Recovery of Palladium and Gold from PGM Ore and Concentrates Using ZnAl-Layered Double Hydroxide@zeolitic Imidazolate Framework-8 Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of Zn-Al LDH and Zn–Al–LDH@ZIF–8 Nanocomposites
2.4. Dispersive µ-Solid-Phase Extraction Method for Recovery of Au and Pd Ions
2.5. Batch Adsorption Experiments
2.6. Regeneration and Stability Studies
2.7. Application to Real Samples
3. Results and Discussion
3.1. Characterisation of the Nanocomposite
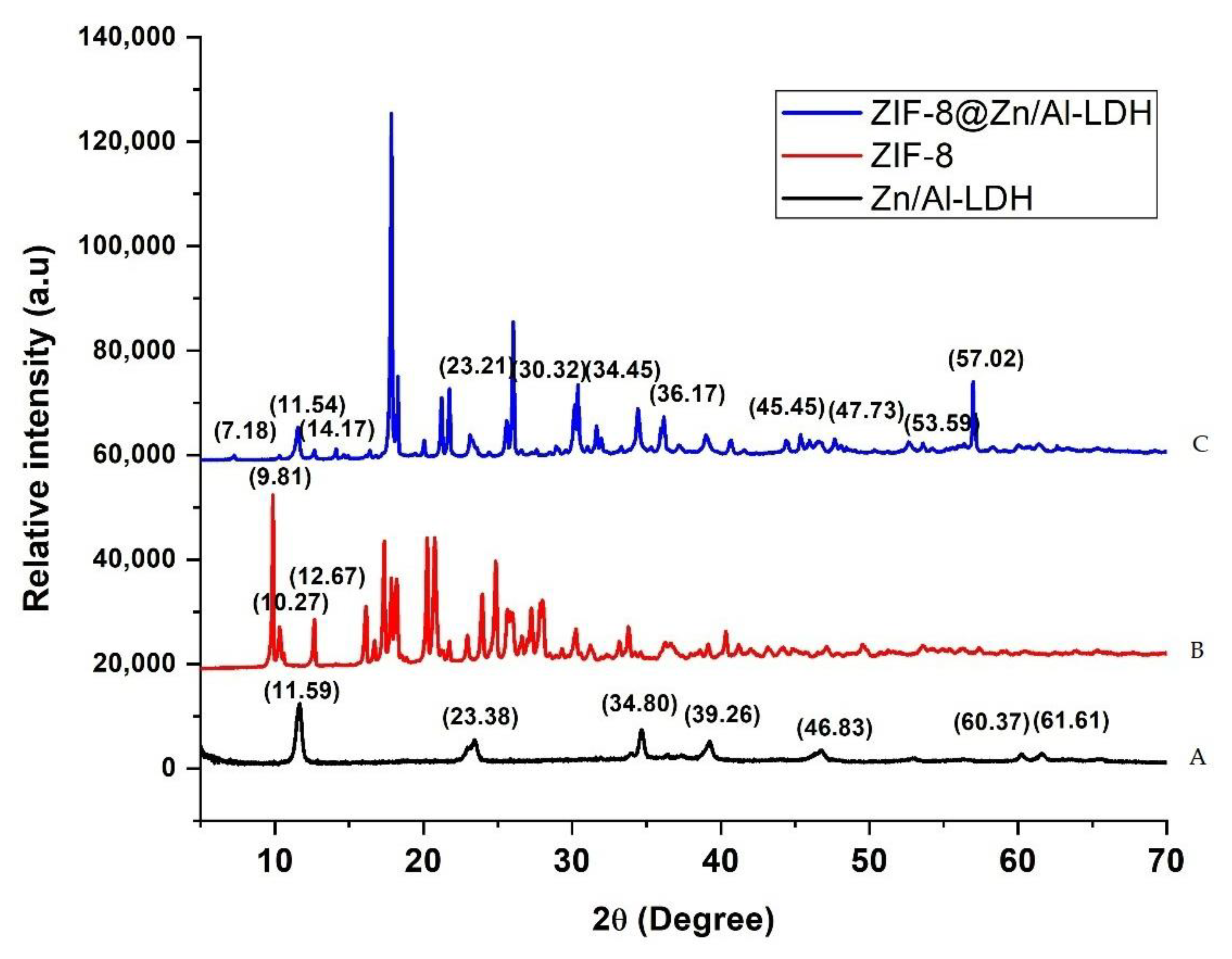
3.1.1. X-ray Diffraction (XRD) Analysis
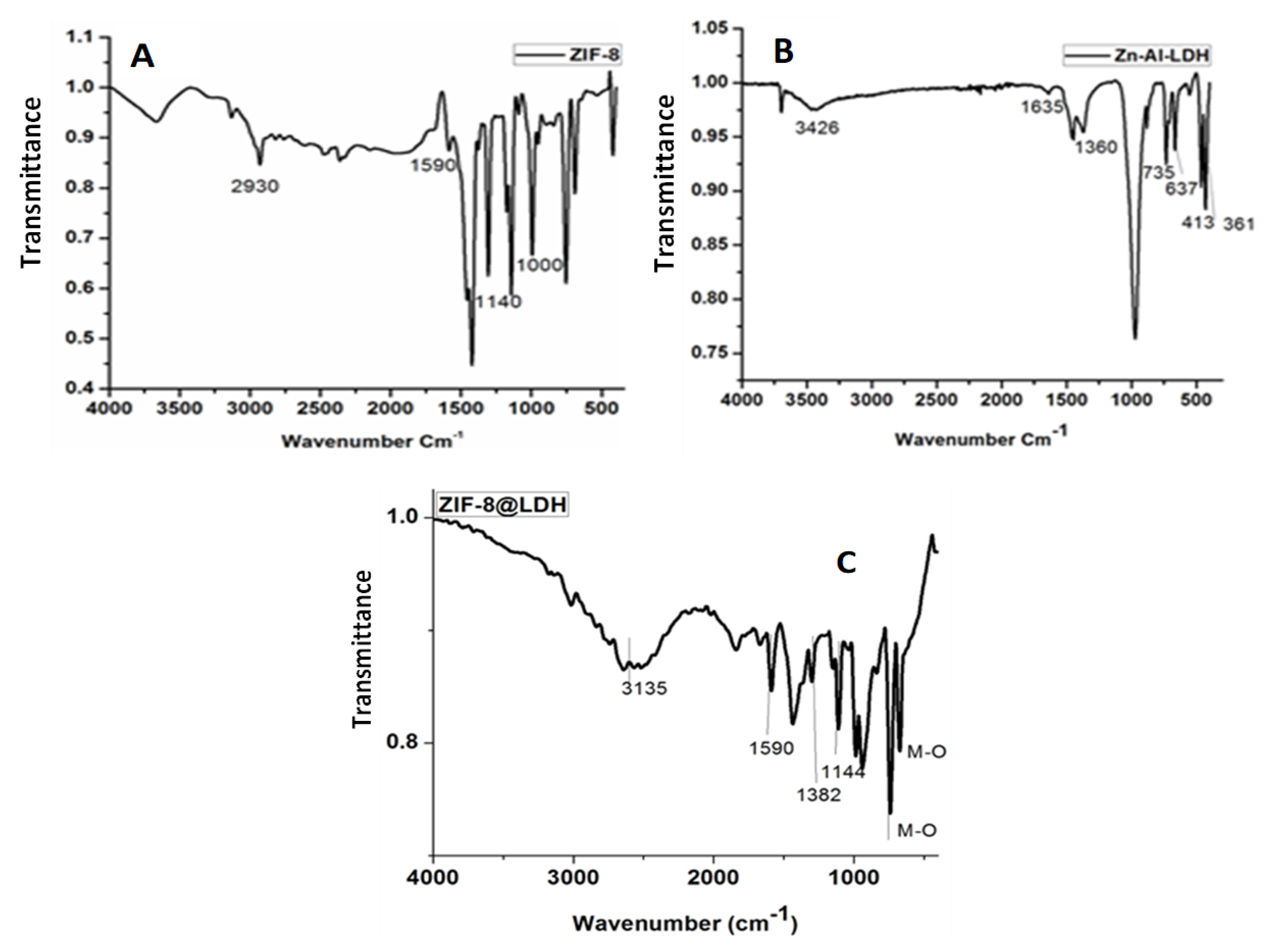
3.1.2. Fourier-Transform Infrared Spectroscopy
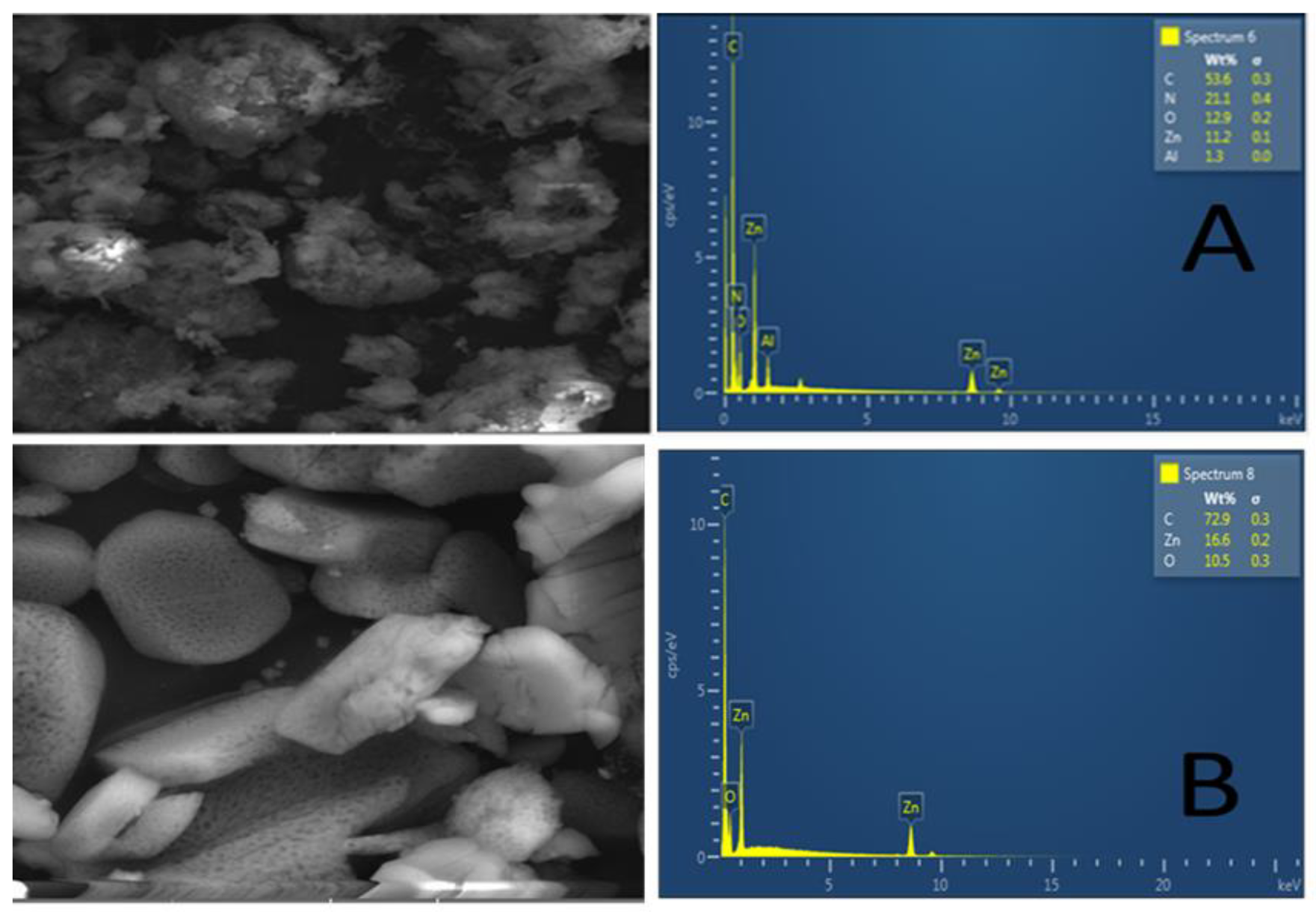
3.1.3. Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy
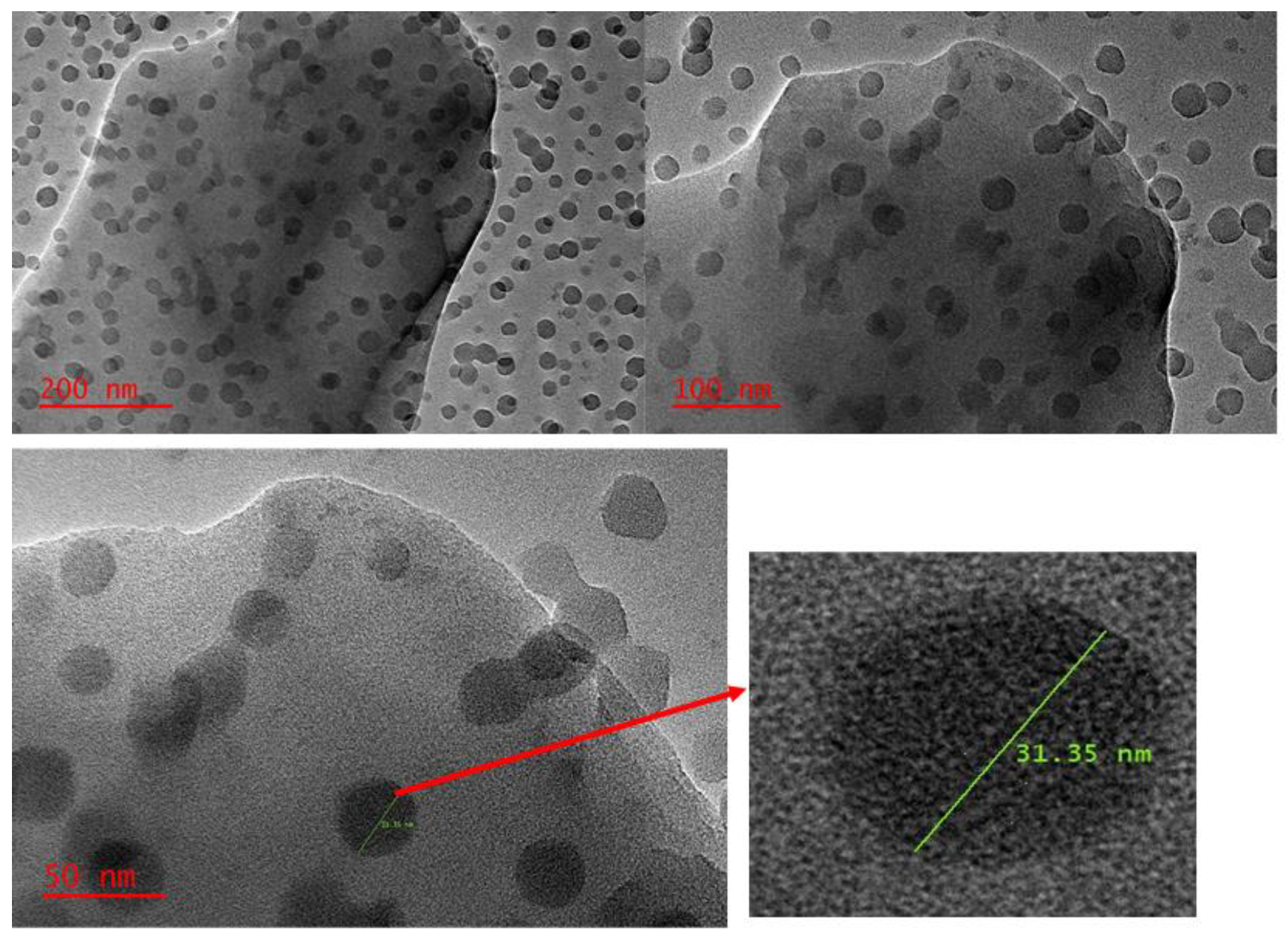
3.1.4. Transmission Electron Microscopy (TEM) Image of Zn–Al–LDH@ZIF–8 Nanocomposite
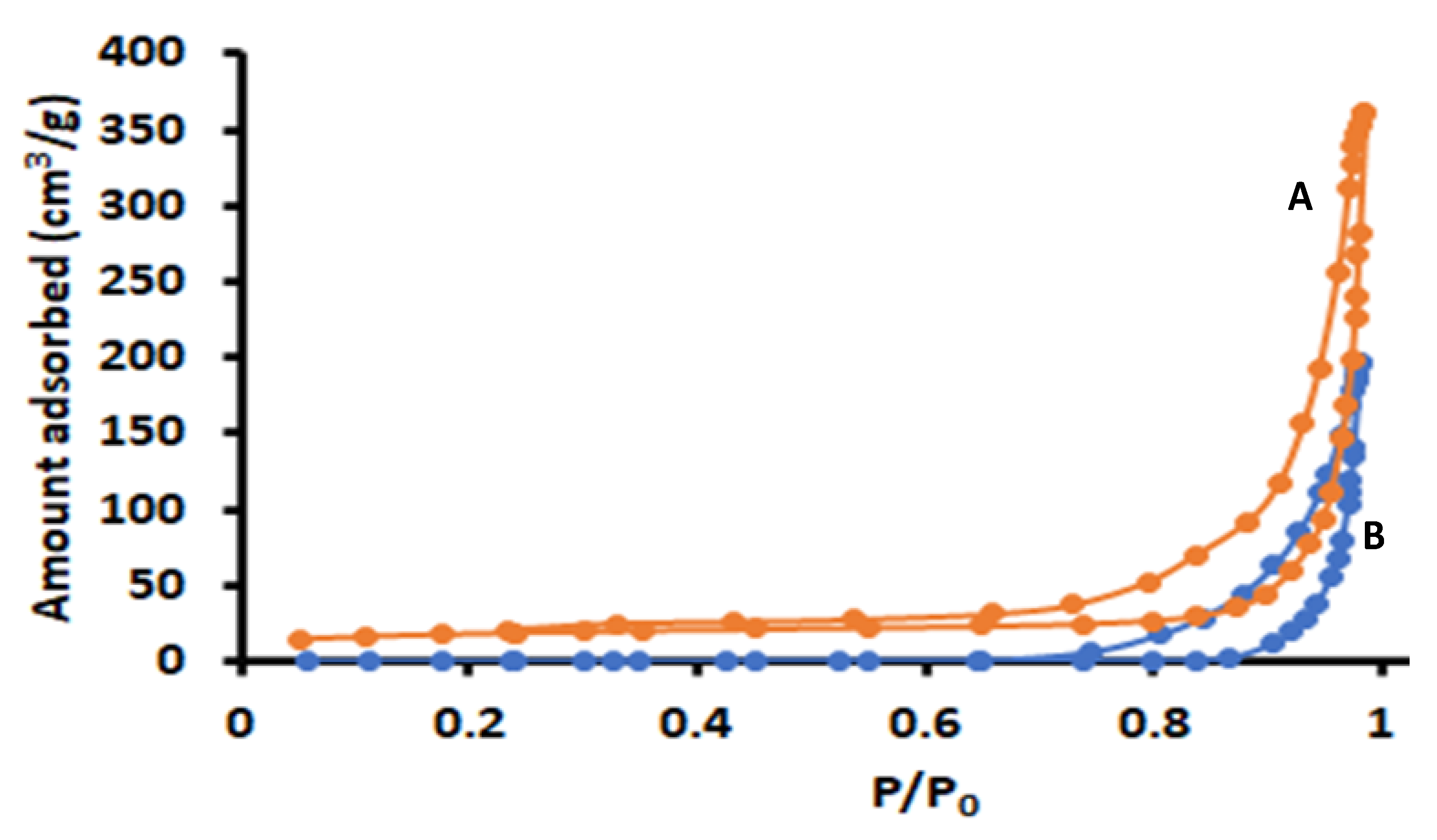
3.1.5. N2-Adsorption/Desorption Analysis
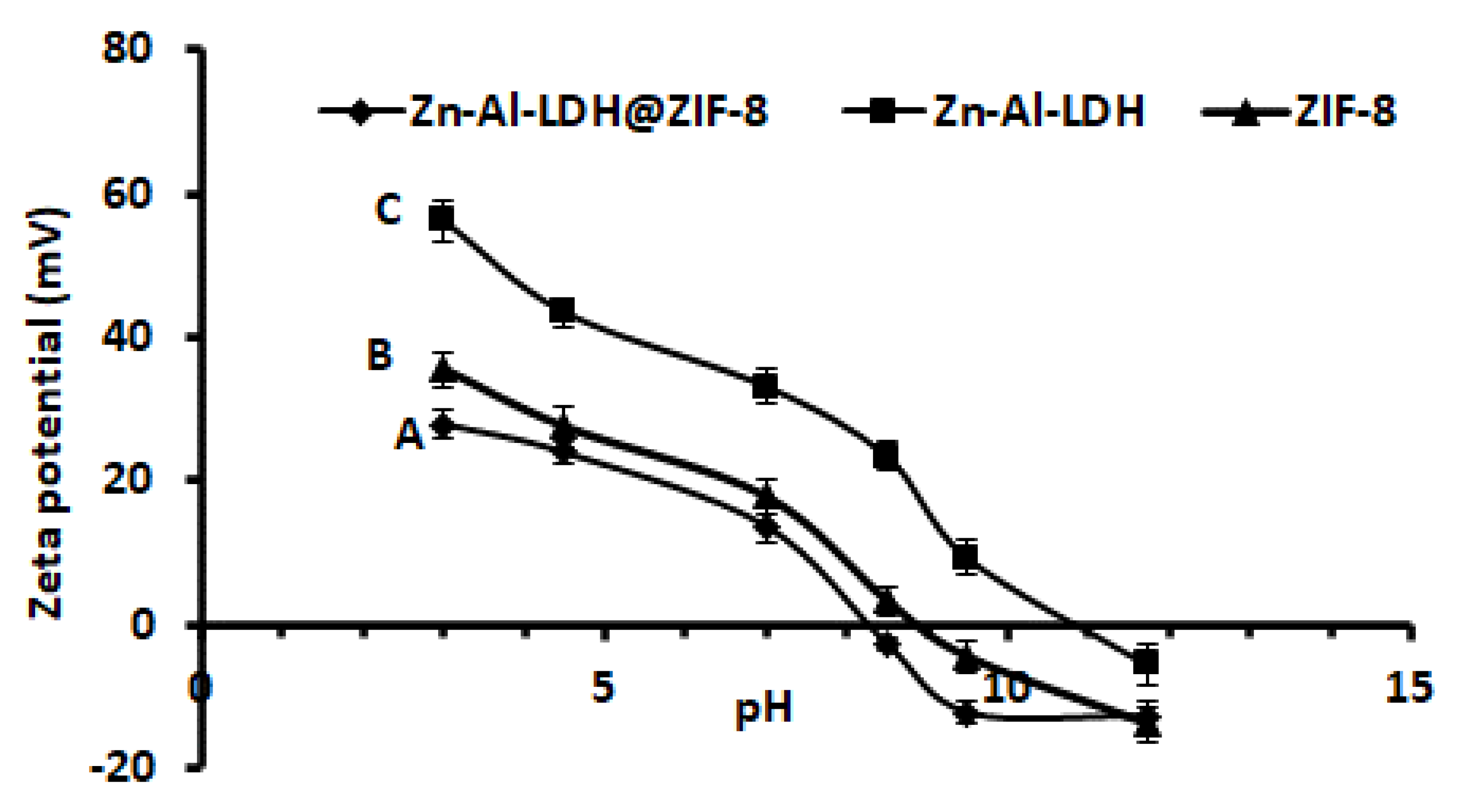
3.1.6. Zeta Potential Experiments
3.2. Optimisation
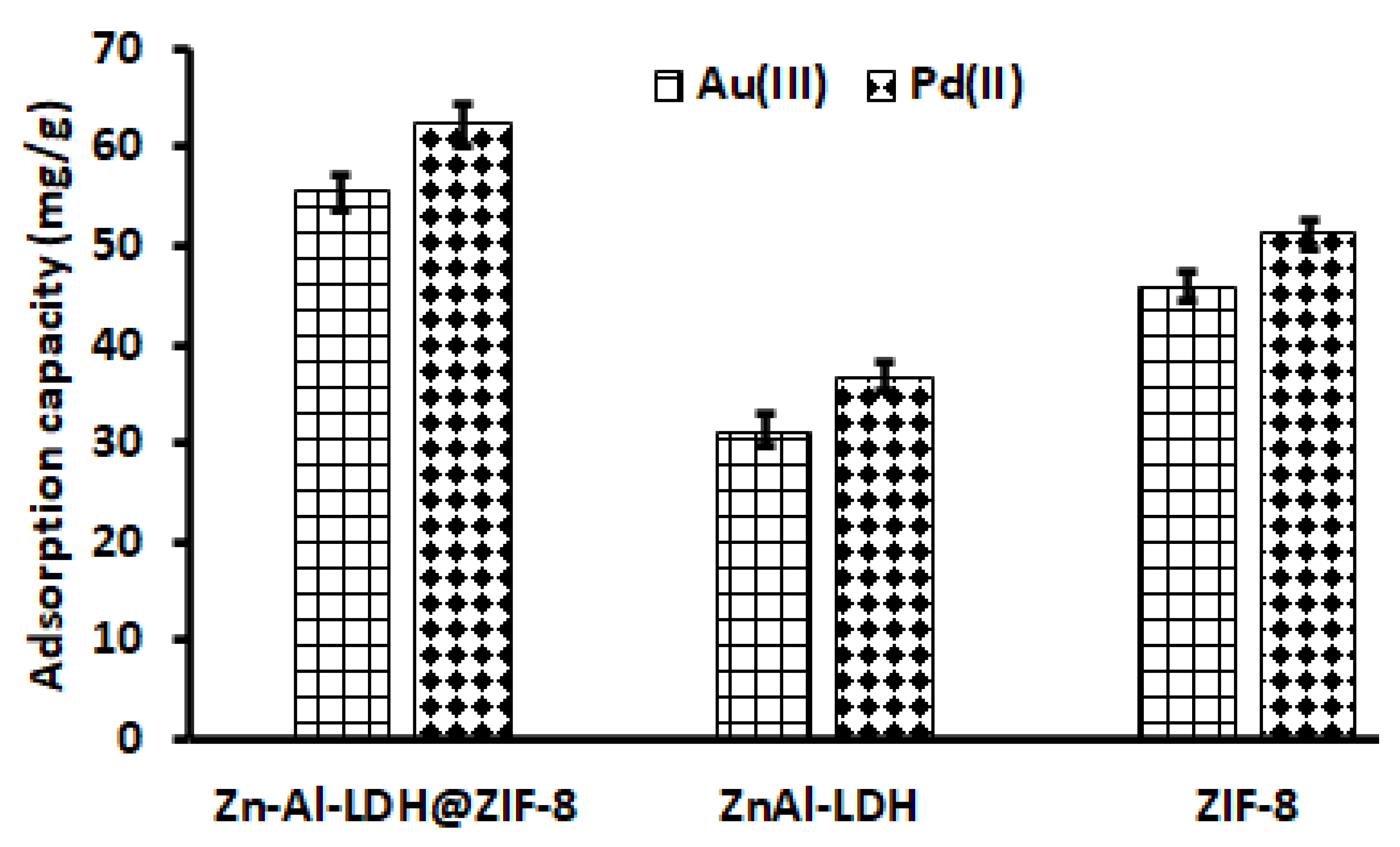
3.2.1. Selection of an Adsorbent
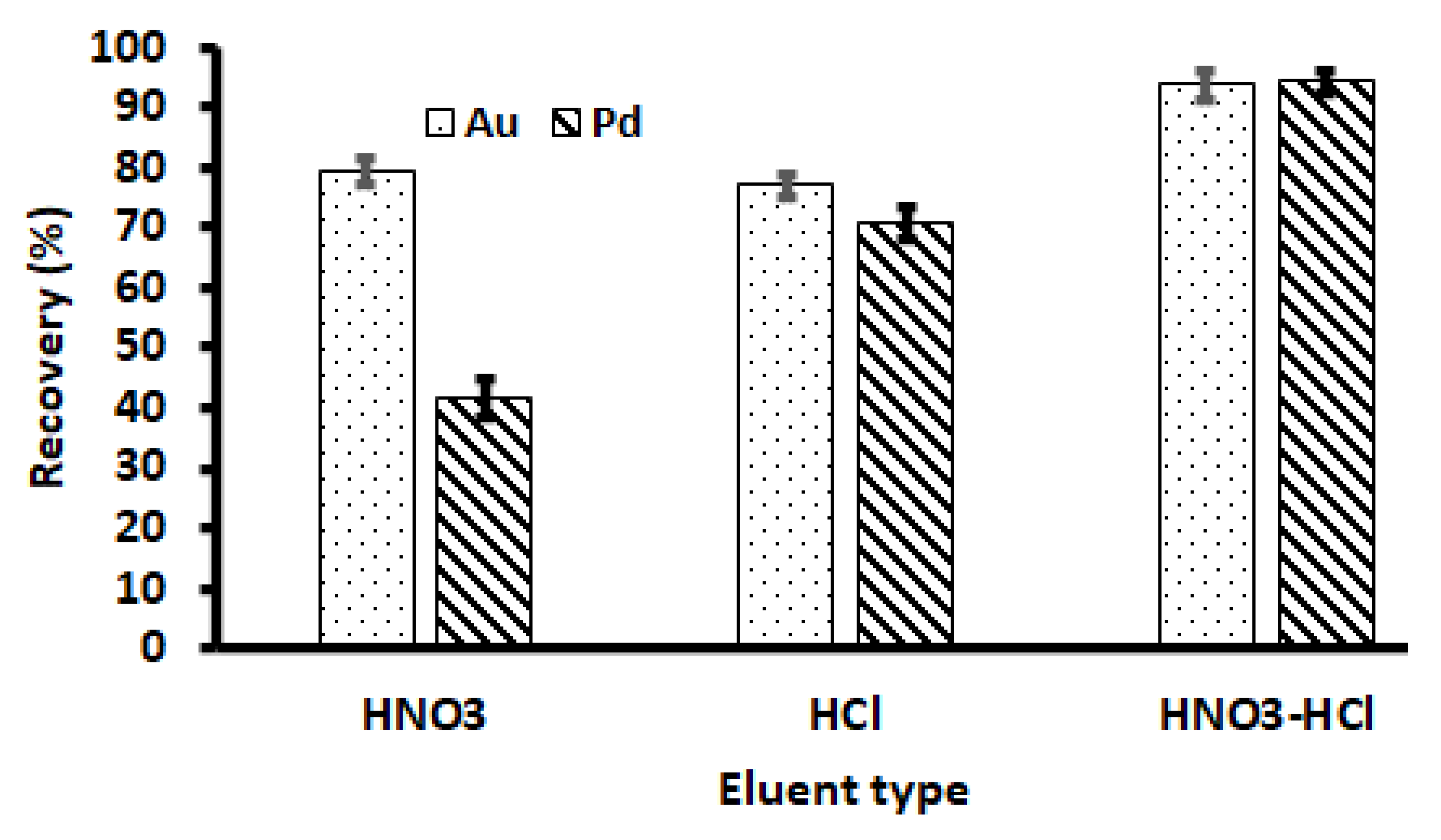
3.2.2. Choice of Elution Solution
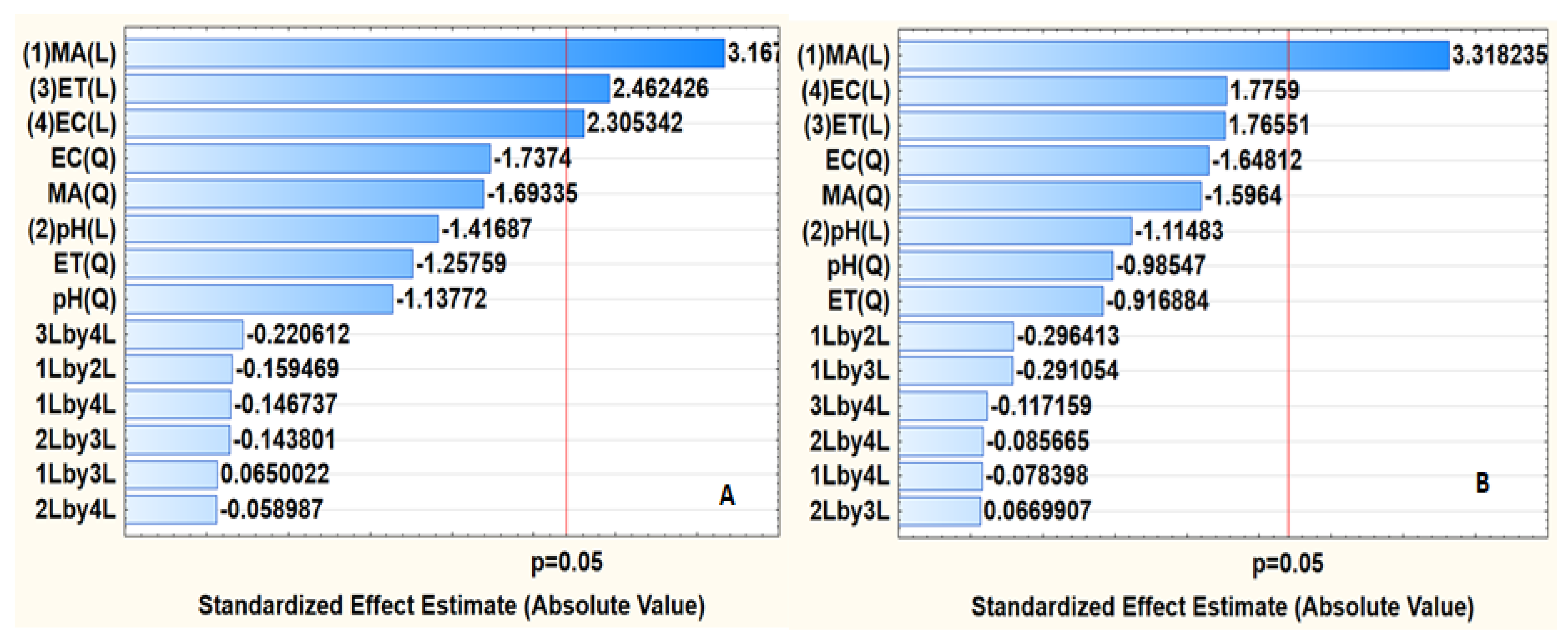
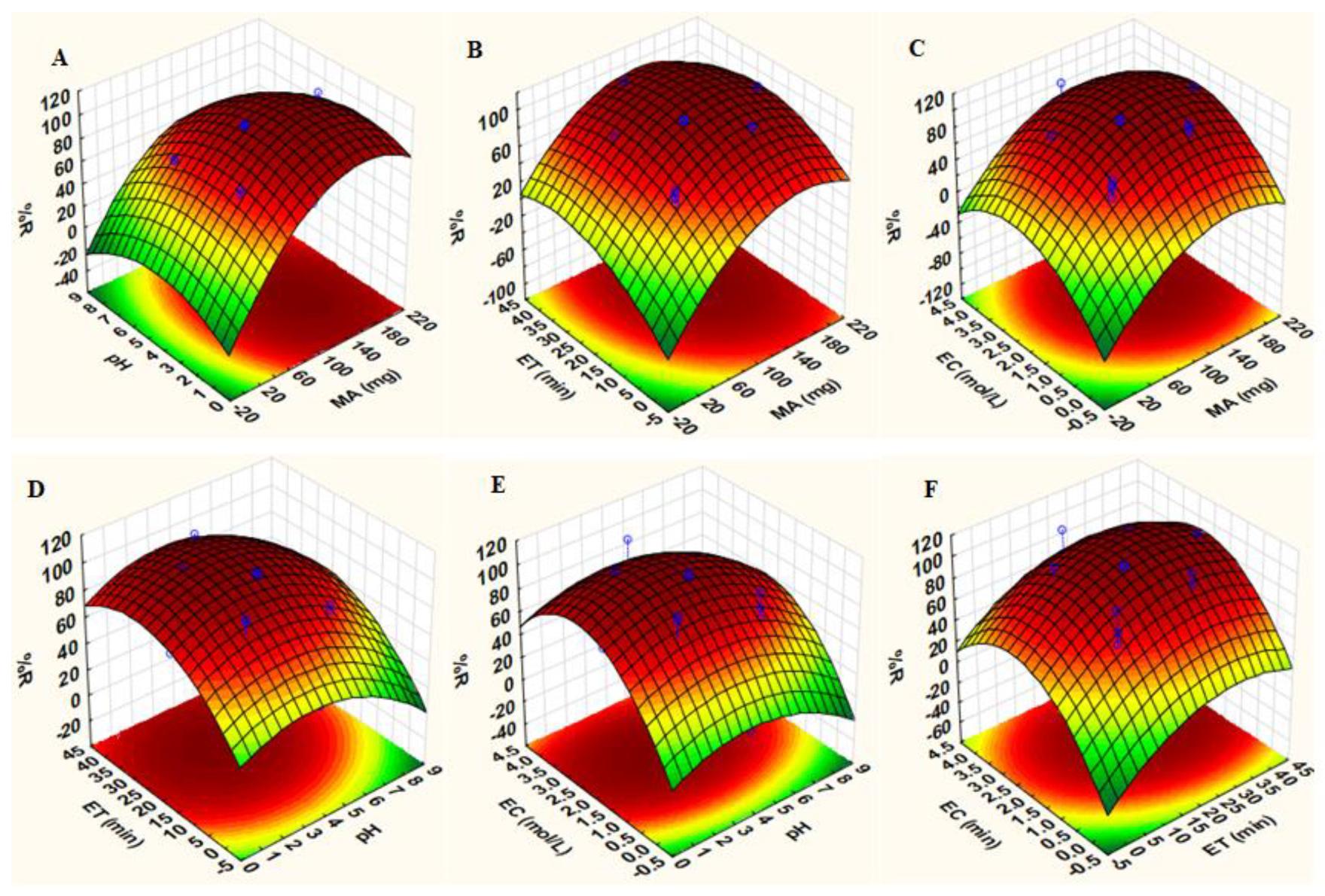
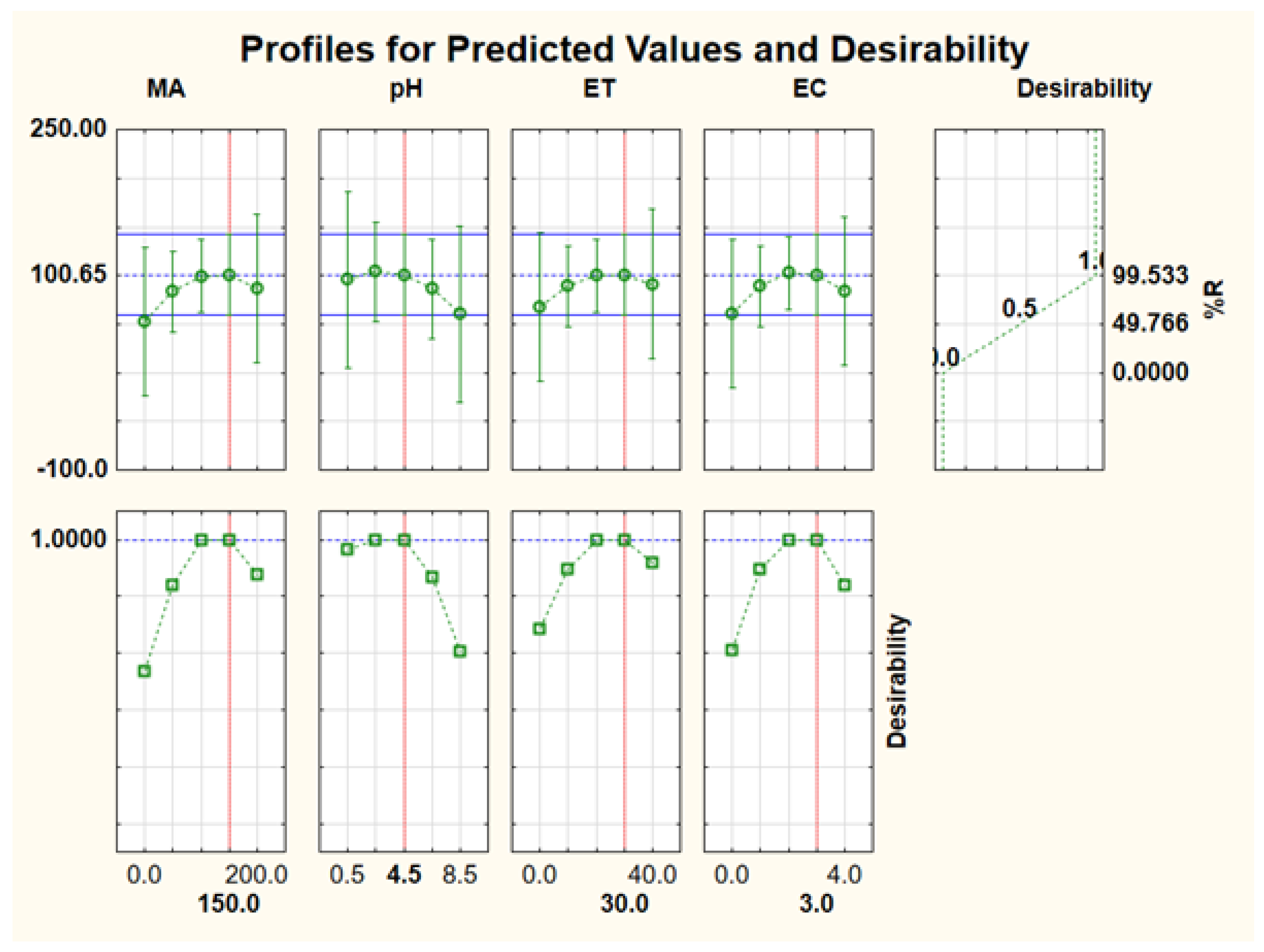
3.2.3. Response Surface Methodology (RSM) Based on Central Composite Design (CCD)
3.3. Batch Adsorption Studies
3.4. Effect of Interfering Ions
3.5. Recovery of Au(III) and Pd(II) from PGM Ore and Concentrate
3.6. Effect of other PGMs Present in Ore Samples on the Extraction and Recovery of Au(III) and Pd(II)
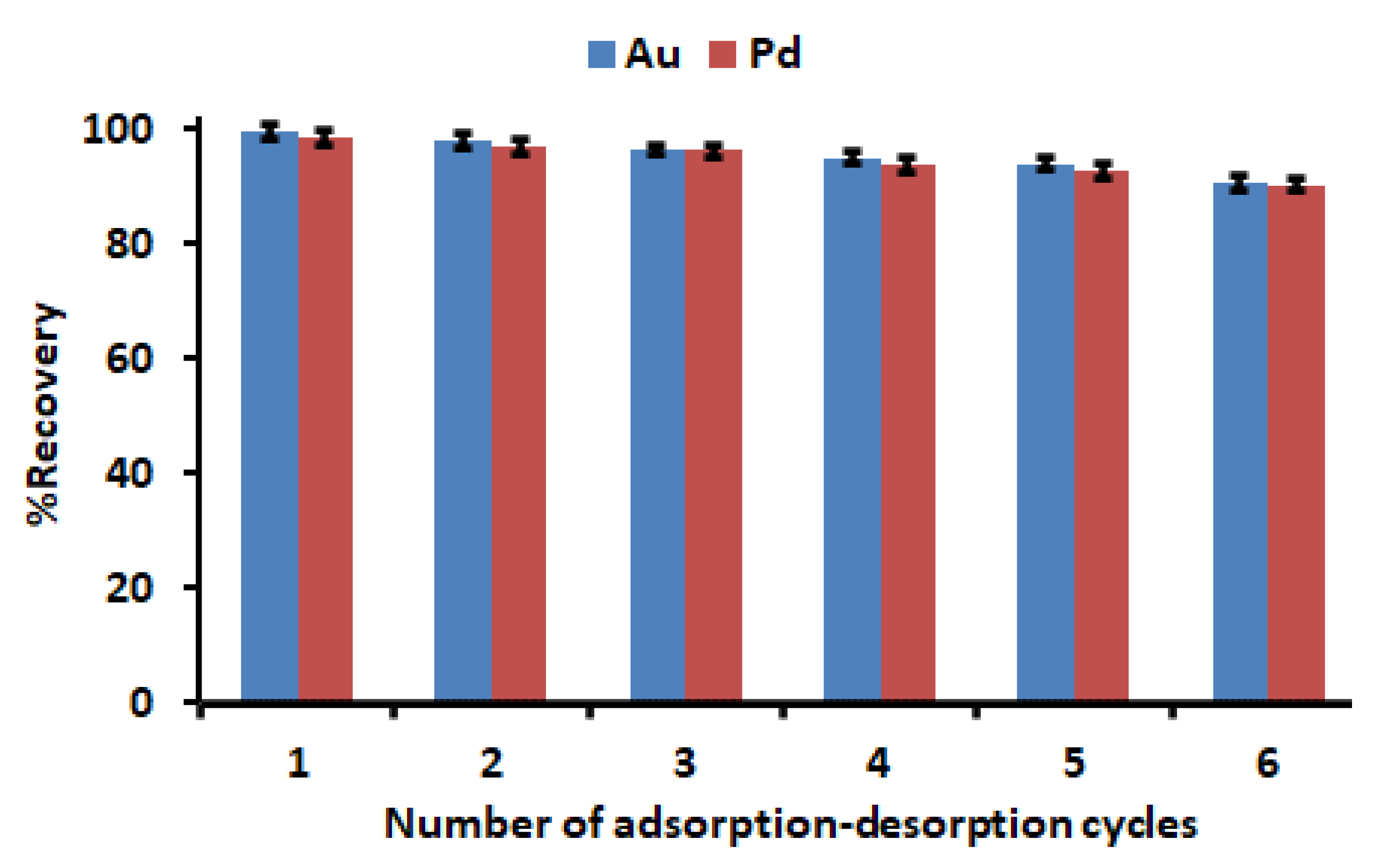
3.7. Regeneration and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nancharaiah, Y.V.; Mohan, S.V.; Lens, P.N.L. Biological and Bioelectrochemical Recovery of Critical and Scarce Metals. Trends Biotechnol. 2016, 34, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, E.; Alorro, R.D.; Encila, A.N.; Yoo, K. Magnetic adsorbents for the recovery of precious metals from leach solutions and wastewater. Metals 2017, 7, 529. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wieczorek, D. Extraction and separation of palladium(II), platinum(IV), gold(III) and rhodium(III) using piperidine-based extractants. Hydrometallurgy 2018, 175, 359–366. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.; Kim, M.; Jeong, J.; Kim, B.-S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Souza, E.J.D.S.; Amaral, C.D.B.D.; Nagata, N.; Grassi, M.T. Cloud point extractors for simultaneous determination of Pd and Pt in water samples by ICP OES with multivariate optimisation. Microchem. J. 2020, 152, 104309. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Separation of Pt(IV), Pd(II), Ru(III) and Rh(III) from model chloride solutions by liquid-liquid extraction with phosphonium ionic liquids. Sep. Purif. Technol. 2019, 212, 791–801. [Google Scholar] [CrossRef]
- Chassary, P.; Vincent, T.; Marcano, J.S.; Macaskie, L.E.; Guibal, E. Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 2005, 76, 131–147. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Hasegawa, H.; Barua, S.; Wakabayashi, T.; Mashio, A.; Maki, T.; Furusho, Y.; Rahman, I.M.M. Selective recovery of gold, palladium, or platinum from acidic waste solution. Microchem. J. 2018, 139, 174–180. [Google Scholar] [CrossRef]
- Othman, N.; Fatiha, N.; Noah, M.; Norimie, R.; Sulaiman, R.; Abdullah, N.A.; Bachok, S.K. Jurnal Teknologi Full paper Liquid-Liquid Extraction of Palladium from Simulated Liquid Waste using Phosphinic Acid as a Carrier. J. Teknol. 2014, 5, 41–45. [Google Scholar]
- Hubicki, Z.; Wołowicz, A.; Leszczyńska, M. Studies of removal of palladium(II) ions from chloride solutions on weakly and strongly basic anion exchangers. J. Hazard. Mater. 2008, 159, 280–286. [Google Scholar] [CrossRef]
- Murakami, H.; Nishihama, S.; Yoshizuka, K. Separation and recovery of gold from waste LED using ion exchange method. Hydrometallurgy 2015, 157, 194–198. [Google Scholar] [CrossRef]
- Mpinga, C.N.; Eksteen, J.J.; Aldrich, C.; Dyer, L. Direct leach approaches to Platinum Group Metal (PGM) ores and concentrates: A review. Miner. Eng. 2015, 78, 93–113. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, D.I.; Hong, S. New industrial application of forward osmosis (FO): Precious metal recovery from printed circuit board (PCB) plant wastewater. J. Membr. Sci. 2018, 552, 234–242. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pandey, A.; Gulati, S.; Adholeya, A. An optimized procedure for preconcentration, determination and on-line recovery of palladium using highly selective diphenyldiketone-monothiosemicarbazone modified silica gel. J. Hazard. Mater. 2012, 209–210, 285–292. [Google Scholar] [CrossRef]
- Soylak, M.; Tuzen, M. Coprecipitation of gold(III), palladium(II) and lead(II) for their flame atomic absorption spectrometric determinations. J. Hazard. Mater. 2008, 152, 656–661. [Google Scholar] [CrossRef]
- Fungene, T. Effluent treatment: What role can modified waste/biomass play in the local platinum industry—A review. In Proceedings of the 4th International Platinum Conference, Sun City, South Africa, 11–14 October 2010. [Google Scholar]
- Devi, A.; Singhal, A.; Gupta, R.; Panzade, P. A study on treatment methods of spent pickling liquor generated by pickling process of steel. Clean Technol. Environ. Policy 2014, 16, 1515–1527. [Google Scholar] [CrossRef]
- Lim, C.-R.; Lin, S.; Yun, Y.-S. Highly efficient and acid-resistant metal-organic frameworks of MIL-101(Cr)-NH2 for Pd(II) and Pt(IV) recovery from acidic solutions: Adsorption experiments, spectroscopic analyses, and theoretical computations. J. Hazard. Mater. 2020, 387, 121689. [Google Scholar] [CrossRef]
- Di Natale, F.; Orefice, M.; la Motta, F.; Erto, A.; Lancia, A. Unveiling the potentialities of activated carbon in recovering palladium from model leaching solutions. Sep. Purif. Technol. 2017, 174, 183–193. [Google Scholar] [CrossRef]
- Liu, J.; Jin, C.; Wang, C. Hyperbranched thiourea-grafted electrospun polyacrylonitrile fibers for efficient and selective gold recovery. J. Colloid Interface Sci. 2020, 561, 449–458. [Google Scholar] [CrossRef]
- Chen, G.; Tan, L.; Xie, M.; Liu, Y.; Lin, Y.; Tan, W.; Huang, M. Direct contact membrane distillation of refining waste stream from precious metal recovery: Chemistry of silica and chromium (III) in membrane scaling. J. Membr. Sci. 2020, 598, 117803. [Google Scholar] [CrossRef]
- Arslanoğlu, H.; Yaraş, A. Recovery of precious metals from spent Mo–Co–Ni/Al2O3 catalyst in organic acid medium: Process optimization and kinetic studies. Pet. Sci. Technol. 2019, 37, 2081–2093. [Google Scholar] [CrossRef]
- Uheida, A.; Iglesias, M.; Fontàs, C.; Hidalgo, M.; Salvadó, V.; Zhang, Y.; Muhammed, M. Sorption of palladium(II), rhodium(III), and platinum(IV) on Fe3O4 nanoparticles. J. Colloid Interface Sci. 2006, 301, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.; Kamal, M.S.; Shehzad, F.; Al-Harthi, M.A. Graphene/layered double hydroxides nanocomposites: A review of recent progress in synthesis and applications. Carbon 2016, 104, 241–252. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, L.; Parashar, V.; Ngila, J.C. Controlled synthesis of microsheets of ZnAl layered double hydroxides hexagonal nanoplates for efficient removal of Cr(VI) ions and anionic dye from water. J. Environ. Chem. Eng. 2017, 5, 1718–1731. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C. Layered double hydroxides: Emerging sorbent materials for analytical extractions. TrAC Trends Anal. Chem. 2016, 75, 174–182. [Google Scholar] [CrossRef]
- Kumar, P.; Gill, K.; Kumar, S.; Ganguly, S.K.; Jain, S.L. Magnetic Fe3O4@MgAl–LDH composite grafted with cobalt phthalocyanine as an efficient heterogeneous catalyst for the oxidation of mercaptans. J. Mol. Catal. A Chem. 2015, 401, 48–54. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, K.; Yu, S.; Hao, Y.; Yu, H.; Du, B. Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution. Chem. Eng. J. 2014, 252, 38–46. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Kuznetsov, B.; Mendis, C.L.; Starykevich, M.; Karpushenkov, S.; Tedim, J.; Ferreira, M.G.S.; Blawert, C.; Zheludkevich, M.L. PEO coatings with active protection based on in-situ formed LDH-nanocontainers. J. Electrochem. Soc. 2017, 164, C36–C45. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Xiao, G.; Su, H. Fabrication of biomaterial/TiO2 composite photocatalysts for the selective removal of trace environmental pollutants. Chin. J. Chem. Eng. 2019, 27, 1416–1428. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Nqombolo, A.; Mpupa, A.; Gugushe, A.S.; Moutloali, R.M.; Nomngongo, P.N. Adsorptive removal of lead from acid mine drainage using cobalt-methylimidazolate framework as an adsorbent: Kinetics, isotherm, and regeneration. Environ. Sci. Pollut. Res. 2019, 26, 3330–3339. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Yan, X.; Kong, L.; Zhang, G.; Liu, H.; Qiu, J.; Yeung, K.L. Synthesis of Fe3O4@ZIF-8 magnetic core–shell microspheres and their potential application in a capillary microreactor. Chem. Eng. J. 2013, 228, 398–404. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. In-situ growth of ZIF-8 on layered double hydroxide: Effect of Zn/Al molar ratios on their structural, morphological and adsorption properties. J. Colloid Interface Sci. 2017, 505, 206–212. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Xiao, H.; Zhang, L.; Qin, Y.; Liu, D.; Hou, W.; Du, N. Synthesis and thermal properties of ZnAl layered double hydroxide by urea hydrolysis. Powder Technol. 2014, 253, 41–45. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; de Witte, K.; van Tendeloo, G.; Cool, P.; Vansant, E.F. Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 2008, 113, 296–304. [Google Scholar] [CrossRef]
- Li, H.; Deng, Q.; Liu, J.; Hou, W.; Du, N.; Zhang, R.; Tao, X. Synthesis, characterization and enhanced visible light photocatalytic activity of Bi2MoO6/Zn–Al layered double hydroxide hierarchical heterostructures. Catal. Sci. Technol. 2014, 4, 1028–1037. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Chen, R.; Liu, J.; Zhang, H.; Li, R.; Takahashi, K.; Liu, P.; Wang, J. Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance. ACS Appl. Mater. Interfaces 2017, 9, 11106–11115. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z. Insitu growth of ZIF-8 on CoAl layered double hydroxide/carbon fiber composites for highly efficient absorptive removal of hexavalent chromium from aqueous solutions. Appl. Clay Sci. 2019, 175, 115–123. [Google Scholar] [CrossRef]
- Zhang, F.; Du, N.; Zhang, R.; Hou, W. Mechanochemical synthesis of Fe3O4@(Mg-Al-OH LDH) magnetic composite. Powder Technol. 2012, 228, 250–253. [Google Scholar] [CrossRef]
- Ghiasi Moaser, A.; Khoshnavazi, R. Facile synthesis and characterization of Fe3O4@MgAl-LDH@STPOM nanocomposites for highly enhanced and selective degradation of methylene blue. New J. Chem. 2017, 41, 9472–9481. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Wang, L.; Li, R.; Chen, P.; Rao, X.; Deng, L.; Rong, L.; Lei, J. NiO-PTA supported on ZIF-8 as a highly effective catalyst for hydrocracking of Jatropha oil. Sci. Rep. 2016, 6, 23667. [Google Scholar] [CrossRef]
- Shou, J.; Jiang, C.; Wang, F.; Qiu, M.; Xu, Q. Fabrication of Fe3O4/MgAl-layered double hydroxide magnetic composites for the effective decontamination of Co(II) from synthetic wastewater. J. Mol. Liq. 2015, 207, 216–223. [Google Scholar] [CrossRef]
- Fan, R.; Min, H.; Hong, X.; Yi, Q.; Liu, W.; Zhang, Q.; Luo, Z. Plant tannin immobilized Fe3O4@SiO2 microspheres: A novel and green magnetic bio-sorbent with superior adsorption capacities for gold and palladium. J. Hazard. Mater. 2019, 364, 780–790. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Bao, C.; Jia, Q.; Xiao, P.; Liu, X.; Zhang, Q. Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 2012, 93, 350–357. [Google Scholar] [CrossRef]
- Neyestani, M.R.; Shemirani, F.; Mozaffari, S.; Alvand, M. A magnetized graphene oxide modified with 2-mercaptobenzothiazole as a selective nanosorbent for magnetic solid phase extraction of gold(III), palladium(II) and silver(I). Microchim. Acta 2017, 184, 2871–2879. [Google Scholar] [CrossRef]
- Awual, M.R. Solid phase sensitive palladium(II) ions detection and recovery using ligand based efficient conjugate nanomaterials. Chem. Eng. J. 2016, 300, 264–272. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, S. Adsorption behavior of Au(III) and Pd(II) on persimmon tannin functionalized viscose fiber and the mechanism. Int. J. Biol. Macromol. 2020, 152, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Trieu, Q.A.; Pellet-Rostaing, S.; Arrachart, G.; Traore, Y.; Kimbel, S.; Daniele, S. Interfacial study of surface-modified ZrO2 nanoparticles with thioctic acid for the selective recovery of palladium and gold from electronic industrial wastewater. Sep. Purif. Technol. 2020, 237, 116353. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Taghizadeh, M.; Hassanpour, S. Preparation of a novel magnetic Pd(II) ion-imprinted polymer for the fast and selective adsorption of palladium ions from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 18493–18508. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Yao, C.; Chen, S.; Luo, R.; Liu, S.; Tong, S. Highly efficient and selective recovery of Au(III) from a complex system by molybdenum disulfide nanoflakes. Chem. Eng. J. 2018, 350, 692–702. [Google Scholar] [CrossRef]
- Kasmiarno, L.D.; Chang, J.S. Adsorption of gold from aqueous systems using microbial thermophilic proteins. J. Eng. Technol. Sci. 2020, 52, 121–135. [Google Scholar] [CrossRef]
| Parameters | Lower Level (−) | Central Point (0) | Higher Level (+) |
|---|---|---|---|
| Adsorbent dosage (mg) | 50 | 125 | 200 |
| Extraction time (min) | 5.0 | 17.5 | 30 |
| pH | 2.0 | 5.5 | 9.0 |
| Eluent concentration (mol L−1) | 0.5 | 1.75 | 3 |
| Adsorbents | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| ZI−-8 | 1234 | 0.98 |
| Zn–Al–LDH | 91.1 | 0.55 |
| Zn–Al–LDH@ZIF–8 | 1032 | 1.43 |
| Isotherms | Parameters | Au | Pd |
|---|---|---|---|
| qcal (mg/g) | 163 | 177 | |
| Langmuir | qmax (mg/g) | 164 | 178 |
| KL | 0.37 | 0.36 | |
| R2 | 0.9950 | 0.9945 | |
| Freundlich | KF (mg/g) | 40.8 | 39.9 |
| n | 2.5 | 2.2 | |
| R2 | 0.9430 | 0.9613 |
| Analyte | Adsorbent | Eluent | qmax (mg g−1) | Refs |
|---|---|---|---|---|
| Au(III) and Pd(II) | Zn–Al–LDH@ZIF–8 | 3.0 mol L−1 HCl-HNO3 | 163 and 177 | This work |
| Au(III) and Pd(II) | Fe3O4@SiO2@PT | 917 and 196 | [48] | |
| Au(III) and Pd(II) | CSGO | 1077 and 217 | [49] | |
| Au(III) | MoS2 | Thiourea | 1133 | [55] |
| Au(III) and Pd(II) | mag-GO@MBT/SDS | 160 and 160 | [50] | |
| Pd(II) | Ligand-based conjugate | 0.10 mol L−1 HCl-0.20 mol L−1 thiourea | 157 | [51] |
| Au(III) and Pd(II) | DAVF-PT | HCl | 535 and 214 | [52] |
| Pd(II) and Au(III) | ZrO2-TOA | 0.2 mol L−1 HCl-/0.5 mol L−1 thiourea | 6.3 and 44 | [53] |
| Pd(II) | Pd(II)-MIIPs | Acidified solution | 65.75 | [54] |
| Au(III) | Microbial thermophilic proteins | 5 mol L−1 HNO3 | 483.2 | [56] |
| Au(III) | Pd(II) | |||||
|---|---|---|---|---|---|---|
| Certified value | Recovered | %R | Certified value | Recovered | %R | |
| SARM 186 | 2.58 ± 0.8 | 2.41 ± 0.15 | 93.4 | 28.1 ± 1.4 | 28.0 ± 0.22 | 99.6 |
| SARM 107 | 0.046 ± 0.010 | 0.044 ± 0.020 | 95.7 | 0.926 ± 0.036 | 0.858 ± 0.050 | 92.7 |
| PGMs | SARM 186 | SARM 107 | ||||
|---|---|---|---|---|---|---|
| Certified Valuemg kg−1 | Recoveredmg kg−1 | %R | Certified Value | Recovered | %R | |
| Pt | 67.1 ± 4.21 | 31.7 ± 1.3 | 31.7 | 1.99± 0.07 | 0.75 ± 0.02 | 37.7 |
| Rh | 7.63 ± 0.596 | 0.17 ± 0.01 | 2.23 | 0.320 ± 0.0396 | 0.12 ± 0.01 | 3.75 |
| Ru | 11.4 ± 0.66 | 0.22 ± 0.01 | 1.93 | 626 ± 0.0087 | 8.23 ± 0.11 | 1.31 |
| Ir | 3.01 ± 0.567 | 0.092 ± 0.002 | 3.05 | 0.14 ± 0.0180 | 0.0021 ± 0.0001 | 1.43 |
| Fe | 10.9 ± 0.46 | ND | ||||
| Co | 0.18 ± 0.014 | ND | ||||
| Cr | 0.59 ± 0.07 | 0.012 ± 9.01 | 2.03 | |||
| Cu | 1.25 ± 0.097 | ND | ||||
| Ni | 2.29 ± 0.21 | ND | ||||
| Al | 1.48 ± 0.032 | ND | ||||
| Ca | 1.29 ± 0.23 | ND | ||||
| Mg | 13.5 ± 0.72 | ND | ||||
| Os | 1.48 ± 0.08 | 0.032 ± 0.001 | 2.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biata, N.R.; Jakavula, S.; Mpupa, A.; Moutloali, R.M.; Nomngongo, P.N. Recovery of Palladium and Gold from PGM Ore and Concentrates Using ZnAl-Layered Double Hydroxide@zeolitic Imidazolate Framework-8 Nanocomposite. Separations 2022, 9, 274. https://doi.org/10.3390/separations9100274
Biata NR, Jakavula S, Mpupa A, Moutloali RM, Nomngongo PN. Recovery of Palladium and Gold from PGM Ore and Concentrates Using ZnAl-Layered Double Hydroxide@zeolitic Imidazolate Framework-8 Nanocomposite. Separations. 2022; 9(10):274. https://doi.org/10.3390/separations9100274
Chicago/Turabian StyleBiata, Nkositetile Raphael, Silindokuhle Jakavula, Anele Mpupa, Richard M. Moutloali, and Philiswa Nosizo Nomngongo. 2022. "Recovery of Palladium and Gold from PGM Ore and Concentrates Using ZnAl-Layered Double Hydroxide@zeolitic Imidazolate Framework-8 Nanocomposite" Separations 9, no. 10: 274. https://doi.org/10.3390/separations9100274
APA StyleBiata, N. R., Jakavula, S., Mpupa, A., Moutloali, R. M., & Nomngongo, P. N. (2022). Recovery of Palladium and Gold from PGM Ore and Concentrates Using ZnAl-Layered Double Hydroxide@zeolitic Imidazolate Framework-8 Nanocomposite. Separations, 9(10), 274. https://doi.org/10.3390/separations9100274









