Cross-Linking Combined with Surfactant Bilayer Assembly Enhances the Hydrophilic and Antifouling Properties of PTFE Microfiltration Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
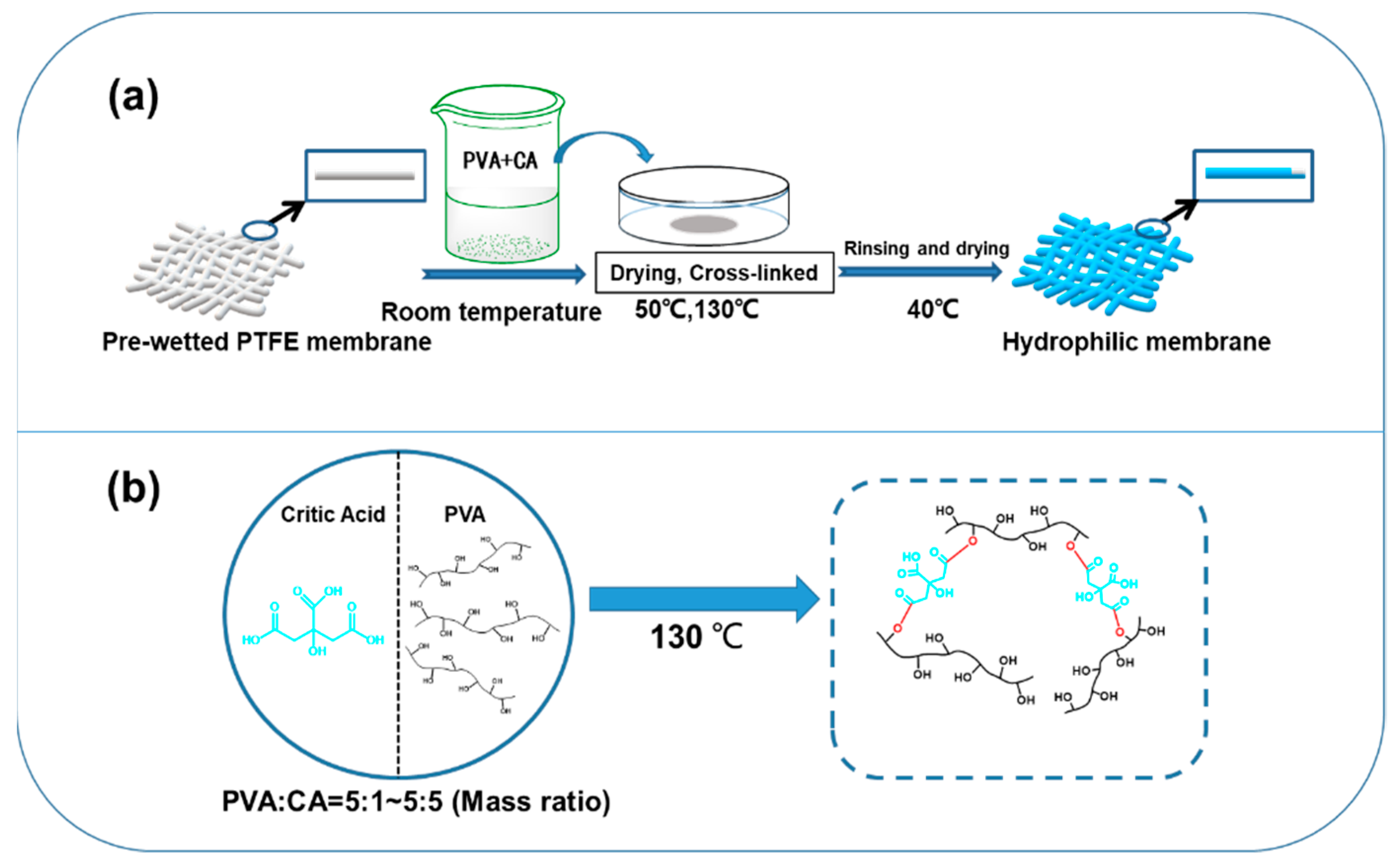
2.2. Preparation of PTFE-Modified Membranes
2.3. Characterization of PTFE Microfiltration Membrane Structure and Surface Chemical Composition
2.4. Surface Wettability and Pure Water Flux Testing of PTFE Microfiltration Membranes
2.5. Anti-Fouling Performance Test of PTFE Microfiltration Membrane
3. Results
3.1. Surface Chemical Composition and Microscopic Morphology Analysis
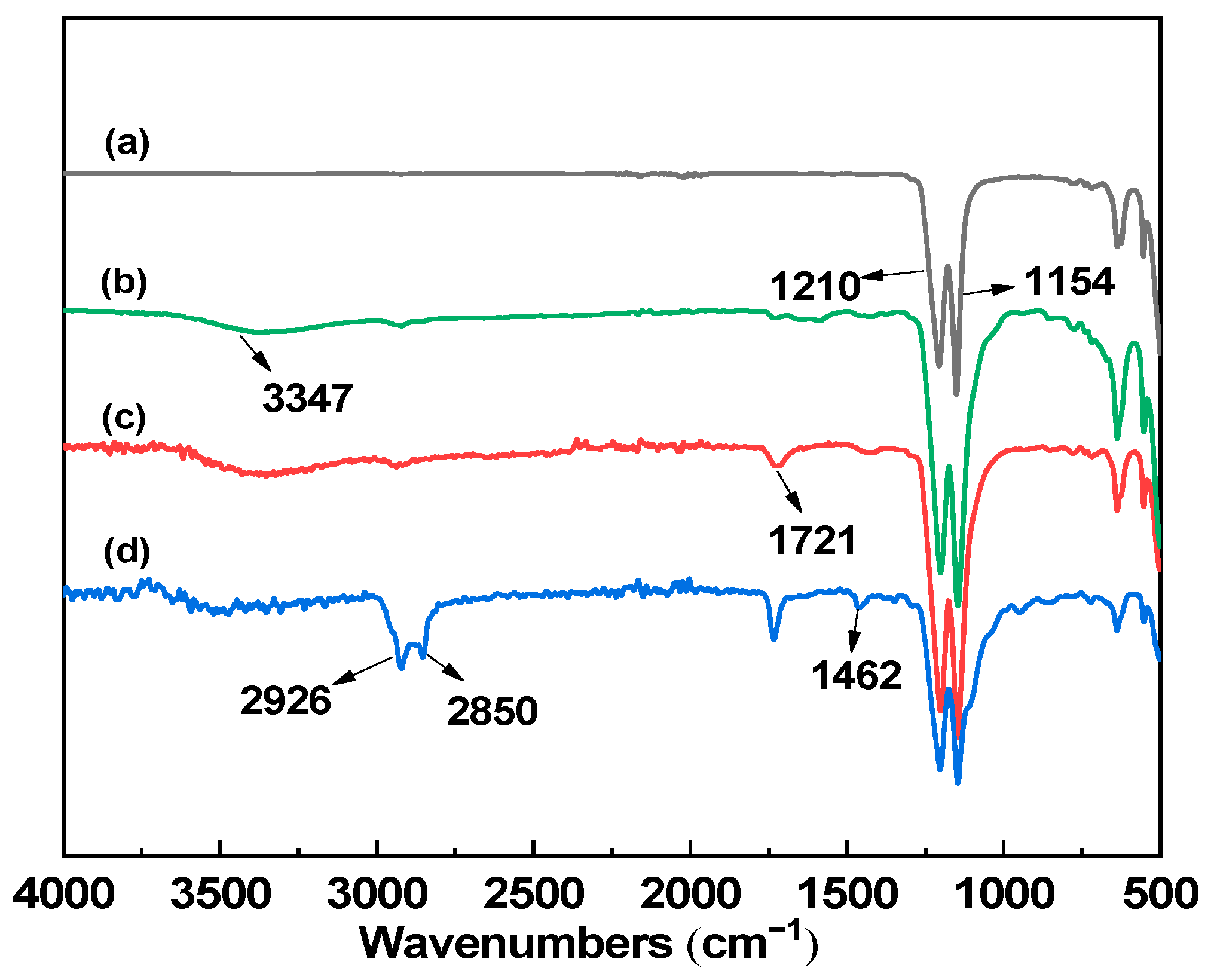
3.1.1. FTIR Analysis
3.1.2. SEM Analysis
3.1.3. Surface Roughness Analysis
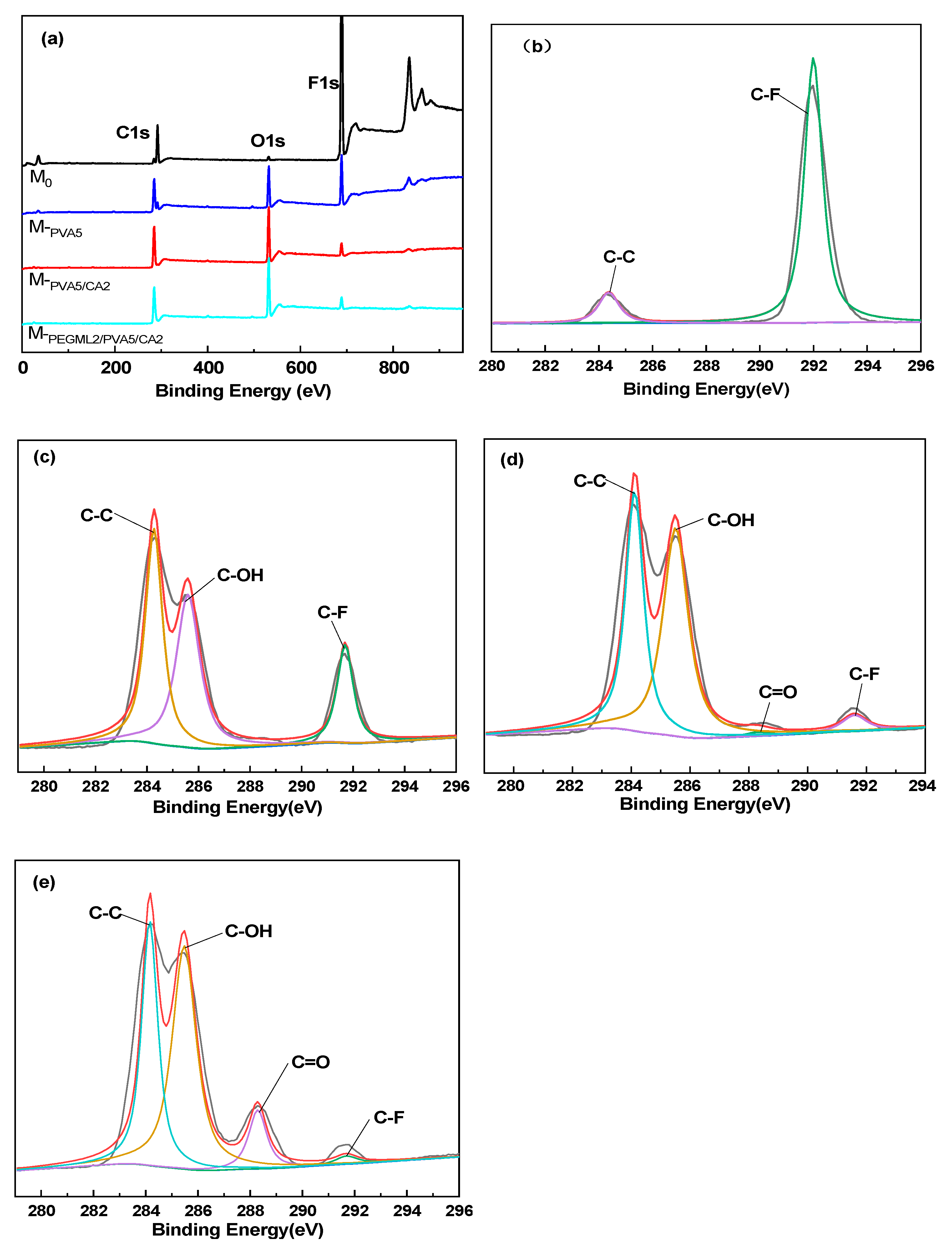
3.1.4. XPS Analysis
3.2. Optimization of Hydrophilic Modification Conditions
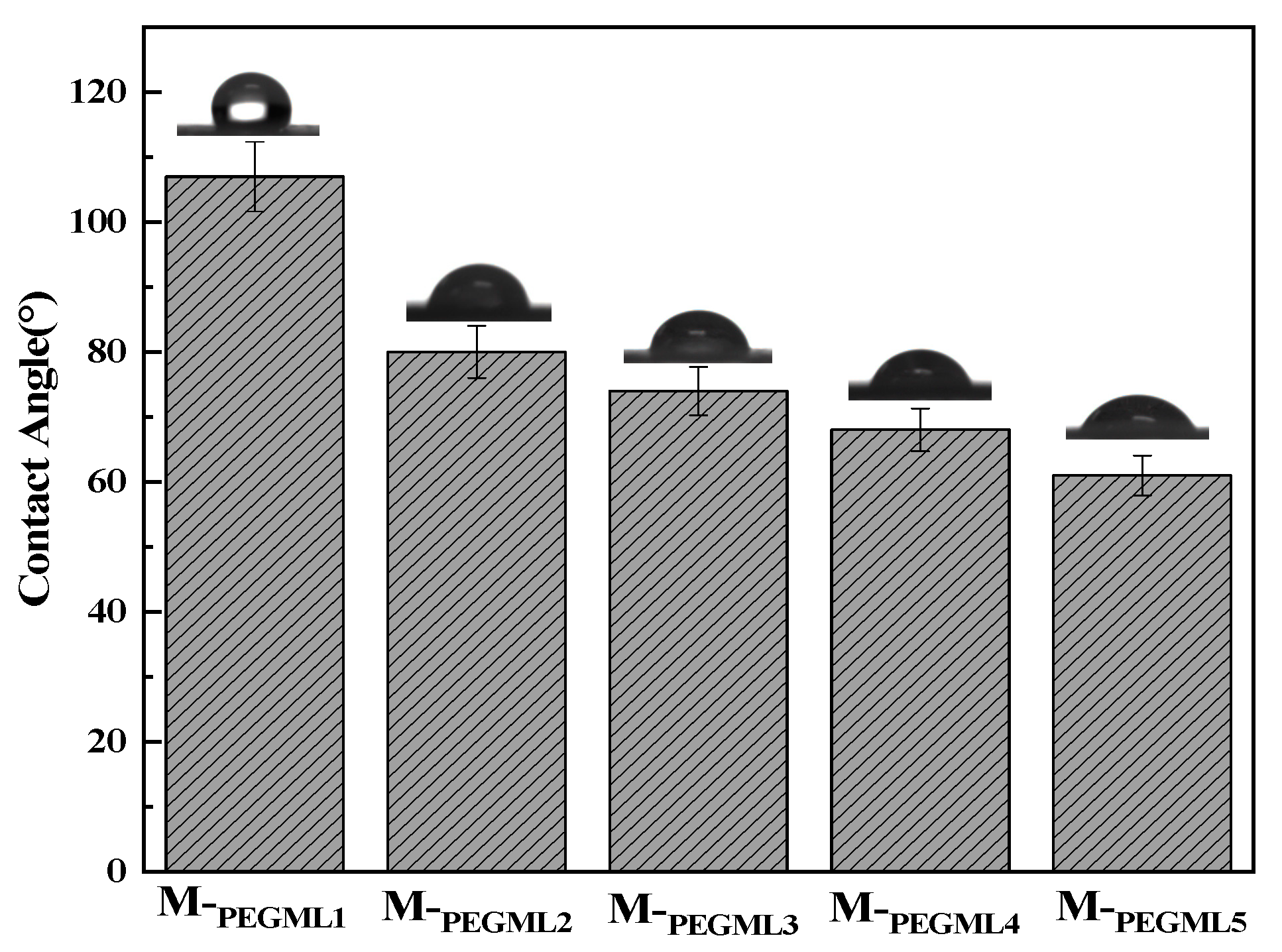
3.2.1. Effect of PEGML Concentration on the Water Contact Angle of PTFE Microfiltration Membrane
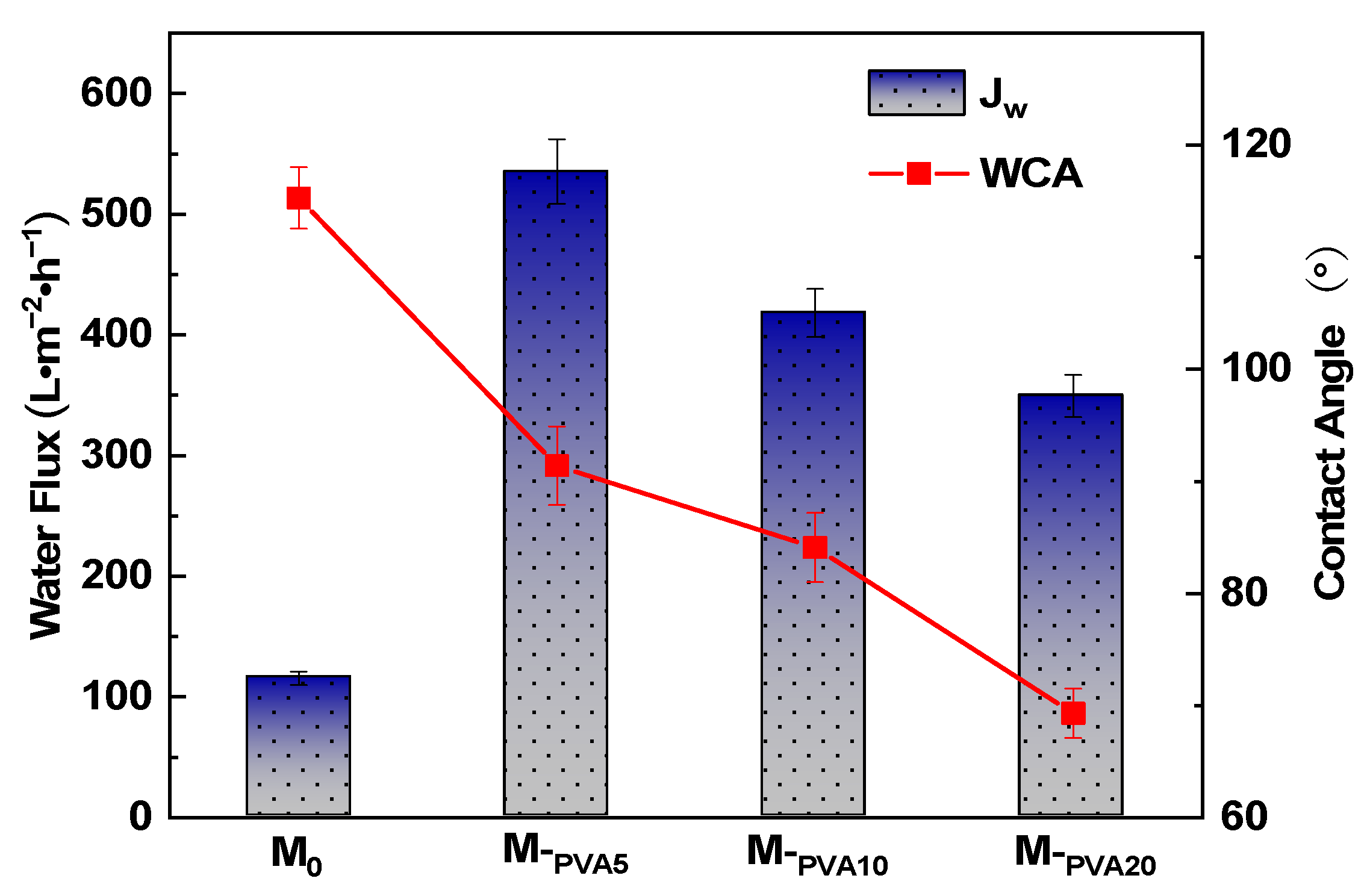
3.2.2. Effect of PVA Content on Hydrophilic Properties of PTFE
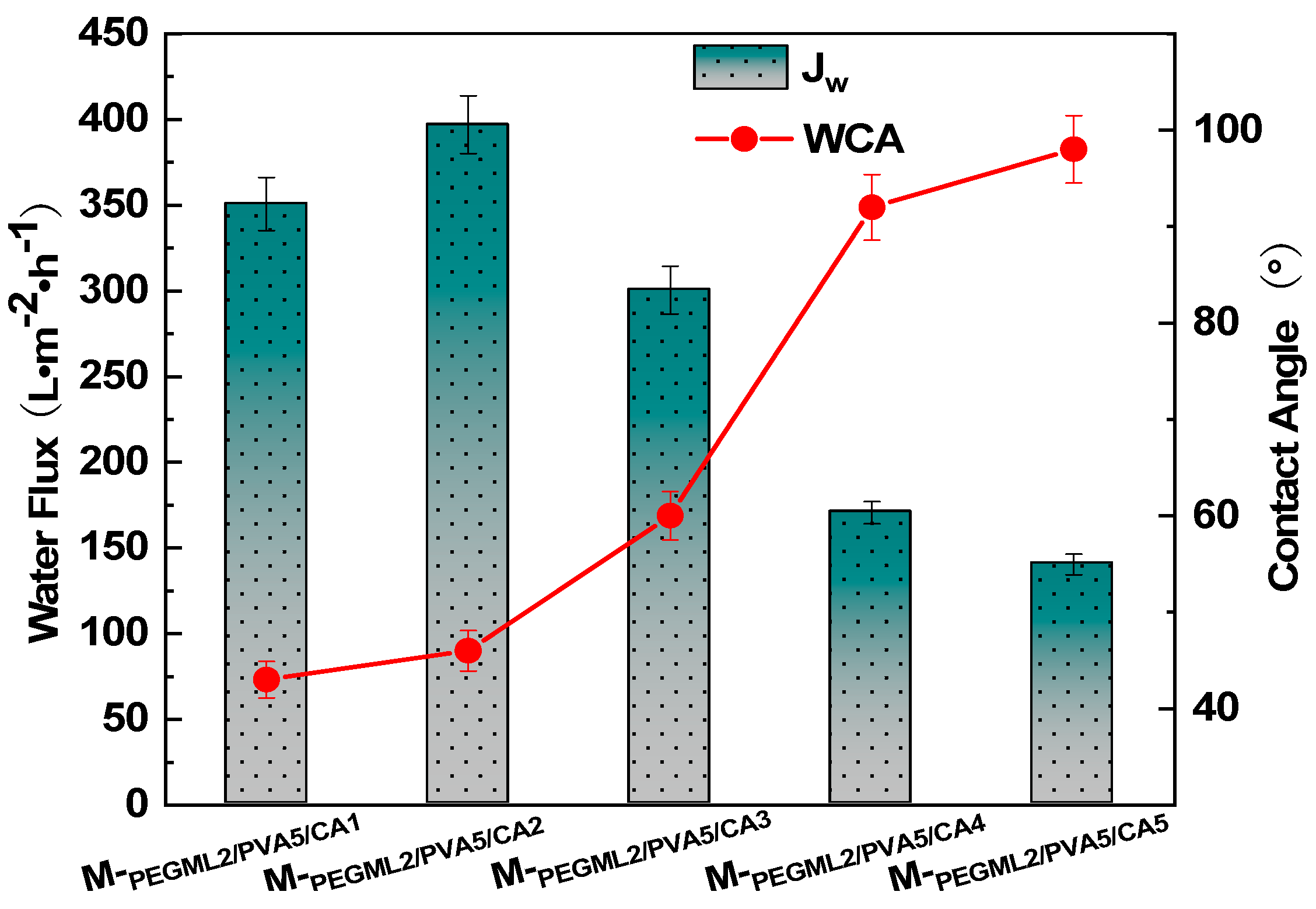
3.2.3. Effect of Crosslinker Content on the Hydrophilic Properties of PTFE
3.3. PTFE Microfiltration Membrane Performance Test
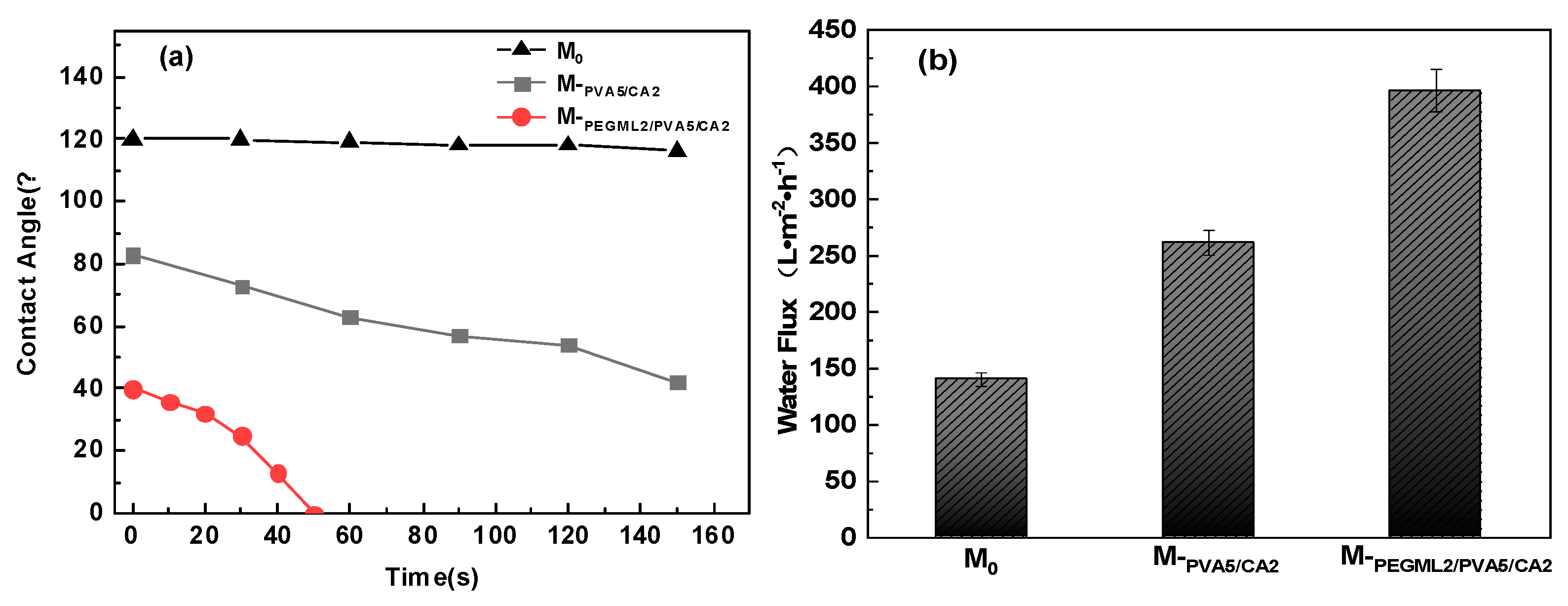
3.3.1. Hydrophilic Performance Test
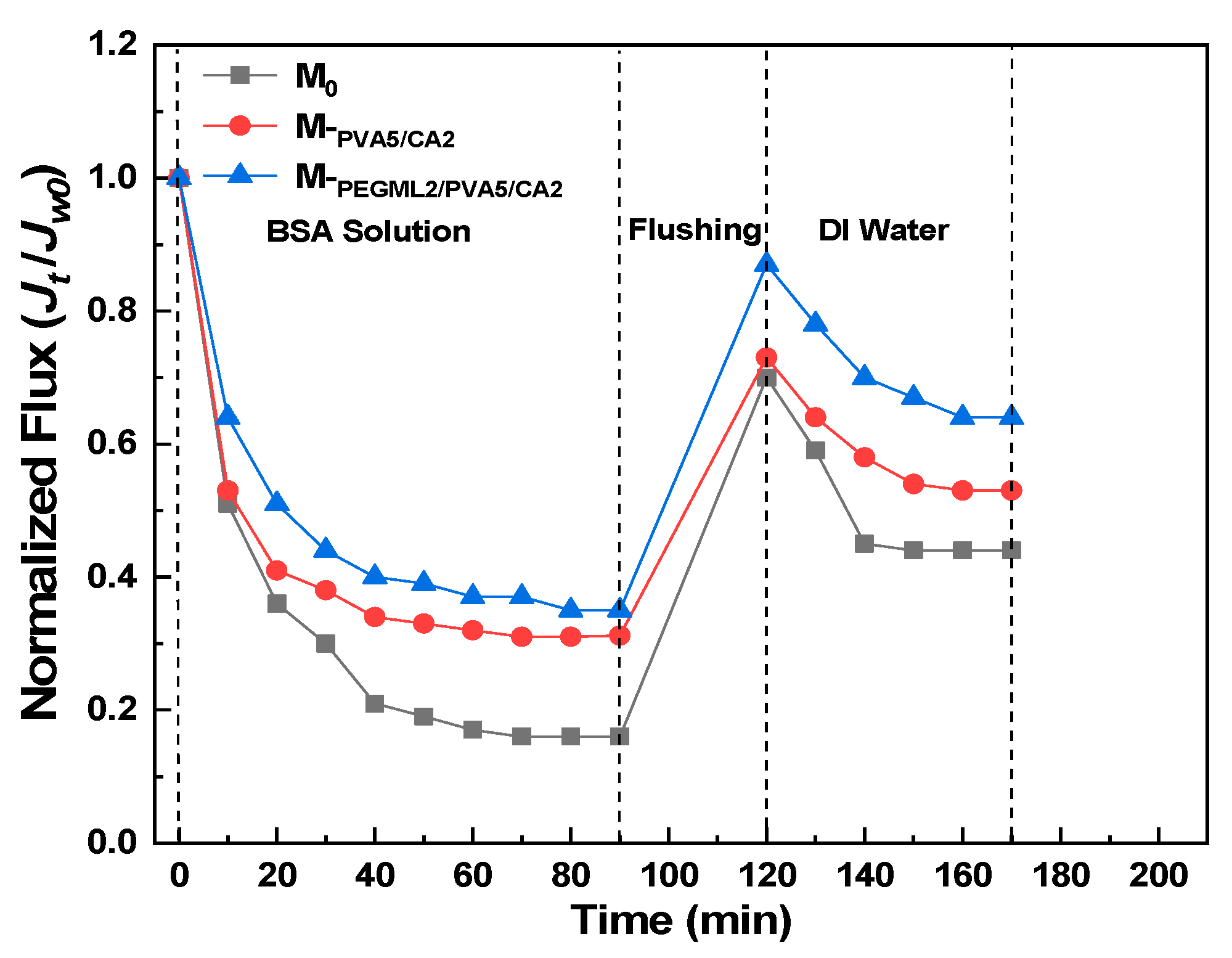
3.3.2. BSA Solution Filtration Performance Test
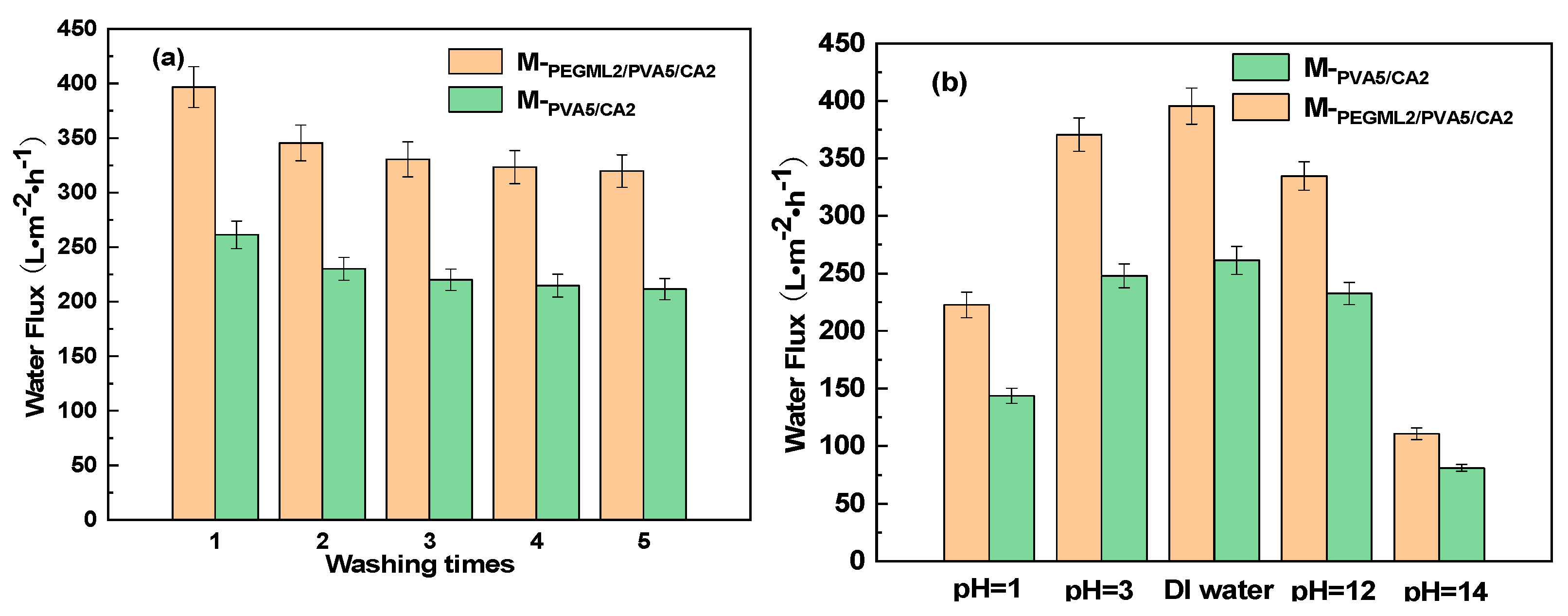
3.3.3. Hydrophilic Coating Stability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Shan, H.; Cao, M.; Li, B. Mussel-inspired modification of PTFE membranes in a miscible THF-Tris buffer mixture for oil-in-water emulsions separation. J. Membr. Sci. 2018, 555, 237–249. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, K.-Y.; Lee, Y.-S.; Nam, J.-W.; Lee, Y.-S.; Kim, H.-S.; Jang, A. A study on the high-flux MBR system using PTFE flat sheet membranes with chemical backwashing. Desalination 2012, 306, 35–40. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Wang, F.; Zhu, H.; Guo, Y.; Chen, M. Laccase-catalyzed homo-polymer of GAL and cross-linking with PEI to enhance hydrophilicity and antifouling property of PTFE flat membrane. Prog. Org. Coat. 2019, 132, 429–439. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Li, J.; Zhu, H.; Guo, Y. A catechol-based biomimetic strategy combined with surface mineralization to enhance hydrophilicity and anti-fouling property of PTFE flat membrane. J. Membr. Sci. 2017, 524, 409–418. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Zhang, M.; Zhao, H.; Jiang, Y.; Gao, J. Rapid preparation of Tannic acid (TA) based zwitterionic nanofiltration membrane via a multiple layer-by-layer (mLBL) assembly strategy for enhanced antifouling performance. Sep. Purif. Technol. 2020, 253, 117519. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H.Q. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, J.; Suo, J.; Luo, T. Surface modification of porous poly(tetrafluoraethylene) film by a simple chemical oxidation treatment. Appl. Surf. Sci. 2010, 256, 2293–2298. [Google Scholar] [CrossRef]
- Liu, K.; Lei, J.; Zheng, Z.; Zhu, Z.; Liu, S. The hydrophilicity improvement of polytetrafluoroethylene by Ar plasma jet: The relationship of hydrophilicity, ambient humidity and plasma parameters. Appl. Surf. Sci. 2018, 458, 183–190. [Google Scholar] [CrossRef]
- Hidzir, N.M.; Lee, Q.; Hill, D.J.T.; Rasoul, F.; Grondahl, L. Grafting of Acrylic Acid-co-Itaconic Acid onto ePTFE and Characterization of Water Uptake by the Graft Copolymers. J. Appl. Polym. Sci. 2015, 132, 41482. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Luo, Y.; Wang, F.; Zhu, H.; Guo, Y. One-bath two step method combined surface micro/nanostructures treatment to enhance antifouling and antibacterial property of PTFE flat membrane. J. Taiwan Inst. Chem. E 2019, 96, 639–651. [Google Scholar] [CrossRef]
- Liu, W.; Lin, H.; Wang, J.; Han, Q.; Liu, F. Polytetrafluoroethylene (PTFE) hollow fibers modified by hydrophilic crosslinking network (HCN) for robust resistance to fouling and harsh chemical cleaning. J. Membr. Sci. 2021, 630, 119301. [Google Scholar] [CrossRef]
- Song, H.M.; Yu, H.W.; Zhu, L.J.; Xue, L.X.; Wu, D.C.; Chen, H. Durable hydrophilic surface modification for PTFE hollow fiber membranes. React. Funct. Polym. 2017, 114, 110–117. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Wang, F.; Yu, B.; Luo, Y.; Zeng, H.; Zhu, H. Hydrophilic modification of PTFE microfiltration flat membrane by crosslinking OCMCS-PEI to enhance anti-fouling property. Prog. Org. Coat. 2019, 135, 565–573. [Google Scholar] [CrossRef]
- Bell, D.J.; Ludwanowski, S.; Luken, A.; Sarikaya, B.; Walther, A.; Wessling, M. Hydrogel membranes made from crosslinked microgel multilayers with tunable density. J. Membr. Sci. 2021, 620, 118912. [Google Scholar] [CrossRef]
- Topuz, F.; Holtzl, T.; Szekely, G. Scavenging organic micropollutants from water with nanofibrous hypercrosslinked cyclodextrin membranes derived from green resources. Chem. Eng. J. 2021, 419, 129443. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Hardian, R.; Szekely, G. Waltzing around the stereochemistry of membrane crosslinkers for precise molecular sieving in organic solvents. J. Membr. Sci. 2021, 638, 119724. [Google Scholar] [CrossRef]
- Chisca, S.; Bettahalli, N.M.S.; Musteata, V.E.; Vasylevskyi, S.; Hedhili, M.N.; Abou-Hamad, E.; Karunakaran, M.; Genduso, G.; Nunes, S.P. Thermal treatment of hydroxyl functionalized polytriazole and its effect on gas transport: From crosslinking to carbon molecular sieve. J. Membr. Sci. 2022, 642, 119963. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, B.; Fu, Z.; Li, J.; Yu, M.; Li, L.; Li, J. Poly (vinyl alcohol) modification of poly(vinylidene fluoride) microfiltration membranes for oil/water emulsion separation via an unconventional radiation method. J. Membr. Sci. 2021, 619, 118792. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Wang, F.; Zhu, H.; Guo, Y.; Chen, M. PVA and CS cross-linking combined with in situ chimeric SiO2 nanoparticle adhesion to enhance the hydrophilicity and antibacterial properties of PTFE flat membranes. RSC Adv. 2019, 9, 19205–19216. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ren, J.L.; Li, W.Y.; Sun, R.C.; Liu, S.J. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Biswal, N.R.; Paria, S. Wetting of TX-100 and lgepal CO-630 Surfactants on a PTFE Surface. Ind. Eng. Chem. Res. 2011, 50, 6138–6145. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Janczuk, B.; Wojcik, W. The wettability of polytetrafluoroethylene by aqueous solutions of sodium dodecyl sulfate and propanol mixtures. J. Colloid Interface Sci. 2005, 281, 465–472. [Google Scholar] [CrossRef]
- Szymczyk, K. Wetting and Adsorption Properties of Aqueous Solutions of Ternary Mixtures of Hydrocarbon and Fluorocarbon Nonionic Surfactants in PTFE-Solution-Air Systems. Ind. Eng. Chem. Res. 2013, 52, 9106–9114. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Sunayana, S.; Paria, S. Wettability of a PTFE surface by cationic-non-ionic surfactant mixtures in the presence of electrolytes. Soft Matter 2012, 8, 5429–5433. [Google Scholar] [CrossRef]
- Chen, X.R.; Su, Y.; Shen, F.; Wan, Y.H. Antifouling ultrafiltration membranes made from PAN-b-PEG copolymers: Effect of copolymer composition and PEG chain length. J. Membr. Sci. 2011, 384, 44–51. [Google Scholar] [CrossRef]
- Ding, X.L.; Kang, T.; Zhao, H.Y.; Wang, Q.; Zhang, Y.Z.; Kang, G.D. Antifouling behavior on the linear suspension PEG-based surface and two-end-linked PEG-based surface of composite nanofiltration membranes via two-step interfacial polymerization. Desalination Water Treat. 2019, 144, 9–25. [Google Scholar] [CrossRef]
- Khoiroh, I.; Lee, S.Y.; Pirdashti, M.; Lee, M.J. Insight into structural properties of polyethylene glycol monolaurate in water and alcohols from molecular dynamics studies. RSC Adv. 2020, 10, 21760–21771. [Google Scholar] [CrossRef]
- Liu, S.; Cui, S.; Qin, Z.; Zhang, X.; Zhao, Y.; Zhao, Y.; Guo, H. Modification of a Poly(tetrafluoroethylene) Porous Membrane to Superhydrophilicity with Improved Durability. Chem. Eng. Technol. 2019, 42, 1027–1036. [Google Scholar] [CrossRef]
- Shafiei, M.; Hajian, M. Poly(vinyl butyral)/zeolitic imidazole framework-8/poly(vinyl alcohol) thin-film nanocomposite nanofiltration membrane: Synthesis and characterization. Iran Polym. J. 2019, 28, 659–672. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B 2020, 188, 110757. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, V.S.; Dias, R.J.; Mali, K.K.; Mulla, S.I. Citric acid crosslinked carboxymethylcellulose-polyvinyl alcohol hydrogel films for extended release of water soluble basic drugs. J. Drug Deliv. Sci. Technol. 2019, 52, 421–430. [Google Scholar] [CrossRef]
- Wang, J.Q.; Dai, Q.L.; Si, R.Z.; Guo, S.C. Investigation of properties and performances of Polyvinyl Alcohol (PVA) fiber-reinforced rubber concrete. Constr. Build. Mater. 2018, 193, 631–642. [Google Scholar] [CrossRef]
- Popescu, M.C. Structure and sorption properties of CNC reinforced PVA films. Int. J. Biol. Macromol. 2017, 101, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Huisman, I.H.; Pradanos, P.; Hernandez, A. The effect of protein-protein and protein-membrane interactions on membrane fouling in ultrafiltration. J. Membr. Sci. 2000, 179, 79–90. [Google Scholar] [CrossRef]
- Zhou, R.; Ren, P.F.; Yang, H.C.; Xu, Z.K. Fabrication of antifouling membrane surface by poly(sulfobetaine methacrylate)/polydopamine co-deposition. J. Membr. Sci. 2014, 466, 18–25. [Google Scholar] [CrossRef]
- Chi, L.N.; Qian, Y.J.; Guo, J.Q.; Wang, X.Z.; Arandiyan, H.; Jiang, Z. Novel g-C3N4/TiO2/PAA/PTFE ultrafiltration membrane enabling enhanced antifouling and exceptional visible-light photocatalytic self-cleaning. Catal. Today 2019, 335, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Qian, Y.; Chi, L.; Zhou, W.; Yu, Z.; Zhang, Z.; Zhang, Z.; Jiang, Z. Fabrication of TiO2-modified polytetrafluoroethylene ultrafiltration membranes via plasma-enhanced surface graft pretreatment. Appl. Surf. Sci. 2016, 360, 749–757. [Google Scholar] [CrossRef] [Green Version]


| Code Name | PEGML(g·L−1) | PVA(g·L−1) | CA(g·L−1) |
|---|---|---|---|
| M0 | 0 | 0 | 0 |
| M-PEGML1 | 1 | 0 | 0 |
| M-PEGML2 | 2 | 0 | 0 |
| M-PEGML3 | 3 | 0 | 0 |
| M-PEGML4 | 4 | 0 | 0 |
| M-PEGML5 | 5 | 0 | 0 |
| M-PVA5 | 0 | 5 | 0 |
| M-PVA10 | 0 | 10 | 0 |
| M-PVA15 | 0 | 15 | 0 |
| M-PVA20 | 0 | 20 | 0 |
| M-PEGML2/PVA5/CA1 | 2 | 5 | 1 |
| M-PEGML2/PVA5/CA2 | 2 | 5 | 2 |
| M-PEGML2/PVA5/CA3 | 2 | 5 | 3 |
| M-PEGML2/PVA5/CA4 | 2 | 5 | 4 |
| M-PEGML2PVA5/CA5 | 2 | 5 | 5 |
| M-PVA5/CA2 | 0 | 5 | 2 |
| Samples | Sq (µm) | Sa (µm) |
|---|---|---|
| M0 | 3.3 | 2.6 |
| M-PVA5 | 4 | 3.2 |
| M-PVA5/CA2 | 4.3 | 3.4 |
| M-PEGML2/PVA5/CA2 | 3.8 | 3 |
| Samples | Content (%) | ||
|---|---|---|---|
| C | F | O | |
| M0 | 31.57 | 66.9 | 1.53 |
| M-PVA5 | 58.53 | 19.73 | 21.74 |
| M-PVA5/CA2 | 64.54 | 5.75 | 29.72 |
| M-PEGML2/PVA5/CA2 | 61.7 | 4.89 | 33.4 |
| Membrane | PWF (L·m−2·h−1) | Operating Pressure (Mpa) | Modification Method | |
|---|---|---|---|---|
| PTFE (0.22 μm) | 397 | 0.05 | PEGML/PVA Co-deposition | This work |
| PTFE (0.5 μm) | 830 | 0.1 | Grafting gC3N4/TiO2/PAA after plasma treatment | [36] |
| PVDF (0.22 μm) | 785 | 0.09 | PDA/KH550/TiO2 Co-deposition | [37] |
| PTFE (0.2 μm) | 175 | 0.01 | P(VP-VTES) Crosslinking | [12] |
| PTFE (0.5 μm) | 750 | 0.1 | Grafting TiO2/PAA after plasma treatment | [38] |
| Samples | FRR (%) | DRt (%) | DRir (%) | Adsorption Quantity (g/m2) |
|---|---|---|---|---|
| M0 | 44 | 83 | 56 | 20.94 |
| M-PVA5/CA2 | 53 | 69 | 47 | 6.25 |
| M-PEGML2/PVA5/CA2 | 64 | 65 | 36 | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Ma, W.; Yang, H.; Cao, Z.; Gong, F.; Liu, C. Cross-Linking Combined with Surfactant Bilayer Assembly Enhances the Hydrophilic and Antifouling Properties of PTFE Microfiltration Membranes. Separations 2022, 9, 2. https://doi.org/10.3390/separations9010002
Xu S, Ma W, Yang H, Cao Z, Gong F, Liu C. Cross-Linking Combined with Surfactant Bilayer Assembly Enhances the Hydrophilic and Antifouling Properties of PTFE Microfiltration Membranes. Separations. 2022; 9(1):2. https://doi.org/10.3390/separations9010002
Chicago/Turabian StyleXu, Shijie, Wenzhong Ma, Haicun Yang, Zheng Cao, Fanghong Gong, and Chunlin Liu. 2022. "Cross-Linking Combined with Surfactant Bilayer Assembly Enhances the Hydrophilic and Antifouling Properties of PTFE Microfiltration Membranes" Separations 9, no. 1: 2. https://doi.org/10.3390/separations9010002
APA StyleXu, S., Ma, W., Yang, H., Cao, Z., Gong, F., & Liu, C. (2022). Cross-Linking Combined with Surfactant Bilayer Assembly Enhances the Hydrophilic and Antifouling Properties of PTFE Microfiltration Membranes. Separations, 9(1), 2. https://doi.org/10.3390/separations9010002







