Greenness Assessment of Chromatographic Methods Used for Analysis of Empagliflozin: A Comparative Study
Abstract
1. Introduction
2. Experimental Methods
2.1. National Environmental Method Index (NEMI)
2.2. Analytical Eco-Scale Assessment (ESA)
2.3. Green Analytical Procedure Index (GAPI)
2.4. Application of Three Greenness Evaluation Tools to Empagliflozin Assessment Methods
3. Results and Discussion
- −
- NEMI tool:
- −
- ESA tool:
- −
- GAPI tool:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | high-performance liquid chromatography |
| RSC | the Royal Society of Chemistry |
| NEMI | national environmental methods index |
| MDCB | methods and data comparability |
| ESA | eco-scale assessment |
| GAPI | green analytical procedure index |
| PBT | persistent, bio-accumulative, and toxic |
| PCT | proximal convoluted tube |
| CVD | cardiovascular disease |
| SGLT2 | sodium glucose cotransporter-2 |
| DM2 | diabetes type 2 |
| GAC | green analytical chemistry |
References
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Biomath, D.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin cardiovascular outcomes and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Wittich, C.M.; Beckman, T.J. Mayo Clinic Internal Medicine Board Review, 12th ed.; Oxford University Press: Oxford, UK, 2020; pp. 168–225. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 391, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Fabjanowicz, M.; Kalinowska, K.; Namieśnik, J. History and milestones of green analytical chemistry. In Green Analytical Chemistry; Springer: Singapore, 2019; pp. 1–17. [Google Scholar]
- Keith, L.H.; Gron, L.U.; Young, J.L. Green analytical methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Marć, M.; Gałuszka, A.; Namieśnik, J. Green chemistry metrics with special reference to green analytical chemistry. Molecules 2015, 20, 10928–10946. [Google Scholar] [CrossRef]
- Tobiszewski, M. Metrics for green analytical chemistry. Anal. Method. 2016, 8, 2993–2999. [Google Scholar] [CrossRef]
- Shah, P.A.; Shrivastav, P.S.; George, A. Mixed-mode solid phase extraction combined with LC-MS/MS for determination of empagliflozin and linagliptin in human plasma. Microchem. J. 2019, 145, 523–531. [Google Scholar] [CrossRef]
- Manoel, J.W.; Primieri, G.B.; Bueno, L.M.; Wingert, N.R.; Volpato, N.M.; Garcia, C.V.; Steppe, M. The application of quality by design in the development of the liquid chromatography method to determine empagliflozin in the presence of its organic impurities. RSC Adv. 2020, 10, 7313–7320. [Google Scholar] [CrossRef]
- Thakor, N.S.; Amrutkar, S.V.; Chaudhari, P.D. Simultaneous estimation of empagliflozin and metformin by high-performance thin-layer chromatography using quality-by-design approach. J. Planar Chromatogr. 2019, 32, 295–307. [Google Scholar] [CrossRef]
- Ayoub, B.M.; Mowaka, S. LC–MS/MS determination of empagliflozin and metformin. J. Chromatogr. Sci. 2017, 55, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Hassib, S.T.; Taha, E.A.; Elkady, E.F.; Barakat, G.H. Validated liquid chromatographic method for the determination of (canagliflozin, dapagliflozin or empagliflozin) and metformin in the presence of (1-cyanoguanidine). J. Chromatogr. Sci. 2019, 57, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.M.; Chhalotiya, U.K.; Jani, H.D.; Kansara, D.; Shah, D.A. Densitometric simultaneous estimation of combination of empagliflozin, linagliptin and metformin hydrochloride used in the treatment of type 2 diabetes mellitus. J. Planar Chromatogr. 2022, 33, 109–118. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.F.; Ayad, M.F.; Tadros, M.M. Liquid chromatographic and spectrofluorimetric assays of empagliflozin: Applied to degradation kinetic study and content uniformity testing. Luminescence 2018, 33, 919–932. [Google Scholar] [CrossRef]
- Van der Aart-van, A.B.; Wessels, A.M.A.; Heerspink, H.J.; Touw, D.J. Simple, fast and robust LC-MS/MS method for the simultaneous quantification of canagliflozin, dapagliflozin and empagliflozin in human plasma and urine. J. Chromatogr. B 2020, 1152, 122257. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Soliman, S.M.; El-Agizy, H.M.; Mansour, F.R. Ultrasound-assisted dispersive liquid–liquid microextraction for determination of three gliflozins in human plasma by HPLC/DAD. J. Chromatogr. B 2020, 1136, 121932. [Google Scholar] [CrossRef]
- Dias, B.C.L.; Fachi, M.M.; de Campos, M.L.; Degaut, F.L.D.; Peccinini, R.G.; Pontarolo, R. A new HPLC–MS/MS method for the simultaneous quantification of SGLT2 inhibitors and metformin in plasma and its application to a pharmacokinetic study in healthy volunteers. Biomed. Chromatogr. 2019, 33, e4663. [Google Scholar] [CrossRef]
- Pandya, P.A.; Shah, P.A.; Shrivastav, P.S. Separation of achiral anti-diabetic drugs using sub/supercritical fluid chromatography with a polysaccharide stationary phase: Thermodynamic considerations and molecular docking study. J. Pharm. Biomed. Anal. 2020, 189, 113452–113464. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.F.; Abdel-Aziz, O.; Ayad, M.F.; Tadros, M.M. New LC–UV methods for pharmaceutical analysis of novel anti-diabetic combinations. Acta Chromatogr. 2017, 29, 448–452. [Google Scholar] [CrossRef]
- Badragheh, S.; Zeeb, M.; Olyai, M.R.T.B. Silica-coated magnetic iron oxide functionalized with hydrophobic polymeric ionic liquid: A promising nanoscale sorbent for simultaneous extraction of antidiabetic drugs from human plasma prior to their quantitation by HPLC. RSC Adv. 2018, 8, 30550–30561. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Shrivastav, P.S.; Sharma, V.; Yadav, M.S. Challenges in simultaneous extraction and chromatographic separation of metformin and three SGLT-2 inhibitors in human plasma using LC–MS/MS. J. Pharm. Biomed. Anal. 2019, 175, 112790–112799. [Google Scholar] [CrossRef]
- Sharif, S.; Bashir, R.; Adnan, A.; Mansoor, S.; Ahmad, I.; Ch, A.R.; Tahir, M.S. Stability Indicating, pH and p K a Dependent HPLC–DAD Method for the Simultaneous Determination of Weakly Ionizable Empagliflozin, Dapagliflozin and Canagliflozin in Pharmaceutical Formulations. Chromatographia 2020, 83, 1453–1465. [Google Scholar] [CrossRef]
- Vankalapati, K.R.; Alegete, P.; Boodida, S. Stability-indicating ultra performance liquid chromatography method development and validation for simultaneous estimation of metformin, linagliptin, and empagliflozin in bulk and pharmaceutical dosage form. Biomed. Chromatogr. 2021, 35, e5019. [Google Scholar] [CrossRef] [PubMed]
- Manoel, J.W.; Primieri, G.B.; Bueno, L.M.; Giordani, C.F.; Sorrentino, J.M.; Dallegrave, A.; Steppe, M. Determination of empagliflozin in the presence of its organic impurities and identification of two degradation products using UHPLC-QTOF/MS. Microchem. J. 2021, 161, 105795–105804. [Google Scholar] [CrossRef]
- Abbas, N.S.; Derayea, S.M.; Omar, M.A.; Saleh, G.A. Innovative TLC-densitometric method with fluorescent detection for simultaneous determination of ternary anti-diabetic mixture in pharmaceutical formulations and human plasma. Microchem. J. 2021, 165, 106131–106143. [Google Scholar] [CrossRef]
- Abou-Omar, M.N.; Kenawy, M.; Youssef, A.O.; Alharthi, S.; Attia, M.S.; Mohamed, E.H. Validation of a novel UPLC-MS/MS method for estimation of metformin and empagliflozin simultaneously in human plasma using freezing lipid precipitation approach and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2021, 200, 114078–114090. [Google Scholar] [CrossRef]
- Burin, S.L.; Lourenço, R.L.; Doneda, M.; Müller, E.I.; Paula, F.R.; Adams AI, H. Development of an HPLC-UV Method to Assay Empagliflozin Tablets and Identification of the Major Photoproduct by Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. Sci. 2021, 59, 526–535. [Google Scholar] [CrossRef]
- Moussa, B.A.; Mahrouse, M.A.; Fawzy, M.G. Application of experimental design in HPLC method optimization and robustness for the simultaneous determination of canagliflozin, empagliflozin, linagliptin, and metformin in tablet. Biomed. Chromatogr. 2021, 35, e5155–e5166. [Google Scholar] [CrossRef]
- Gollu, G.; Gummadi, S. A rapid LC-PDA method for the simultaneous quantification of metformin, empagliflozin and linagliptin in pharmaceutical dosage form. Ann. Pharm. Françaises 2022, 80, 48–58. [Google Scholar] [CrossRef]
- Elnadi, S.; Abdalsabour, S.; Abdalghany, M.F.; Trabik, Y.A. Stability indicating RP-HPLC and spectrophotometric methods for determination of gliflozins in their mixture with metformin. J. Iranian Chem. Soc. 2022, 19, 1723–1735. [Google Scholar] [CrossRef]
- Marie, A.A.; Salim, M.M.; Kamal, A.H.; Hammad, S.F.; Elkhoudary, M.M. Analytical quality by design based on design space in reversed-phase-high performance liquid chromatography analysis for simultaneous estimation of metformin, linagliptin and empagliflozin. R. Soc. Open Sci. 2022, 9, 220215–220228. [Google Scholar] [CrossRef] [PubMed]
- El-Kafrawy, D.S.; El-Shoubashy, O.H.; Issa, A.E.; Beltagy, Y.A. Green chromatographic methods for simultaneous micro-determination of empagliflozin, linagliptin with metformin and its pharmacopoeial impurities in pure form and triple combination tablets: A comparative study. Sust. Chem. Pharm. 2022, 25, 100560–100572. [Google Scholar] [CrossRef]
- The Royal Society of Chemistry. Royal Society of Chemistry. 2020. Available online: https://www.rsc.or (accessed on 14 February 2021).
- Płotka-Wasylka, J.; Kurowska-Susdorf, A.; Sajid, M.; Namieśnik, J.; Tobiszewski, M. Green Chemistry in Higher Education: State of the Art, Challenges, and Future Trends. Chemsuschem 2018, 11, 2845–2858. [Google Scholar] [CrossRef]
- Dempsey, C.R. A comparison of organic emissions from hazardous waste incinerators versus the 1990 toxics release inventory air releases. Air Waste 1993, 10, 1374–1379. [Google Scholar] [CrossRef]

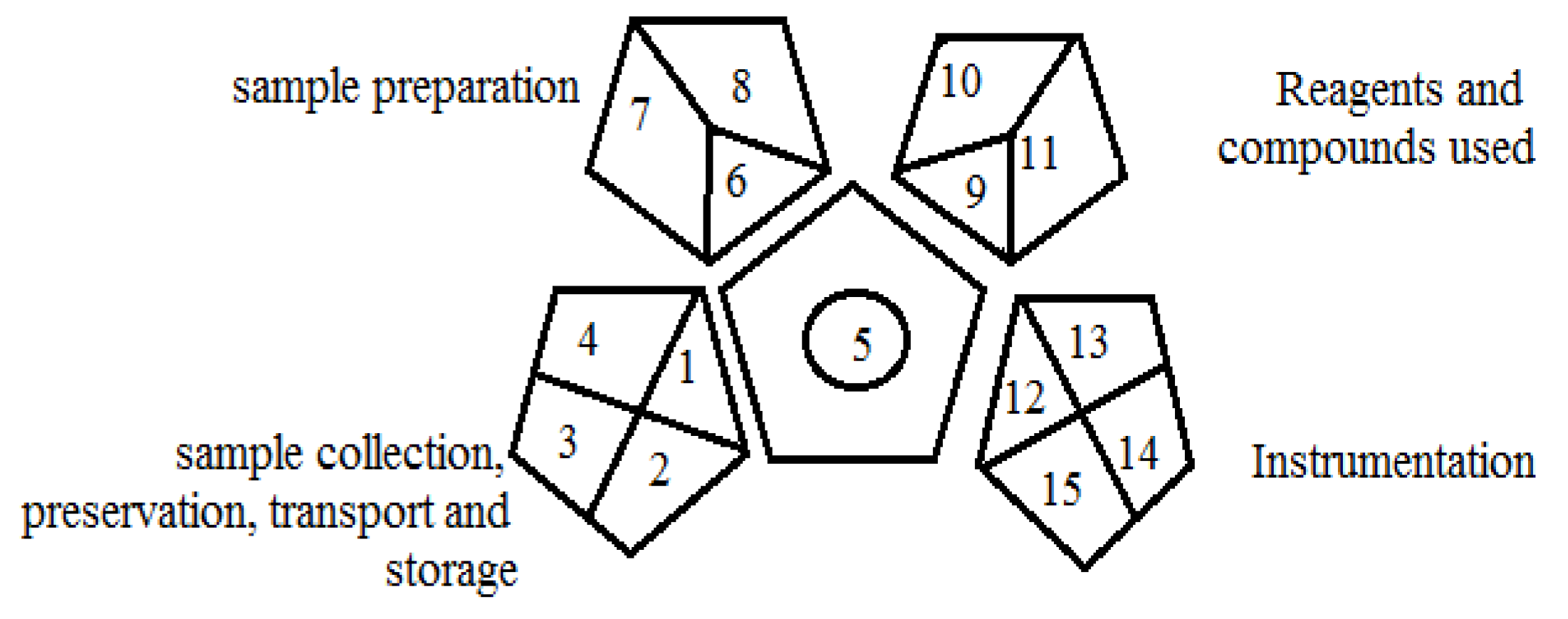
| Study | Applied Instrument and Chromatographic Conditions | NEMI | Eco-Scale | GAPI |
|---|---|---|---|---|
| 1. Mixed-mode solid phase extraction combined with LC-MS/MS for determination of empagliflozin and linagliptin in human plasma [11]. | Analytical method: (HPLC method) Mobile phase: gradient elution (2 M ammonium acetate buffer and acetonitrile) with flow rate: 0.4 mL/min |  | Ammonium acetate buffer 0 Acetonitrile 4 Methanol 6 Occupational hazard 0 Waste 3 Energy 2 Total penalty: 15 Eco-scale: 85 (Green method) | 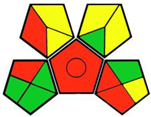 |
| 2. The application of quality by design in the development of a liquid chromatography method to determine empagliflozin in the presence of its organic impurities [12]. | Analytical method: (HPLC method) Mobile phase: acetonitrile: water mixture (72: 28) with flow rate: 0.84 mL/min−1 |  | Acetonitrile 4 Methanol 6 Phosphoric acid 2 Triethylamine 8Occupational hazard 0 Waste 3 Energy 1 Total penalty: 24 Eco-scale: 76 (Green method) | 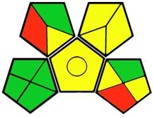 |
| 3. Simultaneous estimation of empagliflozin and metformin with high-performance thin-layer chromatography using quality-by-design approach [13]. | Analytical method: (HPTLC method) Mobile phase: ammonium acetate (2%): methanol: acetonitrile: ethyl acetate (3:1:4.5:1.5) |  | Acetonitrile 4 Methanol 6 Ethyl acetate 4 Ammonium acetate 1 Occupational hazard 0 Waste 3 Energy 0 Total penalty: 18 Eco-scale: 82 (Green method) | 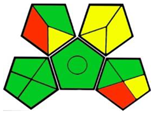 |
| 4. LC-MS/MS determination of empagliflozin and metformin [14]. | Analytical method: (HPLC method) Mobile phase: gradient elution (0.1% aqueous formic acid and acetonitrile) with flow rate: 0.2 mL/min−1 |  | Acetonitrile 4 Formic acid 6 Methanol 6 (applying but not a mobile phase) Occupational hazard 0 Waste 1 Energy 2 Total penalty: 19 Eco-scale: 81 (Green method) | 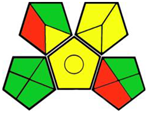 |
| 5. Validated liquid chromatographic method for the determination of canagliflozin, dapagliflozin, or empagliflozin and metformin in the presence of 1-cyanoguanidine [15]. | Analytic method: (HPLC method) Mobile phase: NaH2PO4 buffer (10 mM, pH 2.8): acetonitrile (18.5:81.5, v/v) with flow rate: 2 mL/min−1 |  | Acetonitrile 4 NaH2PO4 (not classified as a hazardous chemical) Orthophosphoric acid 2 Occupational hazard 0 Waste 3 Energy 1 Total penalty: 10 Eco-scale: 90 (Green method) | 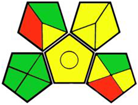 |
| 6. Densitometric simultaneous estimation of combination of empagliflozin, linagliptin, and metformin hydrochloride used in the treatment of type 2 diabetes mellitus [16]. | Analytical method: (HPTLC method) Mobile phase: n-butanol: water: glacial acetic acid (6:3:1, v/v) |  | n-butanol 6 Glacial acetic acid 4 Occupational hazard 0 Waste 3 Energy 2 Total penalty: 15 Eco-scale: 85 (Green method) | 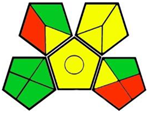 |
| 7. Liquid chromatographic and spectrofluorimetric assays of empagliflozin applied to degradation kinetic study and content uniformity testing [17]. | Analytical method: (HPLC method) Mobile phase: gradient elution (acetonitrile and NaH2PO4 buffer) with flow rate: 1 mL/min |  | Acetonitrile 4 NaH2PO4 0 Orthophosphoric acid 2 Methanol 6 Formic acid 6 Occupational hazard 0 Waste 3 Energy 0 Total penalty: 22 Eco-scale: 78 (Green method) | 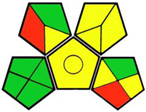 |
| 8. Simple, fast, and robust LC-MS/MS method for the simultaneous quantification of canagliflozin, dapagliflozin, and empagliflozin in human plasma and urine [18]. | Analytic method: (HPLC method) Mobile phase: gradient elution (20 mM ammonium acetate and acetonitrile) with flow rate: 0.8 mL/min |  | Acetonitrile 4 Methanol 6 Ammonium acetate 1 Occupational hazard 0 Waste 1 Energy 2 Total penalty: 14 Eco-scale: 86 (Green method) | 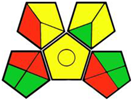 |
| 9. Ultrasound-assisted dispersive liquid–liquid microextraction for determination of three gliflozins in human plasma through HPLC/DAD [19]. | Analytic method: (HPLC method) Mobile phase: acetonitrile: aqueous 0.1% trifluoroacetic acid (40:60, v/v) with flow rate: 1 mL/min |  | Acetonitrile 4 Trifluoroacetic acid 4 Methanol 6 Occupational hazard 0 Waste 3 Energy 1 Total penalty: 18 Eco-scale: 82 (Green method) | 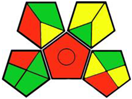 |
| 10. A new HPLC-MS/MS method for the simultaneous quantification of SGLT2 inhibitors and metformin in plasma and its application to a pharmacokinetic study in healthy volunteers [20]. | Analytic method: (HPLC method) Mobile phase: gradient elution (water and acetonitrile, both containing 1 mM ammonium formate and 0.1% formic acid) with flow rate: 0.2 mL min−1 |  | Acetonitrile 4 Formic acid 6 Ammonium formate 1 Methanol 6 Occupational hazard 0 Waste 3 Energy 2 Total penalty: 22 Eco-scale: 78 (Green method) | 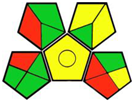 |
| 11-Separation of achiral antidiabetic drugs using sub- or supercritical fluid chromatography with a polysaccharide stationary phase: thermodynamic considerations and molecular docking study [21]. | Analytic method: (supercritical fluid chromatography) Mobile phase: CO2 and 0.1% diethylamine in methanol and isopropanol 50:50 (v/v) in 60:40 ratios with flow rate: 4.0 mL/min |  | CO2 2 Isopropanol 4Diethylamine 6 Methanol 6 Occupational hazard 0 Waste 5 Energy 2 Total penalty: 25 Eco-scale: 75 (Inadequate green method) | 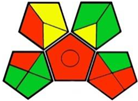 |
| 12. New LC-UV method for pharmaceutical analysis of novel antidiabetic combinations [22]. | Analytical method: (HPLC method) Mobile phase: 0.1 % formic acid: methanol: acetonitrile (40:20:40) by volume with flow rate: 2.0 mL/min−1 |  | Acetonitrile 4 Formic acid 6 Orthophosphoric acid 2 Methanol 6 Occupational hazard 0 Waste 3 Energy 1 Total penalty: 22 Eco-scale: 78 (Green method) | 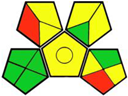 |
| 13. Silica-coated magnetic iron oxide functionalized with hydrophobic polymeric ionic liquid: a promising nanoscale sorbent for simultaneous extraction of antidiabetic drugs from human plasma prior to their quantitation by HPLC [23]. | Analytical method: (HPLC method) Mobile phase: NaH2PO4 and sodium dodecyl sulfate (0.01 M): acetonitrile (55:45, v/v) with flow rate: 1 mL/min−1 |  | Acetonitrile 4 NaH2PO4 0 Sodium dodecyl sulfate 6 Occupational hazard 0 Waste 3 Energy 1 Total penalty: 14 Eco-scale: 86 (Green method) | 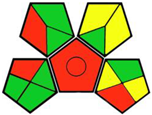 |
| 14. Challenges in simultaneous extraction and chromatographic separation of metformin and three SGLT-2 inhibitors in human plasma using LC-MS/MS [24]. | Analytical method: (HPLC method) Mobile phase: acetonitrile and 10 mM ammonium formate buffer (75:25, v/v) with flow rate: 0.9 mL/min |  | Acetonitrile 4 Ammonium formate 1 Occupational hazard 0 Waste 3 Energy 2 Total penalty: 10 Eco-scale: 90 (Green method) | 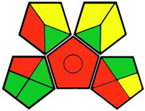 |
| 15. Stability-indicating pH- and pKa-dependent HPLC-DAD method for the simultaneous determination of weakly ionizable empagliflozin, dapagliflozin, and canagliflozin in pharmaceutical formulations [25]. | Analytical method: (HPLC method) Mobile phase: gradient elution (acetonitrile and 0.1% formic acid buffer) with flow rate: 1 mL/min−1 |  | Acetonitrile 4 Formic acid 6 Occupational hazard 0 Waste 3 Energy 1 Total penalty: 14 Eco-scale: 86 (Green method) | 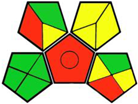 |
| 16. Stability-indicating ultra-performance liquid chromatography method development and validation for simultaneous estimation of metformin, linagliptin, and empagliflozin in bulk and pharmaceutical dosage forms [26]. | Analytical method: (HPLC method) Mobile phase: gradient elution (mixture solution of 40% phosphate buffer and 60% acetonitrile) with flow rate: 0.6 mL/min |  | Acetonitrile 4 Phosphate buffer 0 Occupational hazard 0 Waste 5 Energy 0 Total penalty: 11 Eco-scale: 90 (Green method) | 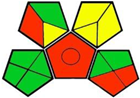 |
| 17. Determination of empagliflozin in the presence of its organic impurities and identification of two degradation products using UHPLC-QTOF/MS [27]. | Analytical method: (UHPLC method) Mobile phase: acetonitrile: water mixture (72:28), with flow rate: 0.84 mL/min−1 |  | Acetonitrile 4 Methanol 6 Phosphoric acid 2 Triethylamine 8 Occupational hazard 0 Waste 3 Energy 0 Total penalty: 23 Eco-scale: 77 (Green method) | 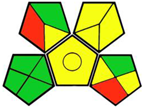 |
| 18. Application of experimental design in HPLC method optimization and robustness for the simultaneous determination of canagliflozin, empagliflozin, linagliptin, and metformin in tablet [31]. | Analytical method: (HPLC method) Mobile phase: Na2HPO4 buffer (0.05 M, adjusted to pH 6 using o-phosphoric acid): acetonitrile: methanol (50:25:25, v/v/v) with flow rate: 1.5 mL/min |  | Na2HPO4 buffer 0 o-phosphoric acid (pH 6) 0 Acetonitrile 4 Methanol 6 Waste 5 Energy 1 Total penalty: 16 Eco-scale: 84 (Green method) | 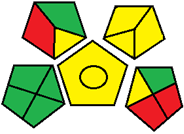 |
| 19. Development of an HPLC-UV method to assay empagliflozin tablets and identification of the major photoproduct by quadrupole time-of-flight mass spectrometry [30]. | Analytical method: (HPLC method) Mobile phase: methanol, acetonitrile, and purified water (60:5:35 v/v) with flow rate: 1 mL/min−1 |  | Purified water 0 Acetonitrile 4 Methanol 6 Waste 3 Energy 1 Total penalty: 14 Eco-scale: 86 (Green method) | 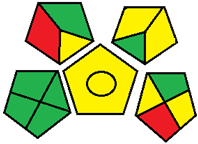 |
| 20. Validation of a novel UPLC-MS/MS method for estimation of metformin and empagliflozin simultaneously in human plasma using freezing lipid precipitation approach and its application to pharmacokinetic study [29]. | Analytical method: (UPLC method) Mobile phase: formic acid (0.01%): acetonitrile (70:30 v/v) with flow rate: 0.3 mL/min |  | Formic acid (0.01%) 0 Acetonitrile 4 Waste 1 Energy 2 Total penalty: 7 Eco-scale: 93 (Green method) | 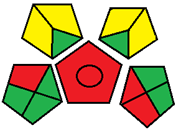 |
| 21. Innovative TLC densitometric method with fluorescent detection for simultaneous determination of ternary antidiabetic mixture in pharmaceutical formulations and human plasma [28]. | Analytical method: (HPTLC method) Mobile phase: toluene: methanol: ethyl acetate (4:3:2 v/v/v) Separation of the cited drugs was performed on aluminum plates precoated with silica gel 60 F254 |  | Toluene 3 Methanol 6 Ethyl acetate 2 Waste 3 Energy 0 Total penalty: 14 Eco-scale: 86 (Green method) | 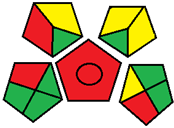 |
| 22. LC-PDA method for the simultaneous quantification of metformin, empagliflozin, and linagliptin in pharmaceutical dosage form [32]. | Analytical method: (HPLC method) Mobile phase: acetonitrile: triethylamine (TEA) (70:30) with flow rate adjusted to 1 mL/ min |  | Acetonitrile 4 TEA 3 Energy 1 Waste 3 Total penalty: 11 Eco-scale score: 89 (Green method) | 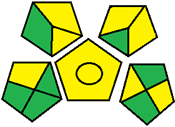 |
| 23. Stability-indicating RP-HPLC for determination of gliflozins in their mixture with metformin [33]. | Analytical method: (HPLC method) Mobile phase: acetonitrile: 0.1% orthophosphoric acid (65:35) with flow rate adjusted to 1 mL/min |  | Acetonitrile 4 Energy 1 Waste 3 Total penalty: 8 Eco-scale score: 92 (Green method) | 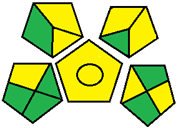 |
| 24. Analytical quality by design based on design space in reversed-phase high-performance liquid chromatography analysis for simultaneous estimation of metformin, linagliptin, and empagliflozin [34]. | Analytical method: (HPLC method) Mobile phase: 0.043 M NaH2PO4 buffer premixed with 0.05% v/v TEA (buffer pH 3.79 adjusted using orthophosphoric acid): methanol (34.4:65.6, v/v) with flow rate: 1 mL/min |  | Orthophosphoric acid 1 Methanol 6 Energy 1 Waste 3 Total penalty: 11 Eco-scale score: 89 (Green method) | 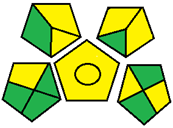 |
| 25. Green chromatographic methods for simultaneous microdetermination of empagliflozin and linagliptin with metformin and its pharmacopeial impurities in pure form and triple-combination tablets: a comparative study (HPLC) [35]. | Analytical method: (HPLC method) Mobile phase: methanol and 0.01 M sodium dihydrogen orthophosphate buffer at pH 2.55 (adjusted with orthophosphoric acid) eluted in a gradient mode |  | Methanol 6 Orthophosphoric acid 1 Energy 1 Waste 5 Total penalty: 12 Eco-scale score: 88 (Green method) | 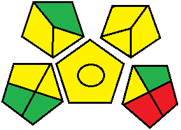 |
| 26. Green chromatographic methods for simultaneous microdetermination of empagliflozin and linagliptin with metformin and its pharmacopeial impurities in pure form and triple-combination tablets: a comparative study (HPTLC) [35]. | Analytical method: (HPTLC method) Mobile phase: ethanol: ethylacetate: water (3.5:3:1, v/v/v, respectively) as mobile phase using Merk TLC plates precoated with 60 F254 silica gel on aluminum sheet as the stationary phase | 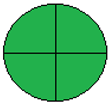 | Ethanol 6 Ethylacetate 1 Energy 0 Waste 5 Total penalty: 12 Eco-scale score: 88 (Green method) | 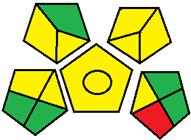 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santali, E.Y.; Naguib, I.A.; Alshehri, A.M.; Alzahrani, Y.A.; Alharthi, A.E.; Alosaimi, T.S.; Alsayali, B.D.; Alsalahat, I.; Almahri, A.; Abourehab, M.A.S.; et al. Greenness Assessment of Chromatographic Methods Used for Analysis of Empagliflozin: A Comparative Study. Separations 2022, 9, 275. https://doi.org/10.3390/separations9100275
Santali EY, Naguib IA, Alshehri AM, Alzahrani YA, Alharthi AE, Alosaimi TS, Alsayali BD, Alsalahat I, Almahri A, Abourehab MAS, et al. Greenness Assessment of Chromatographic Methods Used for Analysis of Empagliflozin: A Comparative Study. Separations. 2022; 9(10):275. https://doi.org/10.3390/separations9100275
Chicago/Turabian StyleSantali, Eman Y., Ibrahim A. Naguib, Abdullah M. Alshehri, Yazeed A. Alzahrani, Abdullah E. Alharthi, Turki S. Alosaimi, Bandar D. Alsayali, Izzeddin Alsalahat, Albandary Almahri, Mohammed A. S. Abourehab, and et al. 2022. "Greenness Assessment of Chromatographic Methods Used for Analysis of Empagliflozin: A Comparative Study" Separations 9, no. 10: 275. https://doi.org/10.3390/separations9100275
APA StyleSantali, E. Y., Naguib, I. A., Alshehri, A. M., Alzahrani, Y. A., Alharthi, A. E., Alosaimi, T. S., Alsayali, B. D., Alsalahat, I., Almahri, A., Abourehab, M. A. S., & Abdallah, F. F. (2022). Greenness Assessment of Chromatographic Methods Used for Analysis of Empagliflozin: A Comparative Study. Separations, 9(10), 275. https://doi.org/10.3390/separations9100275








