Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Instruments and Equipment
2.3. GC-MS Conditions
2.4. Synthesis of Magnetic Amino Modified Multi-Walled Carbon Nanotubes
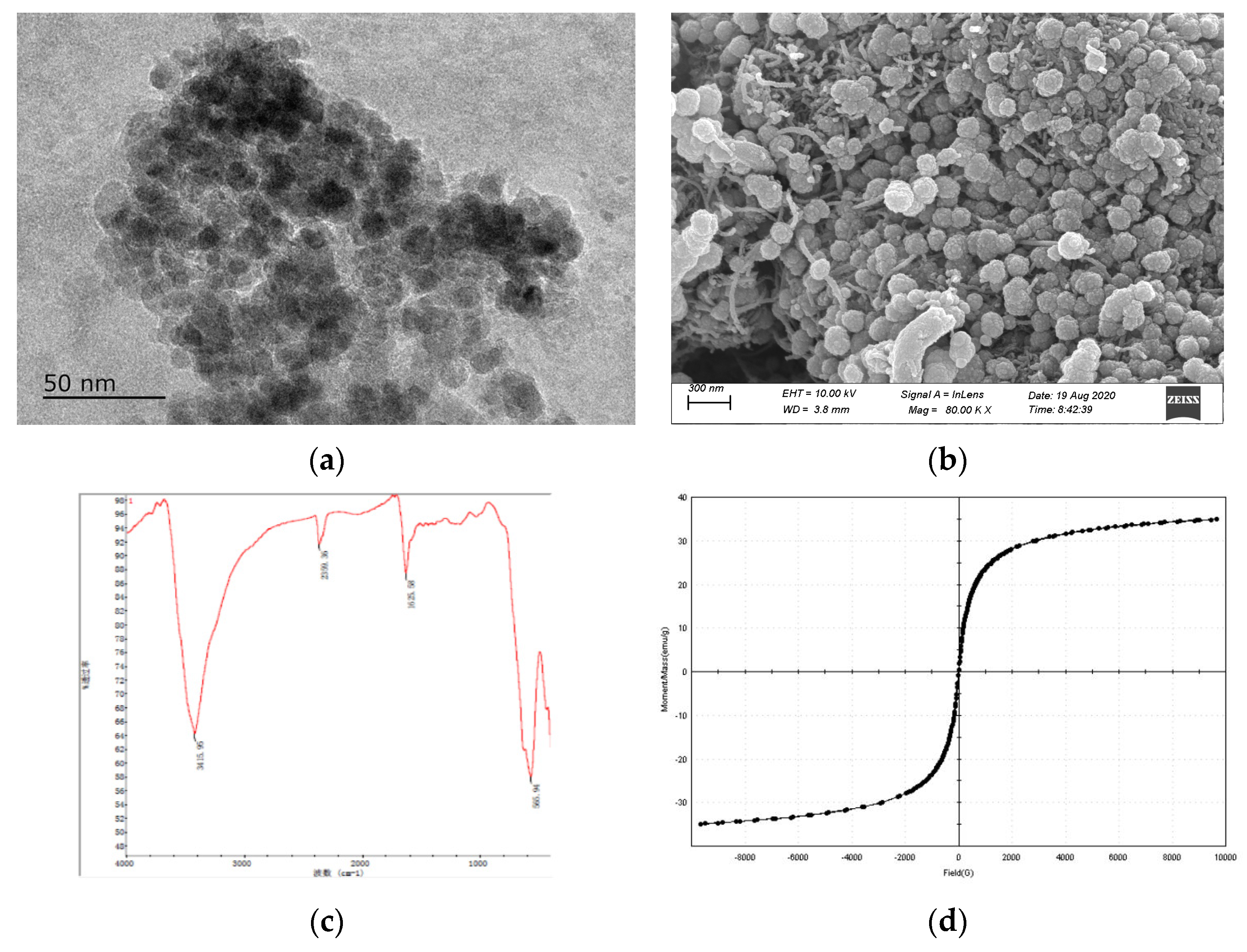
2.5. Characterization of Magnetic Amino Modified Multi-Walled Carbon Nanotubes
2.6. Sample Processing
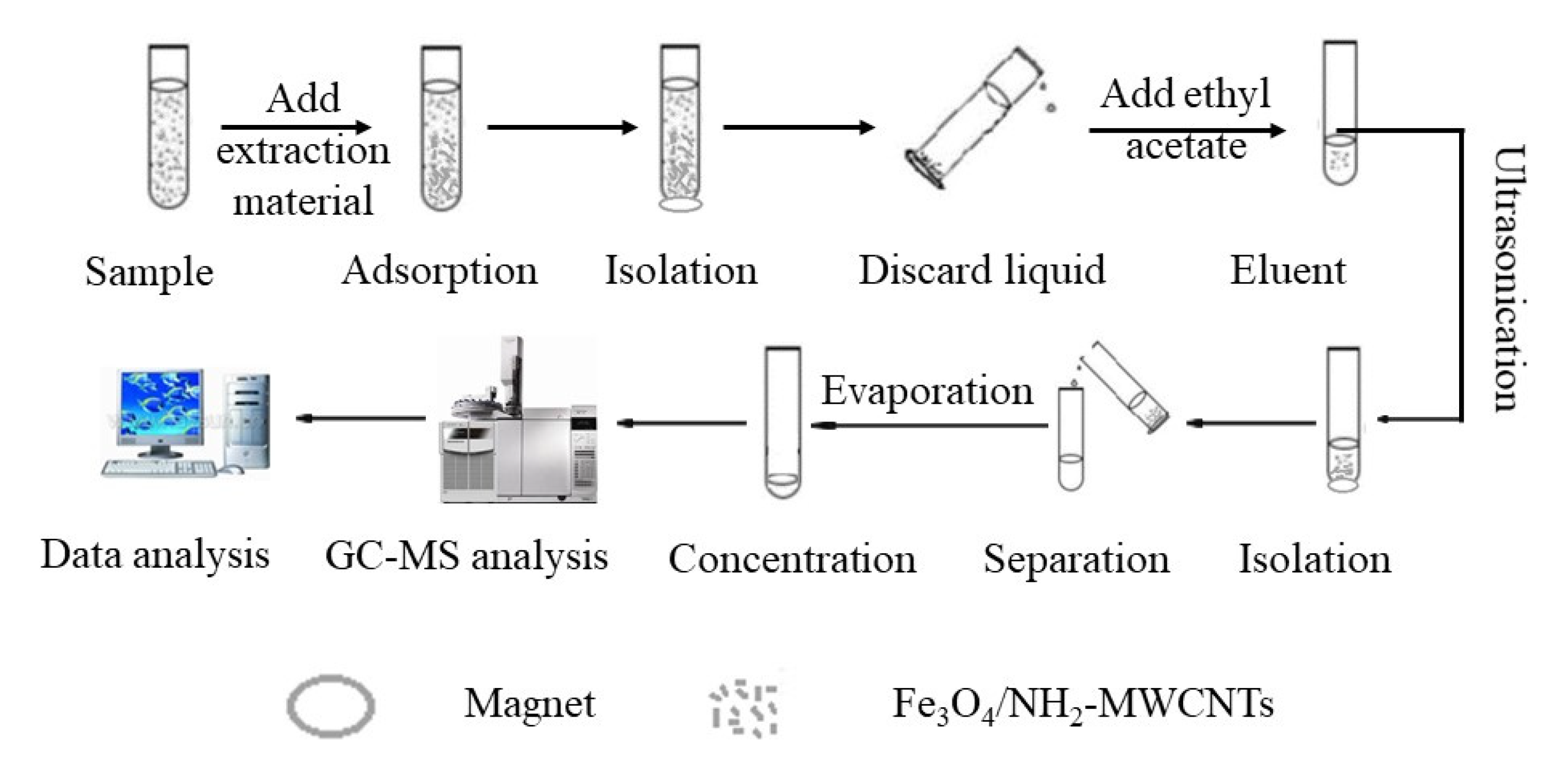
2.7. Magnetic Solid-Phase Extraction Procedures
2.8. Liquid–Liquid Extraction and Solid-Phase Extraction Procedures
3. Results
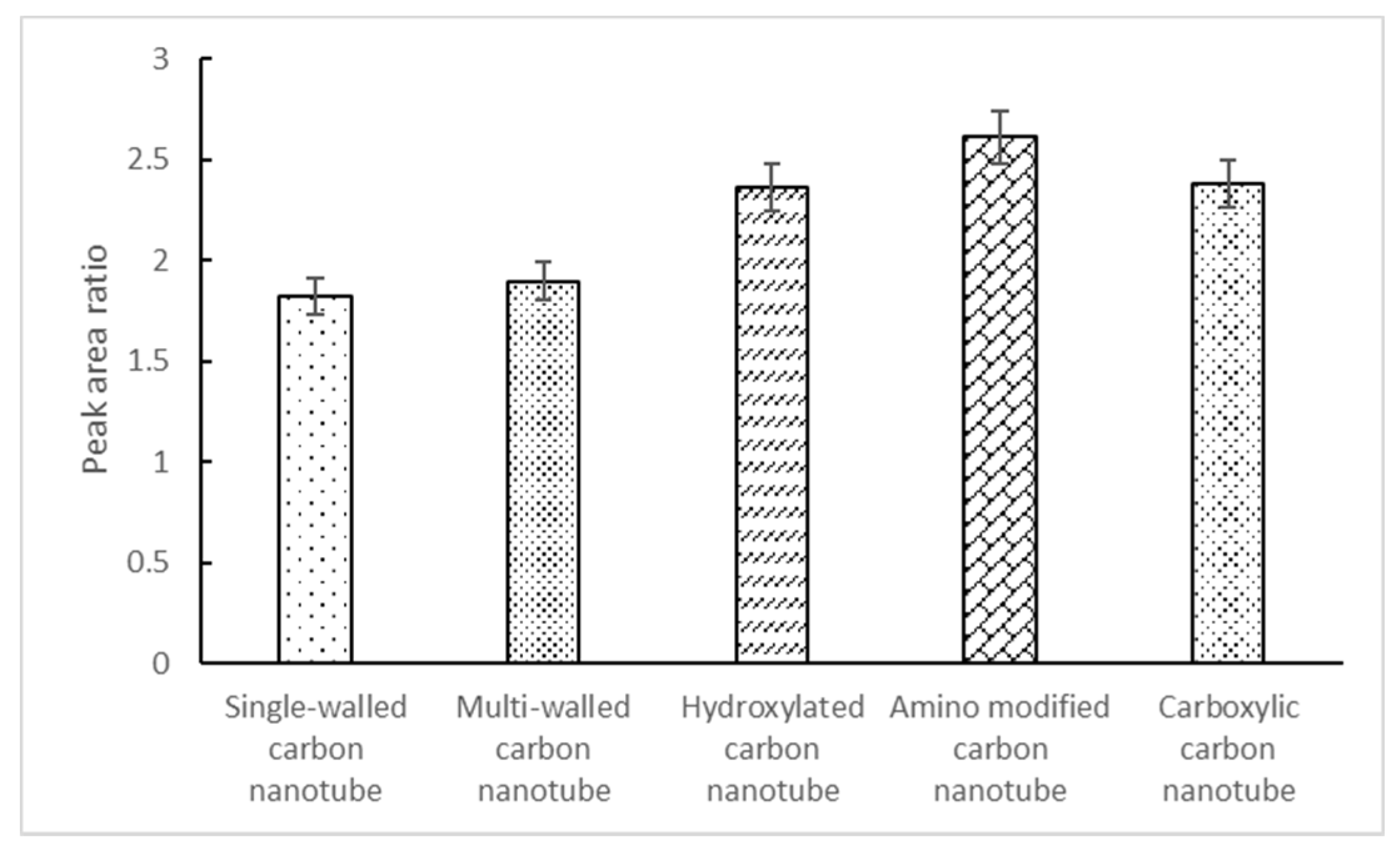
3.1. Selection of Magnetic Carbon Nanotube
3.2. Characterization of Fe3O4/NH2-MWCNTs
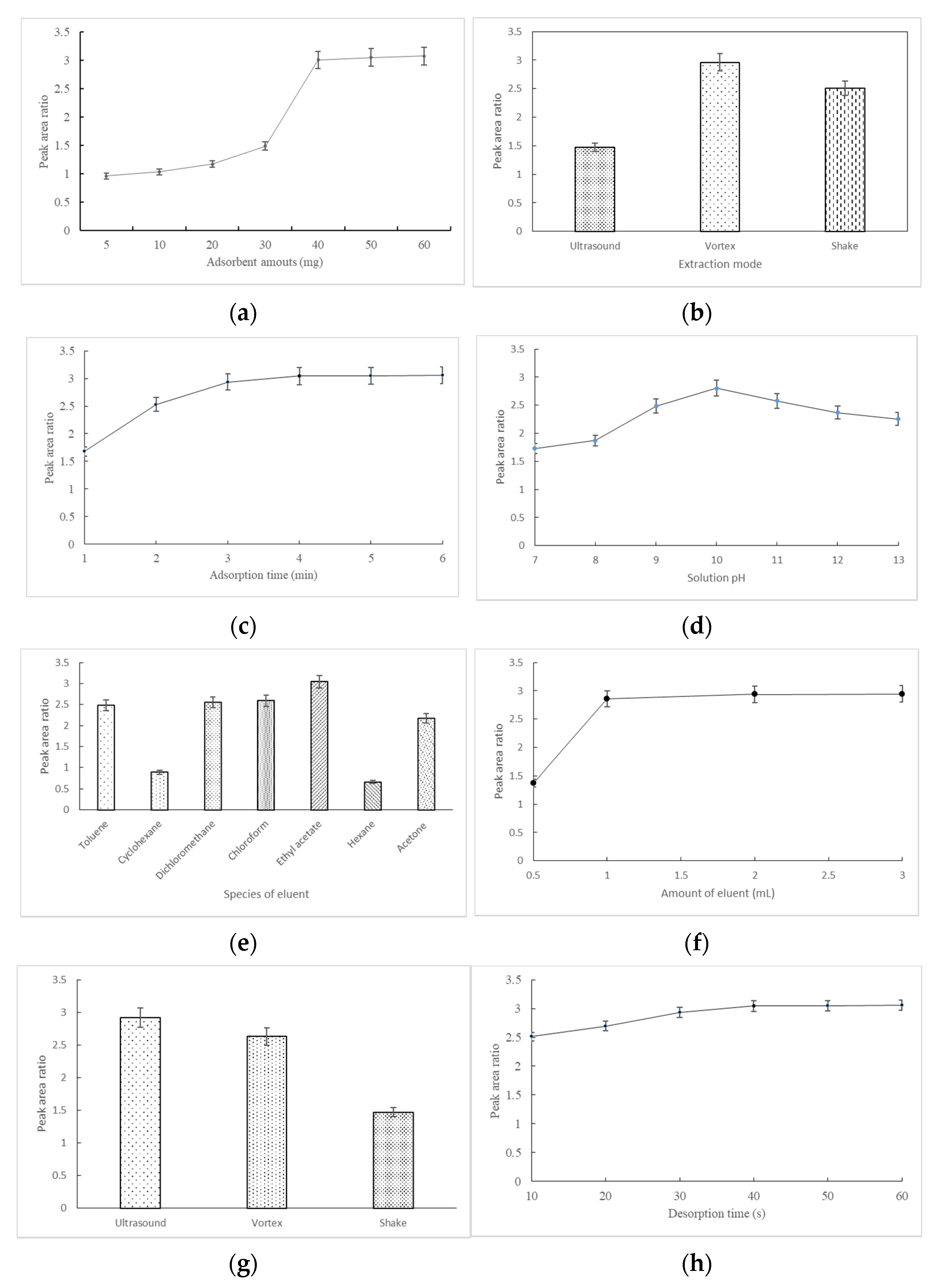
3.3. Optimization of MSPE Conditions
3.4. Comparison with Liquid–Liquid Extraction and Solid-Phase Extraction
3.5. Method Validations
3.6. Application in Real Case
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abebe, W. Khat and synthetic cathinones: Emerging drugs of abuse with dental implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chavant, F.; Boucher, A.; le Boisselier, R.; Deheul, S.; Debruyne, D. New synthetic drugs in addictovigilance. Therapie 2015, 70, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhang, S.J.; Qiu, Y.; Wang, Y.M.; Xu, P.; Shen, H.W. Advances in pharmacological research of synthetic cathinones. Chin. J. Forensic Med. 2019, 34, 279–284. [Google Scholar]
- Riley, A.L.; Nelson, K.H.; To, P.; López-Arnau, R.; Xu, P.; Wang, D.; Wang, Y.M.; Shen, H.W.; Kuhn, D.M.; Angoa-Perez, M.; et al. Hall. Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath salts”). Neurosci. Biobehav. Rev. 2020, 110, 150–173. [Google Scholar] [CrossRef]
- Fujita, Y.; Mita, T.; Usui, K.; Kamijo, Y.; Kikuchi, S.; Onodera, M.; Fujino, Y.; Inoue, Y. Toxicokinetics of the synthetic cathinone α-pyrrolidinohexanophenone. J. Anal. Toxicol. 2018, 42, e1–e5. [Google Scholar] [CrossRef]
- Kenichi, T. Synthetic drugs of abuse. Adv. Clin. Chem. 2021, 103, 191–214. [Google Scholar]
- Ellefsen, K.N.; Concheiro, M.; Huestis, M.A. Synthetic cathinone pharmacokinetics, analytical methods, and toxicological findings from human performance and postmortem cases. Drug Metab. Rev. 2016, 48, 237–265. [Google Scholar] [CrossRef]
- Berger, J.; Staretz, M.E.; Wood, M.; Brettell, T.A. Ultraviolet absorption properties of synthetic cathinones. Forensic Chem. 2020, 21, 100286. [Google Scholar] [CrossRef]
- Sisco, E.; Burns, A.; Moorthy, A.S. Development and evaluation of a synthetic cathinone targeted gas chromatography mass spectrometry (GC-MS) method. J. Forensic Sci. 2021, 66, 1919–1928. [Google Scholar] [CrossRef]
- Ploumen, C.; Marginean, I.; Lurie, I.S. The utility of silica hydride-based stationary phases for dual-mode ultra high performance liquid chromatography separation of synthetic cathinone positional isomers. J. Sep. Sci. 2020, 43, 3449–3457. [Google Scholar] [CrossRef]
- Bade, R.; Abdelaziz, A.; Nguyen, L.; Pandopulos, A.J.; White, J.M.; Gerber, C. Determination of 21 synthetic cathinones, phenethylamines, amphetamines and opioids in influent wastewater using liquid chromatography coupled to tandem mass spectrometry. Talanta 2020, 208, 120479. [Google Scholar] [CrossRef]
- Hägele, J.S.; Hubner, E.M.; Schmid, M.G. Determination of the chiral status of different novel psychoactive substance classes by capillary electrophoresis and β-cyclodextrin derivatives. Chirality 2020, 32, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Camila, D.L.; Rosa, A.S.C.; Luciano, C.A.; Pablo, A.M.; Dilton, M.P.; Quinaz, M.B.; da Silva, R.A.B.; Eduardo, M.R.; Sandro, L.B.; Santos, W.T.P.d. Electrochemical detection of the synthetic cathinone 3,4-methylenedioxypyrovalerone using carbon screen-printed electrodes: A fast, simple and sensitive screening method for forensic samples. Electrochim. Acta 2020, 354, 136728. [Google Scholar]
- Perez-Alcaraz, A.; Borrull, F.; Calull, M.; Aguilar, C. Cathinones in urine samples: A review of recent advances for their determination by chromatographic and related techniques. Trend Anal. Chem. 2021, 143, 116347. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; de Toffoli, A.L.; Neto, E.S.; Nazario, C.E.D.; Lanças, F.M. New materials in sample preparation: Recent advances and future trends. Trend Anal. Chem. 2019, 119, 115633. [Google Scholar] [CrossRef]
- Taghvimi, A.; Ghorbani, M.; Hamishehkar, H. Synthesis of a novel polymeric magnetic solid phase extraction adsorbent for selective extraction of amphetamine from urine samples coupled with high performance liquid chromatography. Drug Test Anal. 2018, 10, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Lian, R.; Yang, F.Y.; Xu, Z.R.; Cao, F.Q.; Wang, R.; Liang, C.; Zhang, Y.R. Rapid quantitation of three synthetic cathinones in urine by magnetic dispersive solid-phase extraction combined with DART-HRMS. Anal. Methods 2021, 13, 5048–5055. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, K.; Luo, C.; Song, G.X.; Hu, Y.M.; Cheng, H.F. Synthesis of Fe3O4@m-SiO2/PSA@Zr-MOF nanocomposites for bifenthrin determination in water Samples. Chromatographia 2017, 80, 463–471. [Google Scholar] [CrossRef]
- Liu, X.X.; Xie, S.Y.; Ni, T.T.; Chen, D.M.; Wang, X.; Pan, Y.H.; Wang, Y.L.; Huang, L.L.; Cheng, G.Y.; Qu, W.; et al. Magnetic solid-phase extraction based on carbon nanotubes for the determination of polyether antibiotic and s-triazine drug residues in animal food with LC-MS/MS. J. Sep. Sci. 2017, 40, 2416–2430. [Google Scholar] [CrossRef]
- Sun, Y.L.; Ilona, P.; Jorn, C.C.Y. Development of magnetic carbon nanotubes for dispersive micro solid phase extraction of the cyanide metabolite, 2-aminothiazoline-4-carboxylic acid, in biological samples. J. Chromatogr. B 2019, 1109, 67–75. [Google Scholar]
- Dong, J.N.; Feng, Z.A.; Kang, S.S.; An, M.; Wu, G.D. Magnetic solid-phase extraction based on magnetic amino modified multiwalled carbon nanotubes for the fast determination of seven pesticide residues in water samples. Anal. Methods 2020, 12, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Erina, K.; Takao, C.; Hiroyuki, T.; Kiyoyuki, K.; Tadashi, H.; Tetsuro, I. Differentiation of the isomers of N-alkylated cathinones by GC-EI-MS-MS and LC-PDA. Anal. Sci. 2016, 32, 831–837. [Google Scholar]
| No | Name | Abbreviate Name | Molecular Formula | Molecular Mass | Chemical Structure | pKa | logP | Boiling Point (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | Cathinone | - | C9H11NO | 149.19 |  | 7.97 | 1.16 | 255.0 |
| 2 | Methcathinone | MC | C10H13NO | 163.22 | 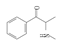 | 7.14 | 1.39 | 254.8 |
| 3 | 4-chloroethcathinone | 4-CEC | C11H14ClNO | 211.69 | 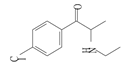 | 7.14 | 2.61 | 317.9 |
| 4 | 4-methylpentedrone | 4-MPD | C13H19NO | 205.29 | 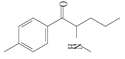 | - | 2.92 | 313.0 |
| 5 | n-ethylhexedrone | NEH | C14H21NO | 219.32 | 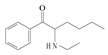 | - | 3.52 | 322.3 |
| 6 | 4-chlorobutylcathinone | 4-CBC | C13H18ClNO | 239.74 |  | - | - | - |
| 7 | 3,4-methylenedioxy-N-methylcathinone | MDMC | C11H13NO3 | 207.22 |  | - | 0.81 | 350.1 |
| 8 | 4′-methyl-α-pyrrolidinopropiophenone | 4-MePPP | C14H19NO | 217.31 |  | - | - | - |
| 9 | 3,4-methylenedioxy-N-ethylcathinone | MDEC | C12H15NO3 | 221.25 | 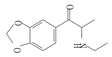 | 7.75 | 1.34 | 362.2 |
| 10 | 4-chloro-α-pyrrolidinopropiophenone | 4-Cl-α-PPP | C13H16ClNO | 237.73 | 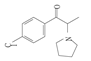 | - | - | - |
| 11 | 4-fluoro-α-pyrrolidinopentiophenone | 4-F-α-PHP; | C16H22FNO | 263.35 | 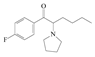 | - | - | - |
| 12 | 4-chloro-α-pyrrolidinopentiophenone | 4-Cl-α-PVP | C15H20ClNO | 265.78 |  | - | 4.33 | 380.7 |
| 13 | 4-chloro-α-pyrrolidinohexanophenone | 4-Cl-α-PHP | C16H22ClNO | 279.81 | 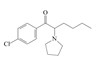 | - | - | - |
| 14 | 3,4-methylenedioxypyrovalerone | MDPV | C16H21NO3 | 275.34 |  | 8.41 | 3.06 | 415.5 |
| 15 | 3,4-methylenedioxy-α-pyrrolidinohexanophenone | MDPHP | C17H23NO3 | 289.37 |  | - | 3.59 | 427.7 |
| 16 | 2-pyrrolidin-1-yl-1-tetralin-6-yl-hexan-1-one | TH-PHP | C20H29NO | 299.45 |  | - | - | - |
| Method | Detection Result (μg/mL) | Extraction Ratio (%) |
|---|---|---|
| MSPE | 4.78 | 95.6 |
| Liquid–liquid extraction | 4.85 | 97.0 |
| Solid-phase extraction | 4.54 | 90.8 |
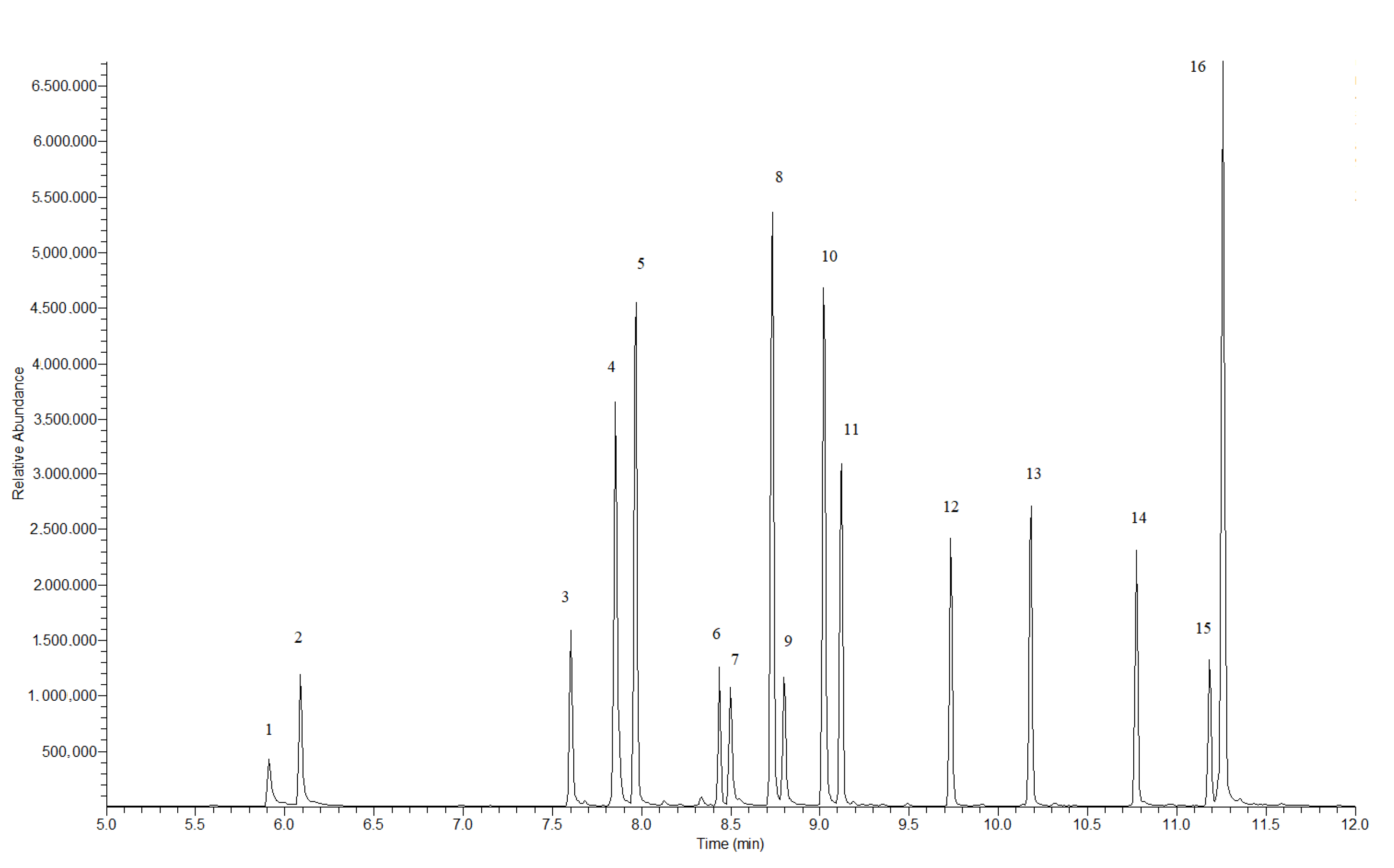
| No | Name | Retention Time (min) | Qualitative Ions (m/z) | Quantitative Ion (m/z) |
|---|---|---|---|---|
| 1 | Cathinone | 5.91 | 44, 77, 51 | 44 |
| 2 | MC | 6.09 | 58, 77, 51 | 58 |
| 3 | 4-CEC | 7.60 | 72, 44, 111 | 72 |
| 4 | 4-MPD | 7.85 | 86, 44, 91 | 86 |
| 5 | NEH | 7.97 | 114, 77, 58 | 114 |
| 6 | 4-CBC | 8.43 | 100, 58, 111 | 100 |
| 7 | MDMC | 8.50 | 58, 149, 121 | 58 |
| 8 | 4-MePPP | 8.73 | 98, 56, 119 | 98 |
| 9 | MDEC | 8.80 | 72, 44, 149 | 72 |
| 10 | 4-Cl-α-PPP | 9.02 | 98, 56, 111 | 98 |
| 11 | 4-F-α-PHP | 9.13 | 140, 84, 123 | 140 |
| 12 | 4-Cl-α-PVP | 9.73 | 126, 84, 98 | 126 |
| 13 | 4-Cl-α-PHP | 10.18 | 140, 84, 98 | 140 |
| 14 | MDPV | 10.78 | 126, 84, 149 | 126 |
| 15 | MDPHP | 11.18 | 140, 84, 98 | 140 |
| 16 | TH-PHP | 11.26 | 112, 70, 91 | 112 |
| No | Name | Linearity Equations | Coefficient (R2) | Linearity Ranges (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|
| 1 | Cathinone | Y = 0.077X + 0.070 | 0.9901 | 0.02–5.00 | 0.01 | 0.02 |
| 2 | MC | Y = 0.067X + 0.052 | 0.9957 | 0.02–5.00 | 0.01 | 0.02 |
| 3 | 4-CEC | Y = 0.76X + 0.16 | 0.992 | 0.02–5.00 | 0.01 | 0.02 |
| 4 | 4-MPD | Y = 1.58X + 0.44 | 0.9949 | 0.01–5.00 | 0.005 | 0.01 |
| 5 | NEH | Y = 5.30X + 1.32 | 0.9901 | 0.005–5.00 | 0.002 | 0.005 |
| 6 | 4-CBC | Y = 2.14X + 0.33 | 0.9922 | 0.01–5.00 | 0.005 | 0.01 |
| 7 | MDMC | Y = 0.088X + 0.039 | 0.9906 | 0.02–5.00 | 0.01 | 0.02 |
| 8 | 4-MePPP | Y = 7.24X + 0.95 | 0.9932 | 0.01–5.00 | 0.005 | 0.01 |
| 9 | MDEC | Y = 0.76X + 0.054 | 0.9945 | 0.02–5.00 | 0.01 | 0.02 |
| 10 | 4-Cl-α-PPP | Y = 6.45X + 1.12 | 0.9938 | 0.01–5.00 | 0.005 | 0.01 |
| 11 | 4-F-α-PHP | Y = 19.44X + 0.72 | 0.9973 | 0.01–5.00 | 0.005 | 0.01 |
| 12 | 4-Cl-α-PVP | Y = 1.56X + 0.49 | 0.9939 | 0.01–5.00 | 0.005 | 0.01 |
| 13 | 4-Cl-α-PHP | Y = 14.82X + 1.40 | 0.9914 | 0.005–5.00 | 0.002 | 0.005 |
| 14 | MDPV | Y = 11.30X + 2.41 | 0.9911 | 0.005–5.00 | 0.002 | 0.005 |
| 15 | MDPHP | Y = 6.95X + 0.57 | 0.9979 | 0.01–5.00 | 0.005 | 0.01 |
| 16 | TH-PHP | Y = 9.64X + 0.64 | 0.9974 | 0.01–5.00 | 0.005 | 0.01 |
| No | Name | Spiked Concentration (µg/mL) | Precision (RSD, %) | Accuracy (Bias) | Recovery (%) | ||
|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||||
| 1 | Cathinone | 0.05 | 6.02 | 7.30 | 0.64 | 0.53 | 89.73 |
| 0.20 | 6.74 | 7.58 | 0.42 | 0.73 | 87.03 | ||
| 5.00 | 3.96 | 5.61 | 0.83 | 0.68 | 95.14 | ||
| 2 | MC | 0.05 | 4.21 | 6.03 | 0.91 | 0.29 | 90.00 |
| 0.20 | 3.28 | 5.91 | 0.32 | 0.65 | 93.75 | ||
| 5.00 | 3.03 | 5.37 | 0.28 | 0.69 | 96.60 | ||
| 3 | 4-CEC | 0.05 | 3.06 | 7.68 | 0.31 | 0.51 | 90.97 |
| 0.20 | 3.33 | 5.75 | 0.21 | 0.85 | 92.88 | ||
| 5.00 | 1.62 | 3.84 | 0.32 | 0.52 | 98.34 | ||
| 4 | 4-MPD | 0.05 | 2.85 | 4.57 | 0.86 | 0.27 | 92.23 |
| 0.20 | 3.36 | 4.37 | 0.51 | 0.36 | 91.64 | ||
| 5.00 | 2.50 | 3.56 | 0.53 | 0.47 | 97.43 | ||
| 5 | NEH | 0.05 | 6.05 | 7.86 | 0.91 | 0.62 | 91.08 |
| 0.20 | 5.47 | 9.52 | 0.25 | 0.29 | 96.23 | ||
| 5.00 | 5.78 | 7.09 | 0.66 | 0.72 | 93.56 | ||
| 6 | 4-CBC | 0.05 | 3.42 | 5.49 | 0.35 | 0.52 | 94.23 |
| 0.20 | 0.62 | 1.70 | 0.54 | 0.45 | 98.88 | ||
| 5.00 | 2.43 | 3.14 | 0.36 | 0.43 | 97.43 | ||
| 7 | MDMC | 0.05 | 4.78 | 6.49 | 0.86 | 0.59 | 88.26 |
| 0.20 | 4.87 | 6.18 | 0.66 | 0.42 | 90.63 | ||
| 5.00 | 0.81 | 2.28 | 0.74 | 0.27 | 99.13 | ||
| 8 | 4-MePPP | 0.05 | 5.10 | 6.23 | 0.57 | 0.44 | 91.88 |
| 0.20 | 1.91 | 4.83 | 0.46 | 0.86 | 96.90 | ||
| 5.00 | 2.91 | 5.35 | 0.22 | 0.31 | 96.40 | ||
| 9 | MDEC | 0.05 | 8.16 | 9.77 | 0.81 | 0.58 | 90.45 |
| 0.20 | 6.50 | 6.85 | 0.18 | 0.25 | 90.56 | ||
| 5.00 | 8.90 | 9.08 | 0.28 | 0.48 | 90.09 | ||
| 10 | 4-Cl-α-PPP | 0.05 | 2.59 | 7.80 | 0.61 | 0.88 | 88.13 |
| 0.20 | 1.34 | 4.60 | 0.63 | 0.58 | 91.02 | ||
| 5.00 | 2.62 | 5.32 | 0.35 | 0.76 | 94.78 | ||
| 11 | 4-F-α-PHP | 0.05 | 1.65 | 3.20 | 0.78 | 0.45 | 97.17 |
| 0.20 | 4.80 | 6.41 | 0.81 | 0.49 | 94.03 | ||
| 5.00 | 1.47 | 2.35 | 0.42 | 0.42 | 98.55 | ||
| 12 | 4-Cl-α-PVP | 0.05 | 4.13 | 5.46 | 0.67 | 0.23 | 88.36 |
| 0.20 | 3.91 | 4.10 | 0.19 | 0.13 | 89.58 | ||
| 5.00 | 4.04 | 5.14 | 0.57 | 0.31 | 95.87 | ||
| 13 | 4-Cl-α-PHP | 0.05 | 4.82 | 5.17 | 0.19 | 0.45 | 96.58 |
| 0.20 | 2.75 | 4.52 | 0.89 | 0.36 | 95.84 | ||
| 5.00 | 4.19 | 5.20 | 0.53 | 0.36 | 95.54 | ||
| 14 | MDPV | 0.05 | 5.06 | 8.25 | 0.59 | 0.29 | 89.34 |
| 0.20 | 4.44 | 6.42 | 0.68 | 0.34 | 91.21 | ||
| 5.00 | 5.38 | 6.63 | 0.63 | 0.25 | 94.08 | ||
| 15 | MDPHP | 0.05 | 5.69 | 6.72 | 0.52 | 0.28 | 93.25 |
| 0.20 | 3.47 | 4.64 | 0.59 | 0.62 | 94.94 | ||
| 5.00 | 5.91 | 7.75 | 0.87 | 0.38 | 93.62 | ||
| 16 | TH-PHP | 0.05 | 3.52 | 8.53 | 0.51 | 0.35 | 92.10 |
| 0.20 | 3.68 | 6.60 | 0.45 | 0.26 | 94.92 | ||
| 5.00 | 5.26 | 7.18 | 0.66 | 0.44 | 94.94 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Chen, X.; Ming, Z.; Jiang, L.; Zhou, Y. Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry. Separations 2022, 9, 3. https://doi.org/10.3390/separations9010003
Wang D, Chen X, Ming Z, Jiang L, Zhou Y. Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry. Separations. 2022; 9(1):3. https://doi.org/10.3390/separations9010003
Chicago/Turabian StyleWang, Dan, Xueguo Chen, Zhu Ming, Limin Jiang, and Yan Zhou. 2022. "Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry" Separations 9, no. 1: 3. https://doi.org/10.3390/separations9010003
APA StyleWang, D., Chen, X., Ming, Z., Jiang, L., & Zhou, Y. (2022). Simultaneous Determination of 16 Kinds of Synthetic Cathinones in Human Urine Using a Magnetic Nanoparticle Solid-Phase Extraction Combined with Gas Chromatography-Mass Spectrometry. Separations, 9(1), 3. https://doi.org/10.3390/separations9010003





