DES Based Efficient Extraction Method for Bioactive Coumarins from Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav.
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of DESs
2.3. Experimental Design
2.3.1. Preparation of Standard Solution
2.3.2. Preparation of Sample Solution
2.3.3. Traditional Extraction Method Comparison
2.4. Single-Factor Experimental Analysis of Extraction of Coumarins from A. dahurica
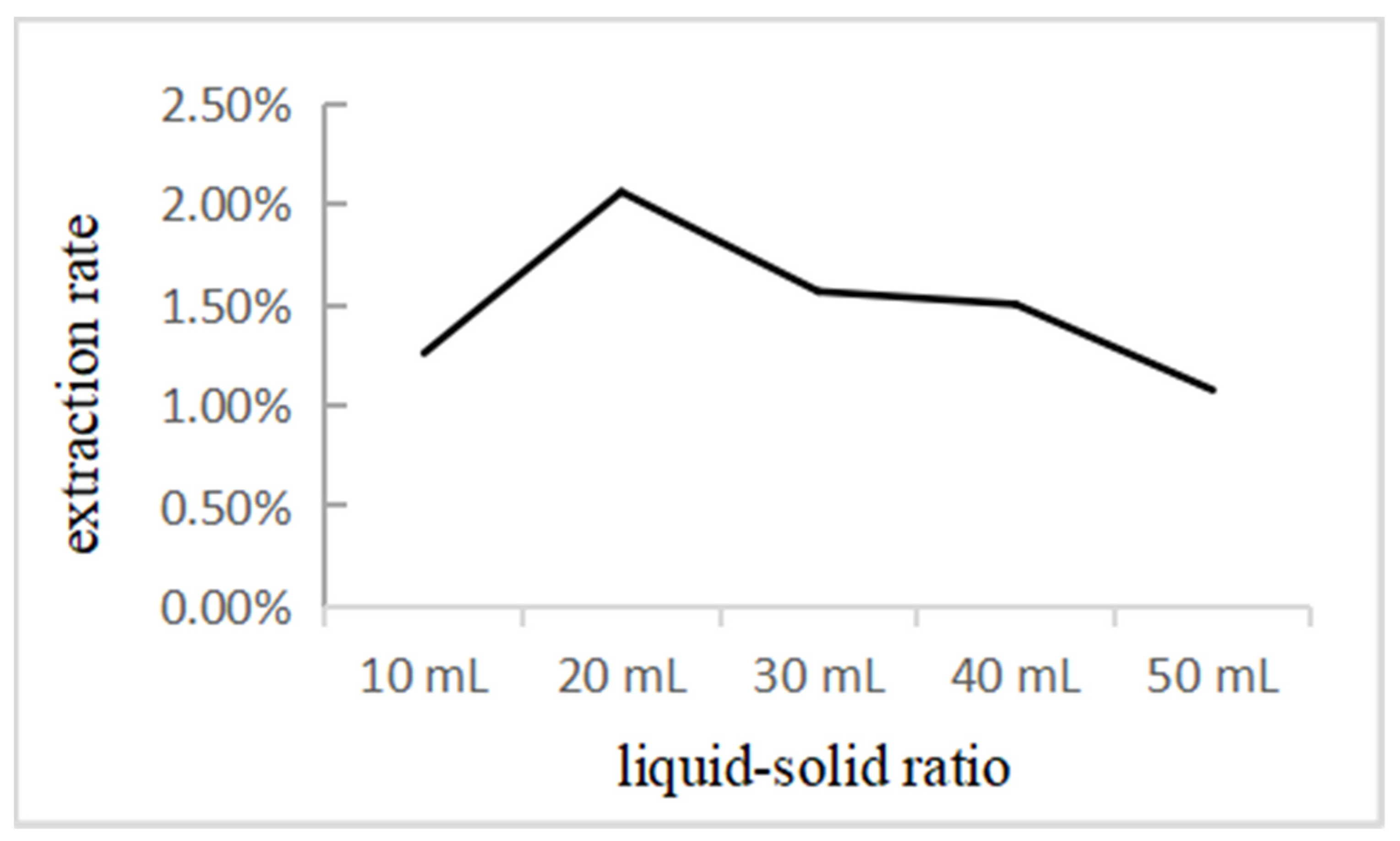
2.4.1. Effect of Liquid–Solid Ratio
2.4.2. Effect of Extraction Temperature
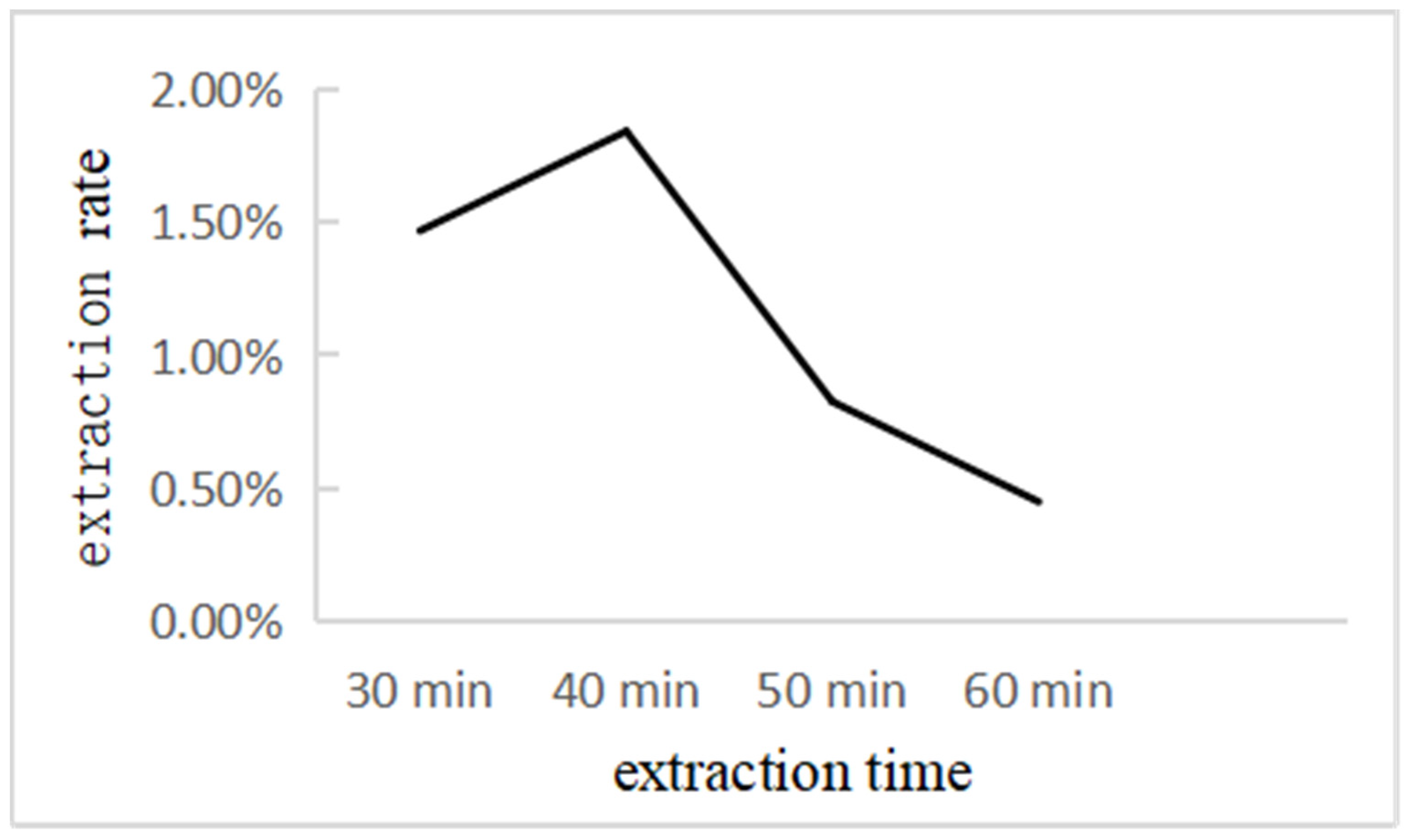
2.4.3. Effect of Extraction Time
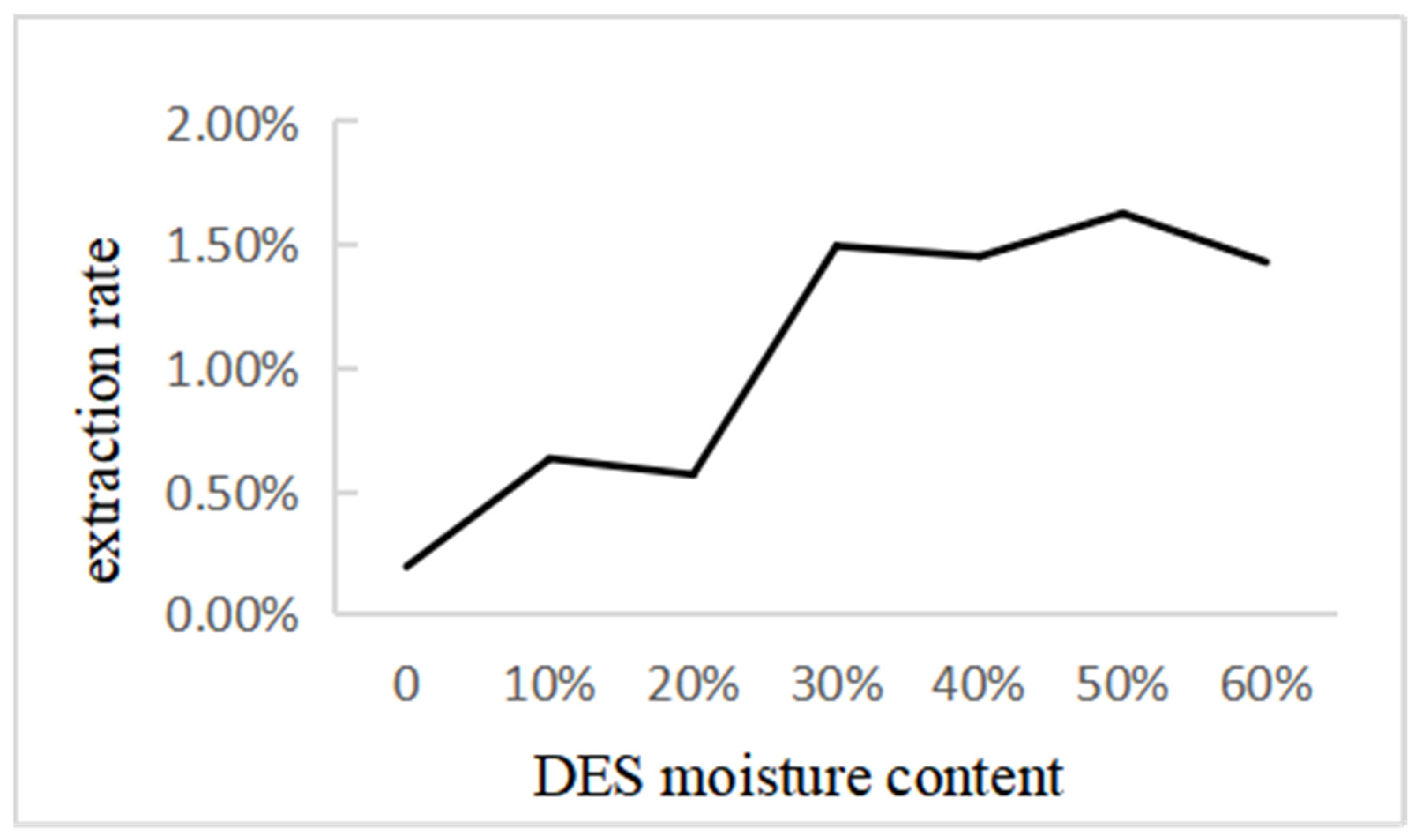
2.4.4. Effect of DES Moisture Content
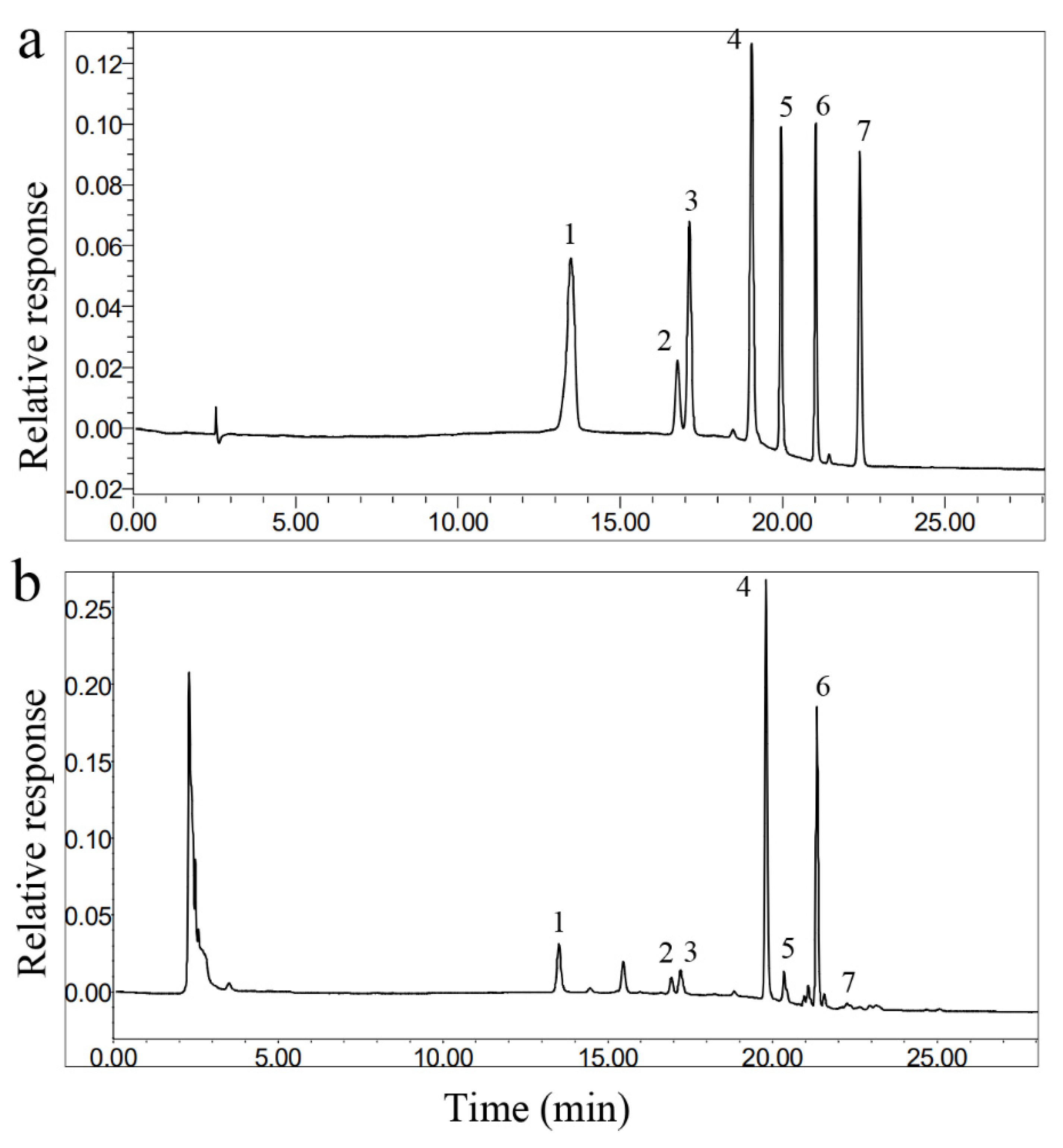
2.5. HPLC Conditions and Method Validation
2.6. Determination of Antioxidant Activity of Plant Extracts
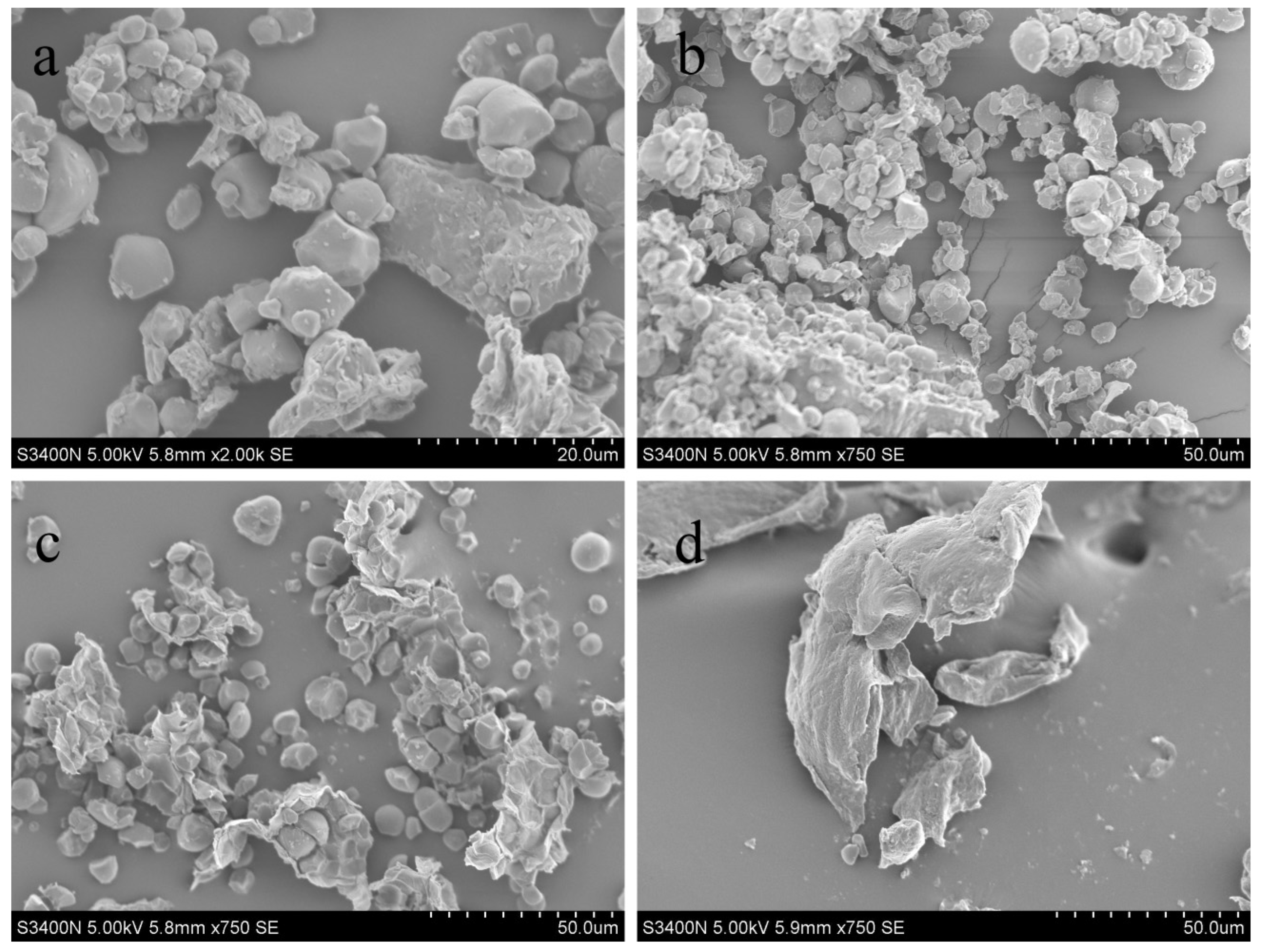
2.7. Microstructure of Plant Material
3. Results and Discussion
3.1. Screening of DESs
3.2. Optimization of the Extraction Conditions by Response Surface Methodology
3.3. Fitting the Response Surface Model
3.4. Verification of Predictive Model
3.5. 3D Response Surface
3.6. Microstructure of Plant Material
3.7. Antioxidant Activity of Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bai, Y.; Li, D.; Zhou, T.; Qin, N.; Li, Z.; Yu, Z.; Hua, H. Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. J. Funct. Foods 2016, 20, 453–462. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, G.; Chen, B.; Xie, Y.; Wu, H.; Wu, Y.; Yan, C.; Wang, J. Separation and quantitative analysis of coumarin compounds from Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook. f by pressurized capillary electrochromatography. J. Pharm. Biomed. Anal. 2006, 41, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, S.S.; Kim, C.M. Coumarin glycosides from the roots of Angelica dahurica. Arch. Pharmacal Res. 1992, 15, 73–77. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Sheng, X.; Yuan, Z.; Yang, W.; Wang, Q.; Zhang, L. Simultaneous characterization and quantitation of 11 coumarins in Radix Angelicae Dahuricae by high performance liquid chromatography with electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010, 51, 599–605. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- Obluchinskaya, E.; Pozharitskaya, O.; Zakharova, L.; Daurtseva, A.; Flisyuk, E.; Shikov, A. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef] [PubMed]
- Boyko, N.; Zhilyakova, E.; Malyutina, A.; Novikov, O.; Pisarev, D.; Abramovich, R.; Potanina, O.; Lazar, S.; Mizina, P.; Sahaidak-Nikitiuk, R. Studying and Modeling of the Extraction Properties of the Natural Deep Eutectic Solvent and Sorbitol-Based Solvents in Regard to Biologically Active Substances from Glycyrrhizae Roots. Molecules 2020, 25, 1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.H.; Yao, X.H.; Zhang, Y.H.; Zhao, C.G.; Zu, Y.G.; Fu, Y.J. Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as Extraction Media for Valuable Flavonoids from Natural Sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.-Y.; Song, J.-N.; Chang, Y.-Q.; Wang, L.; Zheng, Y.-G.; Zhang, D.; Guo, L. Natural Deep Eutectic Solvents for the Extraction of Bioactive Steroidal Saponins from Dioscoreae Nipponicae Rhizoma. Molecules 2021, 26, 2079. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, H.; Tong, M.; Gong, Y. Green and enhanced extraction of coumarins from Cortex Fraxini by ultrasound-assisted deep eutectic solvent extraction. J. Sep. Sci. 2020, 43, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Razboršek, M.I.; Ivanović, M.; Krajnc, P.; Kolar, M. Choline Chloride Based Natural Deep Eutectic Solvents as Extraction Media for Extracting Phenolic Compounds from Chokeberry (Aronia melanocarpa). Molecules 2020, 25, 1619. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Arifianti, A.E.; Sakti, A.S.; Saputri, F.C.; Mun’im, A. Simultaneous Natural Deep Eutectic Solvent-Based Ultrasonic-Assisted Extraction of Bioactive Compounds of Cinnamon Bark and Sappan Wood as a Dipeptidyl Peptidase IV Inhibitor. Molecules 2020, 25, 3832. [Google Scholar] [CrossRef]
- Aryati, W.D.; Nadhira, A.; Febianli, D.; Fransisca, F.; Mun’im, A. Natural deep eutectic solvents ultrasound-assisted extraction (NADES-UAE) of trans-cinnamaldehyde and coumarin from cinnamon bark [Cinnamomum burmannii (Nees T. Nees) Blume]. J. Res. Pharm. 2020, 24, 389–398. [Google Scholar] [CrossRef]
- Chen, L.; Jian, Y.; Wei, N.; Yuan, M.; Zhuang, X.; Li, H. Separation and simultaneous quantification of nine furanocoumarins from Radix Angelicae dahuricae using liquid chromatography with tandem mass spectrometry for bioavailability determination in rats. J. Sep. Sci. 2016, 38, 4216–4224. [Google Scholar] [CrossRef]
- Pfeifer, I.; Murauer, A.; Ganzera, M. Determination of coumarins in the roots of Angelica dahurica by supercritical fluid chromatography. J. Pharm. Biomed. Anal. 2016, 129, 246–251. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.Y.; Li, N.; Zhang, L.J.; Qian, Y. Ultrasound-Assisted Extraction of Imperatorin and Isoimperatorin from Roots of Angelica dahurica. Adv. Mater. Res. 2012, 550–553, 1845–1851. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Hamid, A.A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Al-Haiqi, A. Determination of DPPH free radical scavenging activity: Application of artificial neural networks. Food Chem. 2016, 194, 705–711. [Google Scholar] [CrossRef]
- Bishnoi, A.; Chawla, H.M.; Pant, N.; Mrig, S.; Kumar, S. Evaluation of the radical scavenging activity of resorcinarenes by DPPH• free radical assay. J. Chem. Res. 2010, 34, 440–444. [Google Scholar] [CrossRef]
- Shi, H.; Yang, H.; Zhang, X.; Yu, L. Identification and Quantification of Phytochemical Composition and Anti-inflammatory and Radical Scavenging Properties of Methanolic Extracts of Chinese Propolis. J. Agric. Food Chem. 2012, 60, 12403–12410. [Google Scholar] [CrossRef]
- Xu, Z.; Cai, Y.; Ma, Q.; Zhao, Z.; Yang, D.; Xu, X. Optimization of Extraction of Bioactive Compounds from Baphicacanthus cusia Leaves by Hydrophobic Deep Eutectic Solvents. Molecules 2021, 26, 1729. [Google Scholar] [CrossRef]
- Duan, H.; Zhai, K.F.; Cao, W.G.; Gao, G.Z.; Shan, L.L.; Zhao, L. The Optimum Extraction Process for Radix glycyrrhizae and Angelica dahurica (Fisch.) Benth.et Hook with Orthogonal Design. Indian J. Pharm. Educ. Res. 2017, 51, S631–S636. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.-F.; Jing, N.; Li, Z.-G.; Wei, D.; Lee, M.-R. Ultrasound-Microwave Hybrid-Assisted Extraction Coupled to Headspace Solid-Phase Microextraction for Fast Analysis of Essential Oil in Dry Traditional Chinese Medicine by GC–MS. Chromatographia 2014, 77, 619–628. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, J.; Huang, Y.; Zhang, Y.; Wan, H.; Li, C. Green and Efficient Ultrasonic-Assisted Extraction of Bioactive Components from Salvia miltiorrhiza by Natural Deep Eutectic Solvents. Molecules 2019, 25, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
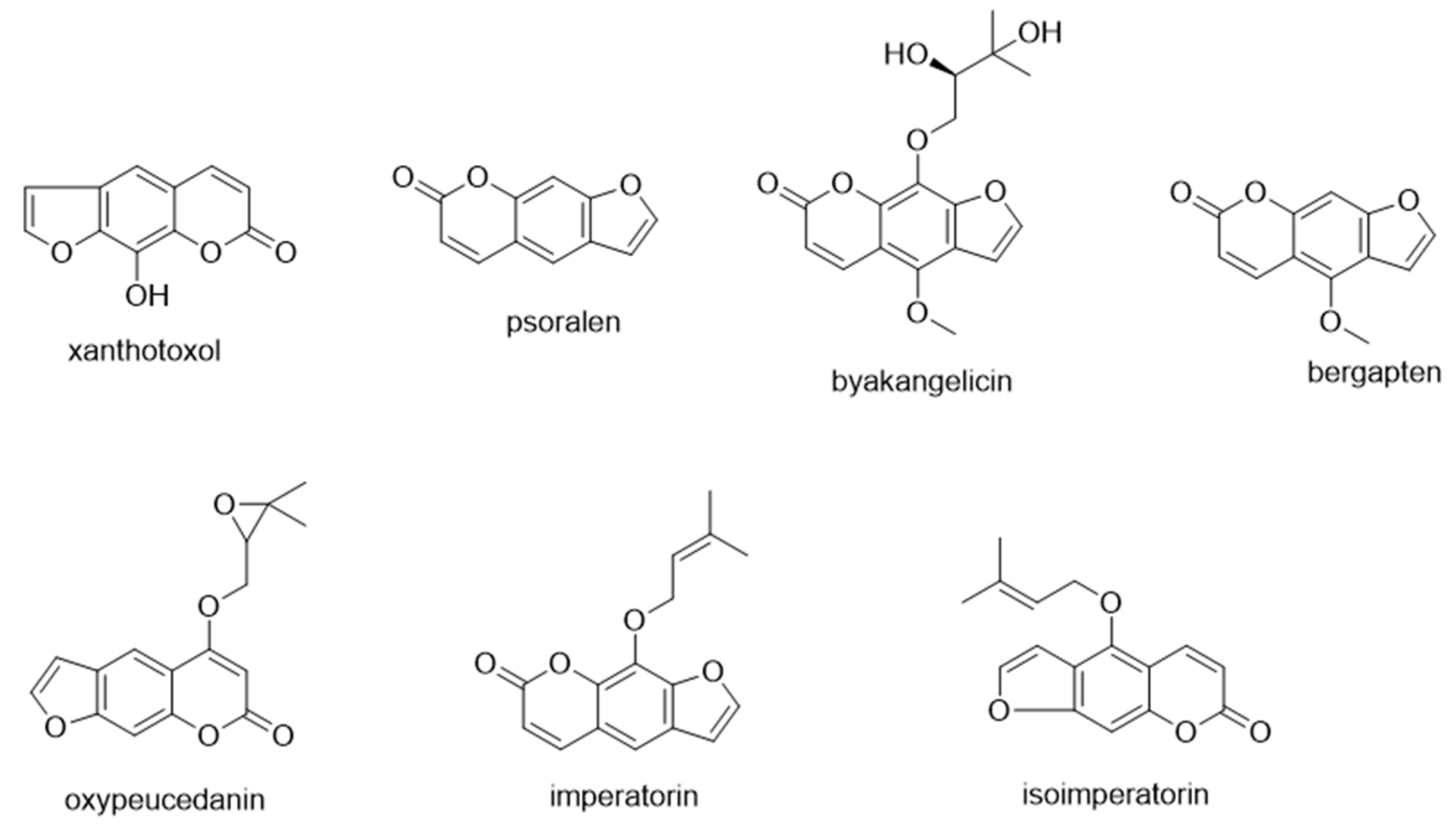




| No. | Hydrogen Bond Acceptors (HBAs) | Hydrogen Bond Donors (HBDs) | HBA/HBD (Water) Ratio | Appearance at RoomTemperature |
|---|---|---|---|---|
| 1 | Choline chloride | Sucrose | 1:1:(2) | Transparent liquid |
| 2 | Choline chloride | Xylitol | 1:1:(1) | Transparent liquid |
| 3 | Choline chloride | Citric acid | 1:1:(2) | Transparent liquid |
| 4 | Choline chloride | D-Glucose | 1:1:(2) | Transparent liquid |
| 5 | Choline chloride | DL-Malic acid | 1:1:(2) | Transparent yellow liquid |
| 6 | Choline chloride | Acetic acid | 1:2 | Transparent liquid |
| 7 | Choline chloride | Lactic acid | 1:3 | Transparent liquid |
| 8 | Choline chloride | Lactic acid | 1:2 | Transparent liquid |
| 9 | Choline chloride | Ethylene glycol | 1:2 | Transparent liquid |
| 10 | Choline chloride | 1,2-Propanediol | 1:2 | Transparent liquid |
| 11 | Choline chloride | 1,4-Butanediol | 1:2 | Viscous liquid |
| 12 | Choline chloride | 1,3-Butanediol | 1:2 | Viscous liquid |
| 13 | Choline chloride | Glycerol | 1:2 | Transparent liquid |
| 14 | Choline chloride | Glycerol | 1:3 | Transparent liquid |
| 15 | Choline chloride | Fructose | 1:2 | Transparent liquid |
| 16 | Choline chloride | Urea | 1:2 | Transparent liquid |
| No. | Analyte | Regression Equation | R2 | Linear Range (mg/mL) | Precision | Repeatability RSD (%) | Stability RSD (%) | |
|---|---|---|---|---|---|---|---|---|
| Intra-Day RSD (%) | Inter-Day RSD (%) | |||||||
| 1 | Xanthotoxol | Y = 9286.6X + 43348 | 0.9948 | 0.040–0.200 | 0.26 | 1.46 | 1.13 | 0.22 |
| 2 | Psoralen | Y = 2232.3X − 8340.4 | 0.9998 | 0.036–0.180 | 0.54 | 1.74 | 1.37 | 1.23 |
| 3 | Byakangelicin | Y = 5192.5X − 3041.4 | 0.9996 | 0.040–0.200 | 0.47 | 1.62 | 0.57 | 1.09 |
| 4 | Bergapten | Y = 8584.5X + 21114 | 0.9998 | 0.040–0.200 | 0.83 | 2.13 | 0.47 | 0.68 |
| 5 | Oxypeucedanin | Y = 4801.5X + 2149.1 | 0.9996 | 0.040–0.200 | 1.24 | 1.82 | 0.44 | 0.34 |
| 6 | Imperatorin | Y = 4442.6X − 7511.1 | 0.9999 | 0.040–0.200 | 0.20 | 1.78 | 0.45 | 0.94 |
| 7 | Isoimperatorin | Y = 5522.3X − 10479 | 0.9999 | 0.040–0.200 | 0.23 | 1.57 | 0.47 | 0.95 |
| Factors | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A Liquid–solid ratio (mg/mL) | 10 | 20 | 30 |
| B Extraction temperature (°C) | 40 | 50 | 60 |
| C Extraction time (min) | 30 | 40 | 50 |
| D DES moisture content (%) | 40 | 50 | 60 |
| Run | Liquid–Solid Ratio (A, mL/g) | Extraction Temperature (B, °C) | Extraction Time (C, min) | DES Moisture Content (D, %) | Total Extraction Yields (mg/g) |
|---|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 0 | 0.92 |
| 2 | 0 | 0 | 1 | −1 | 1.17 |
| 3 | 0 | 0 | −1 | 1 | 1.07 |
| 4 | −1 | 0 | 1 | 0 | 1.13 |
| 5 | −1 | 0 | 0 | −1 | 1.07 |
| 6 | 0 | 1 | 1 | 0 | 1.31 |
| 7 | 0 | 0 | 0 | 0 | 1.02 |
| 8 | 0 | 0 | 1 | 1 | 1.24 |
| 9 | −1 | 1 | 0 | 0 | 1.26 |
| 10 | 1 | 0 | 0 | −1 | 0.5 |
| 11 | 1 | 0 | 1 | 0 | 0.71 |
| 12 | 1 | 0 | −1 | 0 | 0.75 |
| 13 | 0 | 1 | −1 | 0 | 1.26 |
| 14 | 0 | 0 | −1 | −1 | 0.88 |
| 15 | 0 | 1 | 0 | −1 | 0.46 |
| 16 | 0 | 0 | 0 | 0 | 0.94 |
| 17 | 1 | 0 | 0 | 1 | 1.07 |
| 18 | 0 | 0 | 0 | 0 | 0.88 |
| 19 | 0 | −1 | 0 | 1 | 0.95 |
| 20 | 0 | 1 | 0 | 1 | 1.04 |
| 21 | −1 | 0 | −1 | 0 | 0.95 |
| 22 | 1 | −1 | 0 | 0 | 0.68 |
| 23 | 0 | −1 | 0 | −1 | 0.52 |
| 24 | 1 | 1 | 0 | 0 | 0.85 |
| 25 | 0 | −1 | 1 | 0 | 1.19 |
| 26 | −1 | 0 | 0 | 1 | 0.98 |
| 27 | −1 | −1 | 0 | 0 | 0.89 |
| 28 | 0 | 0 | 0 | 0 | 1.09 |
| 29 | 0 | 0 | 0 | 0 | 1.11 |
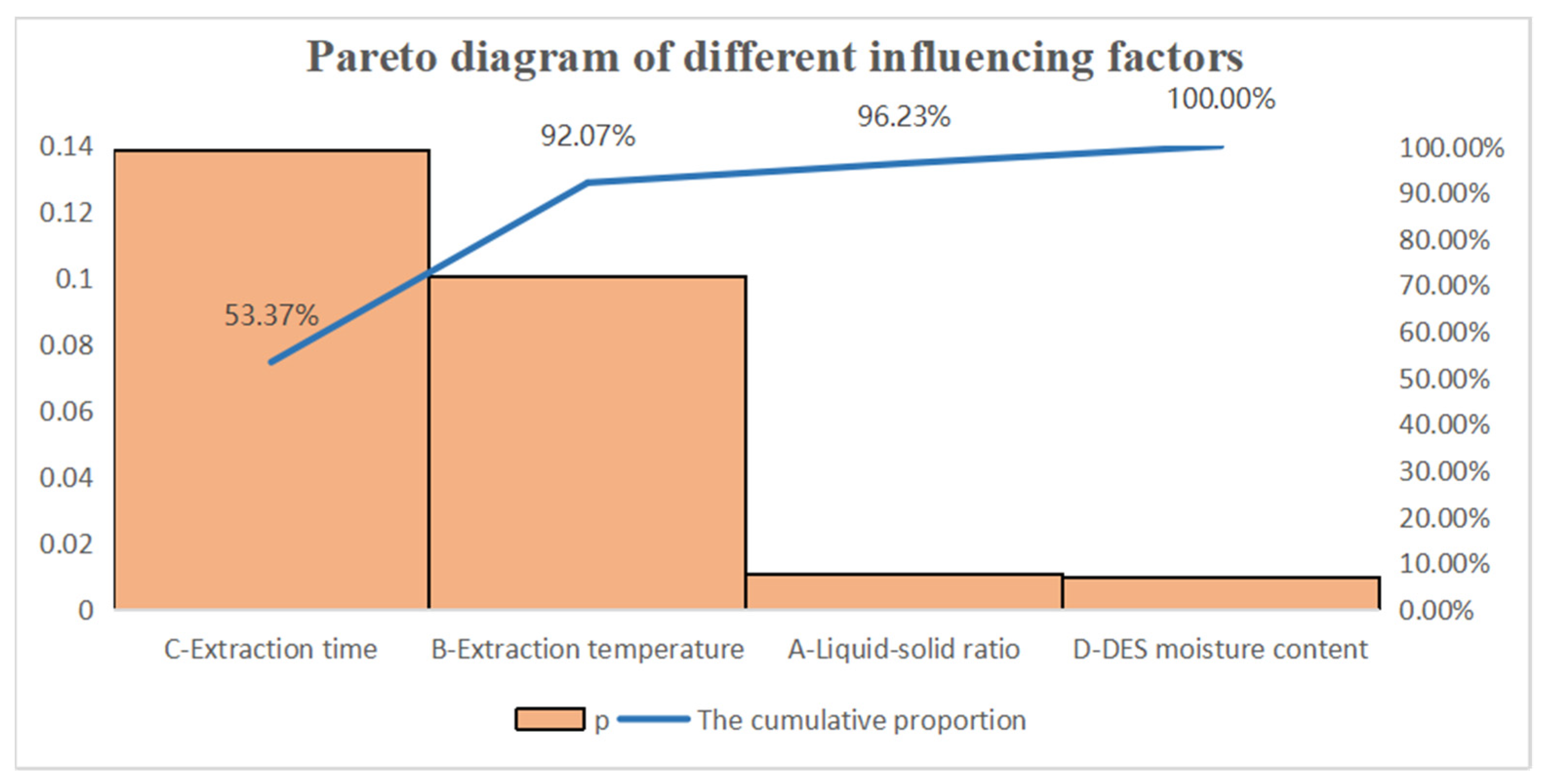
| Variables | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1.06 | 14 | 0.076 | 2.66 | 0.0388 | significant |
| A-Liquid-solid ratio | 0.25 | 1 | 0.25 | 8.62 | 0.0108 | |
| B-Extraction temperature | 0.088 | 1 | 0.088 | 3.09 | 0.1005 | |
| C-Extraction time | 0.071 | 1 | 0.071 | 2.47 | 0.1386 | |
| D-DES moisture content | 0.26 | 1 | 0.26 | 8.93 | 0.0098 | |
| AB | 0.010 | 1 | 0.010 | 0.35 | 0.5636 | |
| AC | 0.012 | 1 | 0.012 | 0.42 | 0.5258 | |
| AD | 0.11 | 1 | 0.11 | 3.81 | 0.0713 | |
| BC | 0.012 | 1 | 0.012 | 0.42 | 0.5258 | |
| BD | 0.006 | 1 | 0.006 | 0.20 | 0.6641 | |
| CD | 0.004 | 1 | 0.004 | 0.13 | 0.7280 | |
| A2 | 0.066 | 1 | 0.066 | 2.32 | 0.1501 | |
| B2 | 0.010 | 1 | 0.010 | 0.36 | 0.5581 | |
| C2 | 0.088 | 1 | 0.088 | 3.08 | 0.1013 | |
| D2 | 0.049 | 1 | 0.049 | 1.73 | 0.2095 | |
| Residual | 0.40 | 14 | 0.029 | |||
| Lake of fit | 0.36 | 10 | 0.036 | 3.78 | 0.1058 | Not significant |
| R2 | 0.974 | |||||
| Adj R2 | 0.817 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Li, Q. DES Based Efficient Extraction Method for Bioactive Coumarins from Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. Separations 2022, 9, 5. https://doi.org/10.3390/separations9010005
Wang T, Li Q. DES Based Efficient Extraction Method for Bioactive Coumarins from Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. Separations. 2022; 9(1):5. https://doi.org/10.3390/separations9010005
Chicago/Turabian StyleWang, Ting, and Qian Li. 2022. "DES Based Efficient Extraction Method for Bioactive Coumarins from Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav." Separations 9, no. 1: 5. https://doi.org/10.3390/separations9010005
APA StyleWang, T., & Li, Q. (2022). DES Based Efficient Extraction Method for Bioactive Coumarins from Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. Separations, 9(1), 5. https://doi.org/10.3390/separations9010005






