module at 60°C and cooling crystallization in the crystallizer. The system was able to achieve a water flux of 5.9 kg/m²/h, salt rejection of 99.97%, and crystallization of NaCl and Na
2SO
4. The authors of [
16] studied the application of MDC to recover crystals from shale-gas-produced water containing 30,000 ppm at 60 °C. The system uses a hydrophobic hollow-fiber polypropylene DCMD module. The system was able to recover 84% of NaCl and CaCO
3 salts at a rate of 2.72 kg/m²/h with specific energy consumption (SEC) of 28.2 kWh/m
3, but membrane scaling by NaCl and CaCO
3 could significantly damage the membrane module. Scaling can be avoided with temperature control or by transferring the crystals to the crystallizer by filtration. The authors of [
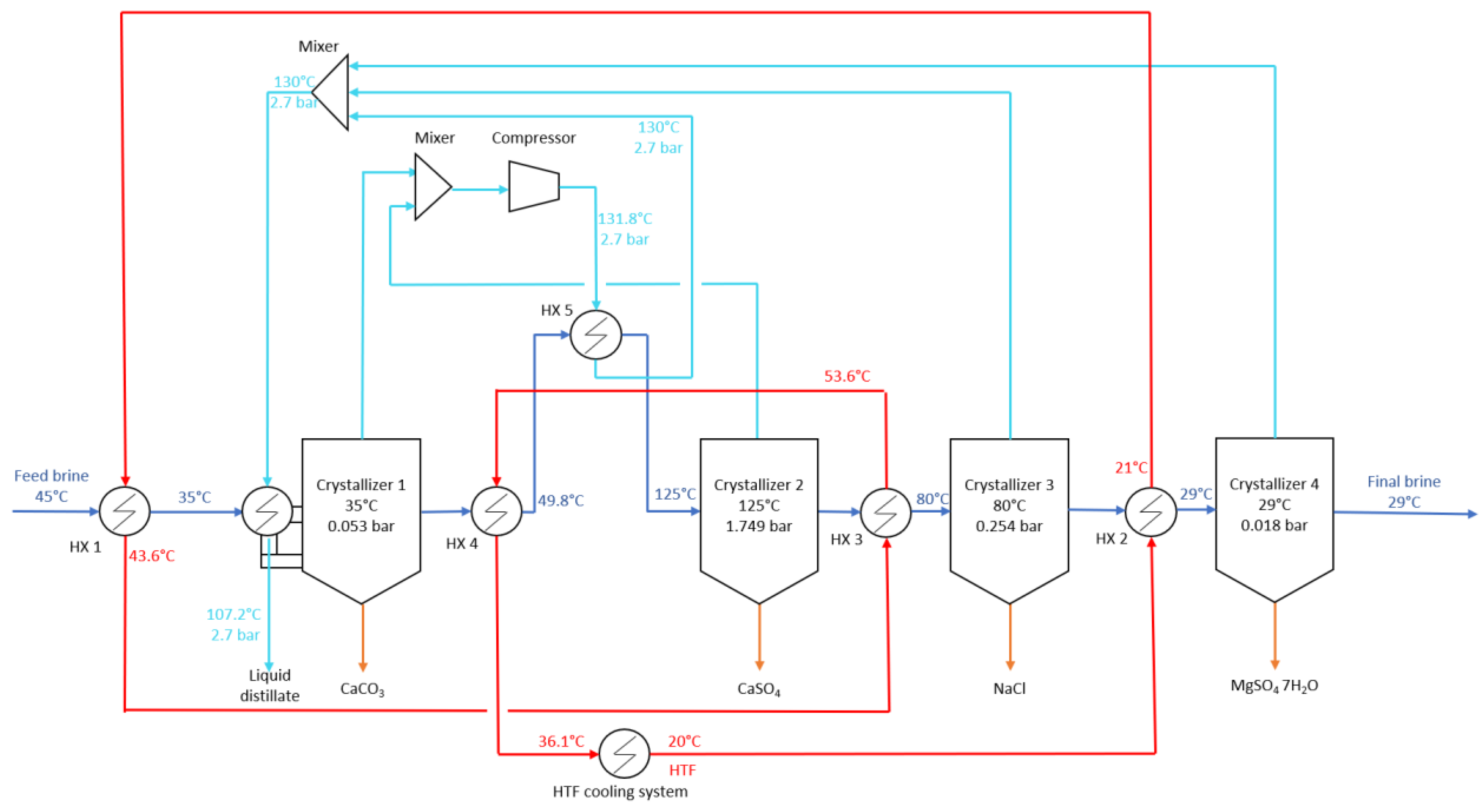
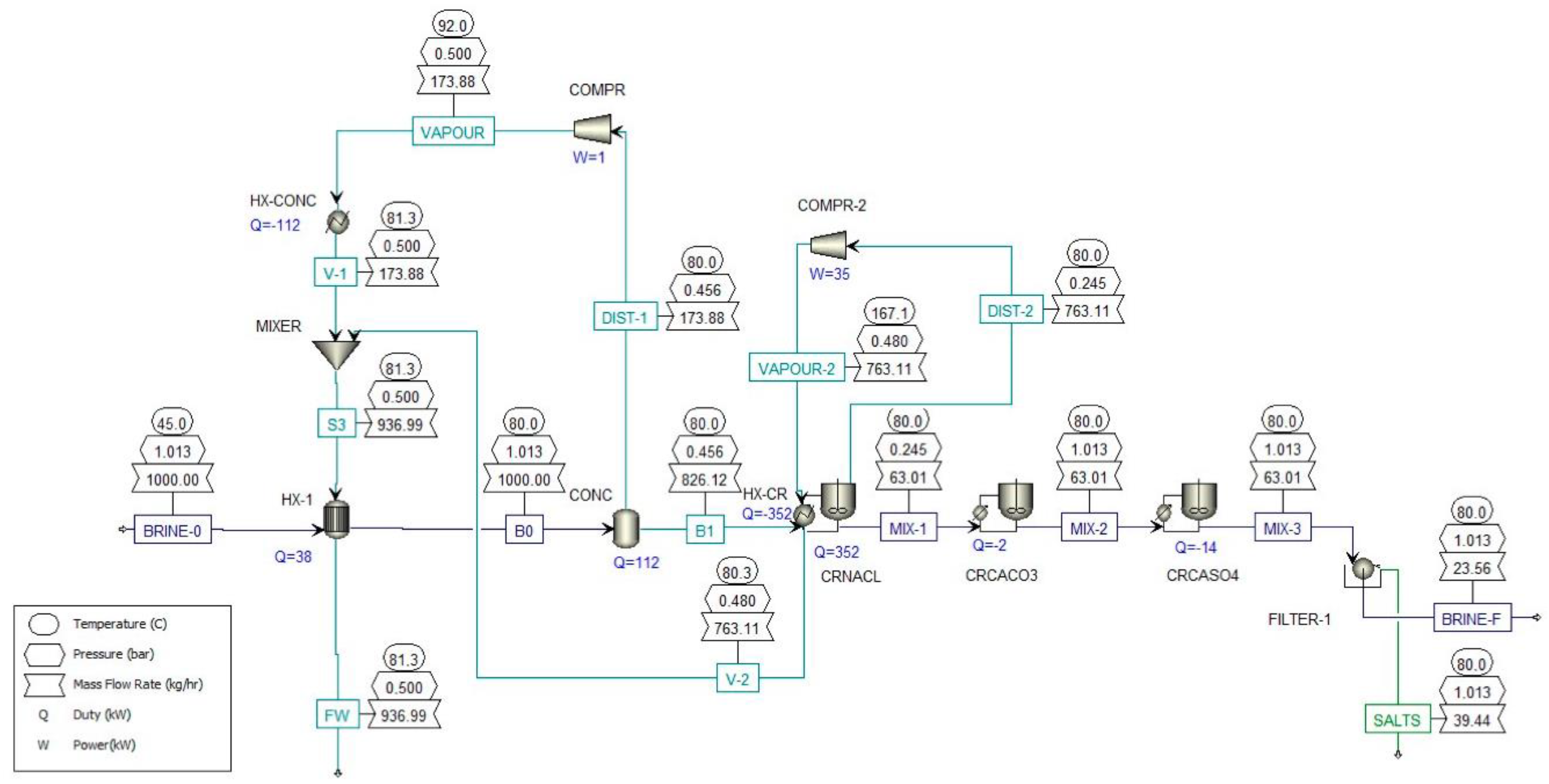
17] studied the application of a MED and crystallizer system to treat desalination brines at 70,000 ppm to achieve ZLD and recover NaCl and water. Forward-feed configuration was chosen to have the highest brine concentration in the last effect, which is at the lowest temperature, to reduce scaling risks. The brine coming out of the last evaporator is heated and fed to the crystallizer operating under vacuum pressure to allow flashing to achieve supersaturation. The formed crystals are separated from the slurry. Depending on the number of effects, the SEC varies between 166–306 kWh
th/m
3. The LCOW of the system was estimated at 4.17 USD/m
3. Cost reduction is possible by scaling up, increasing the number of effects, and using waste heat. The advantage of MED over MD is improved stability, higher energy efficiency, and lower scaling risks (no membrane scaling). The authors of [
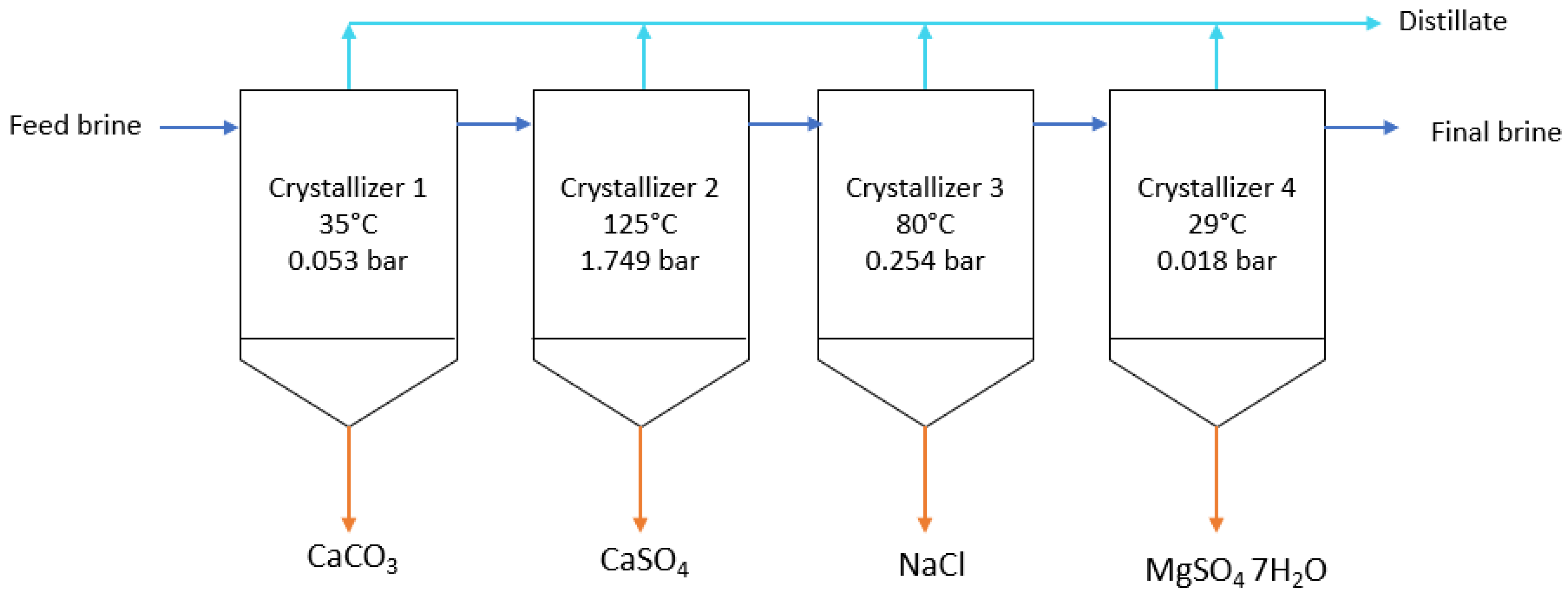
18] studied the application of a custom-made crystallizer consisting of several internal chambers acting as MSF stages to recover
crystals from SWRO brines at 282,600 ppm TDS. A MD pre-treatment of the brine is used to concentrate the feed brine to 282,600 ppm before entering the crystallizer. With 40 MSF stages, the system can achieve a GOR of four and recover 89% of water and 25.05 kg/h of
which can be sold at 90 USD/kg. In the optimal case, selling those crystals reduces the brine treatment cost per cubic meter of treated feed from 1.17 USD/m
3 to 0.35 USD/m
3 which is highly competitive with other brine management methods. Selling salts accounts for 26% of the income meaning salt recovery is profitable and can offset treatment costs. The authors of [
19] tested the application of a MED system to recover NaCl and Na
2SO
4 crystals from brines at 306,000 ppm NaCl. A pre-treatment was used to remove 97.88% of Ca
2+ and 94.1% of Mg
2+ by precipitation with NaOH and Na
2CO
3 to avoid scaling. The first five effects of the MED system specifically recover NaCl, while the last three effects specifically recover Na
2SO
4 crystals. The temperature from the first to the fifth effects decreases gradually from 130–15 °C, and that from the parallel three other effects decreases from 50–30 °C. This allows the separation of both crystals by acting on the difference in T–S behavior as Na
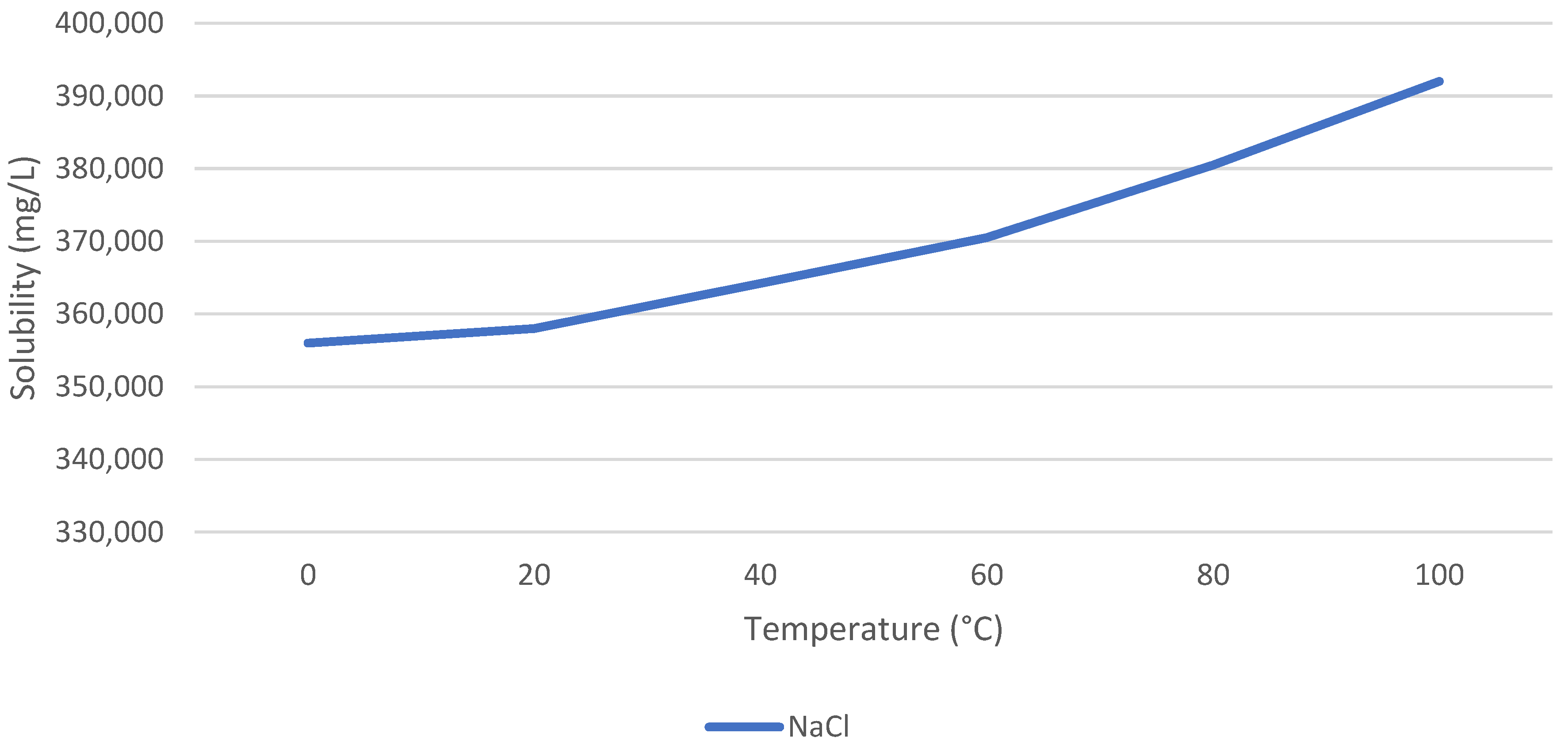
2SO
4 has a positive T–S behavior, and NaCl has a neutral T–S behavior. The authors of [
20] studied the separated precipitation of Mg(OH)
2 and gypsum from waste streams using a two-step precipitation process. MgO or Mg(OH)
2 is first injected into the first crystallizer to create a MgSO
4-rich solution while simultaneously precipitating heavy metal hydroxides. Lime is then added in the second crystallizer to induce the reaction:
. Magnesia precipitates first after a few minutes and is quickly vacuum-filtered and extracted while gypsum starts to precipitate after about 2 h after which it is vacuum-filtered and extracted at a relatively pure state.