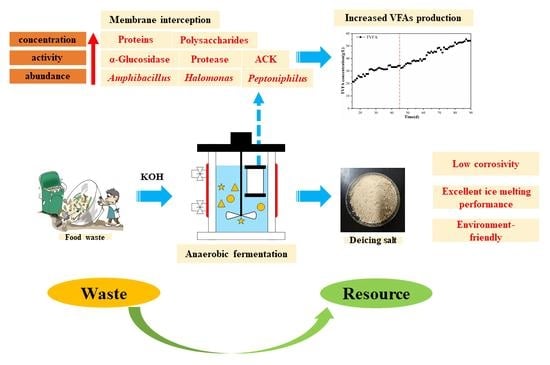
A Novel and Green Method for Turning Food Waste into Environmentally-Friendly Organic Deicing Salts: Enhanced VFA Production through AnMBR
Abstract
:1. Introduction
2. Materials and Methods
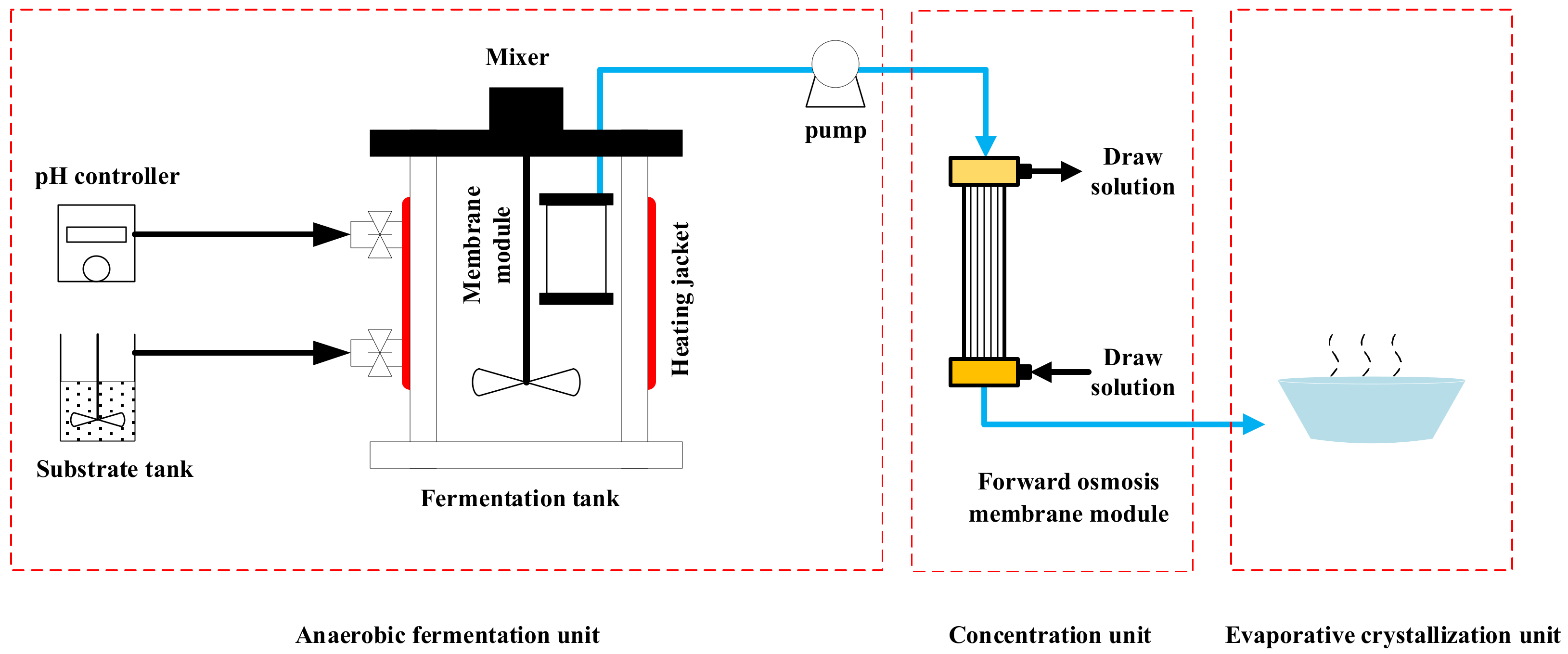
2.1. Production of VFAs by Anaerobic Fermentation
2.1.1. Substrate and Inoculum
2.1.2. Anaerobic Fermentation Experiment Procedure
2.2. Preparation of Organic Deicing Salt
2.2.1. Concentration of VFAs
2.2.2. Evaporative Crystallization of Concentrated Liquid
2.3. Analytical Methods
2.3.1. Measurements of Conventional Indices and Statistical Analysis
2.3.2. Microbial Community Analysis
2.3.3. Characterization of Metal Corrosion Properties
2.3.4. Salt Frost Resistance Test of Concrete
3. Results and Discussions
3.1. Influence and Mechanism Analysis of AnMBR on Food Waste Fermentation
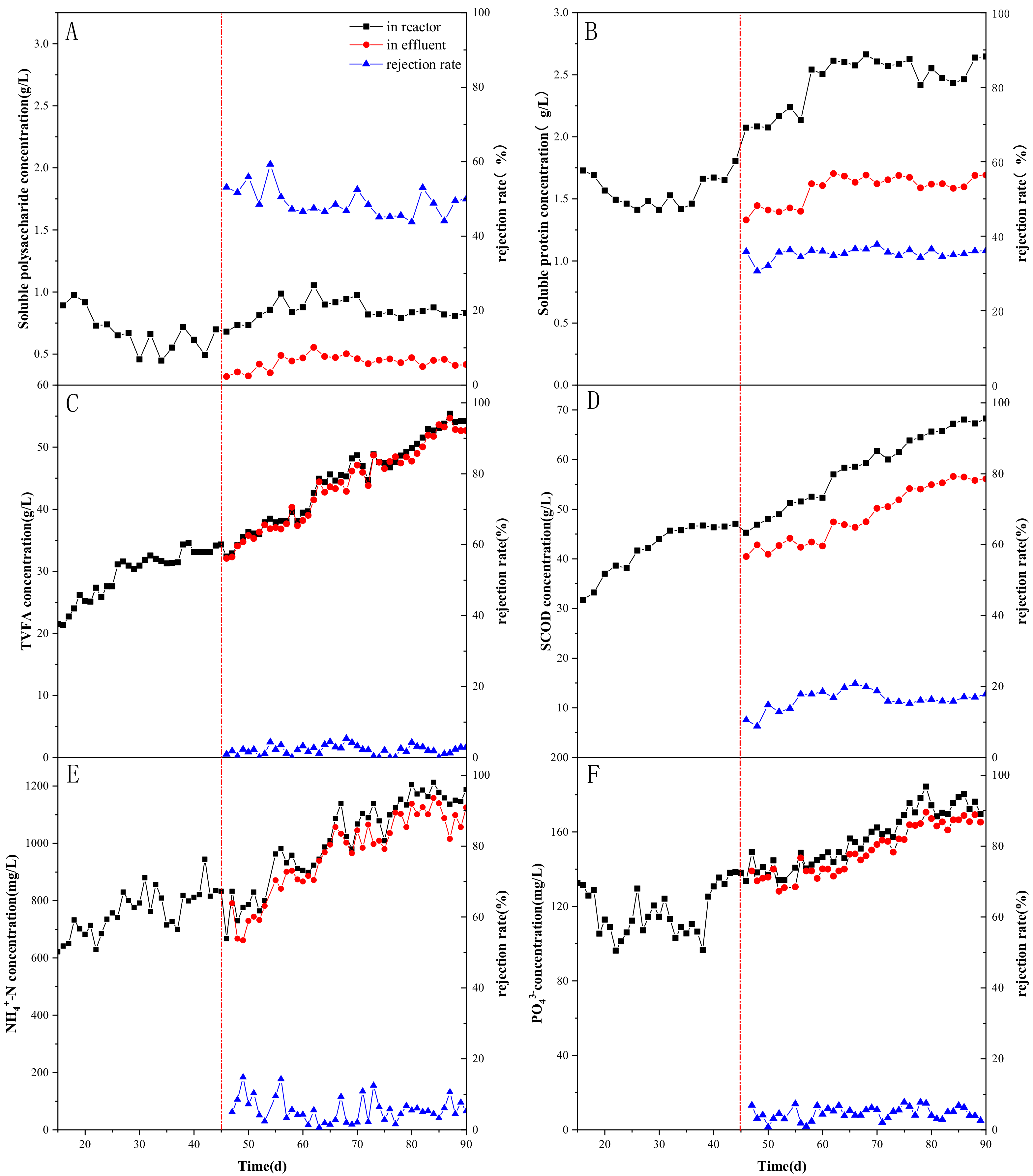
3.1.1. Effect of AnMBR on the VFAs’ Concentration and Distribution
3.1.2. Effect of AnMBR on Retention and Selective Diffusion
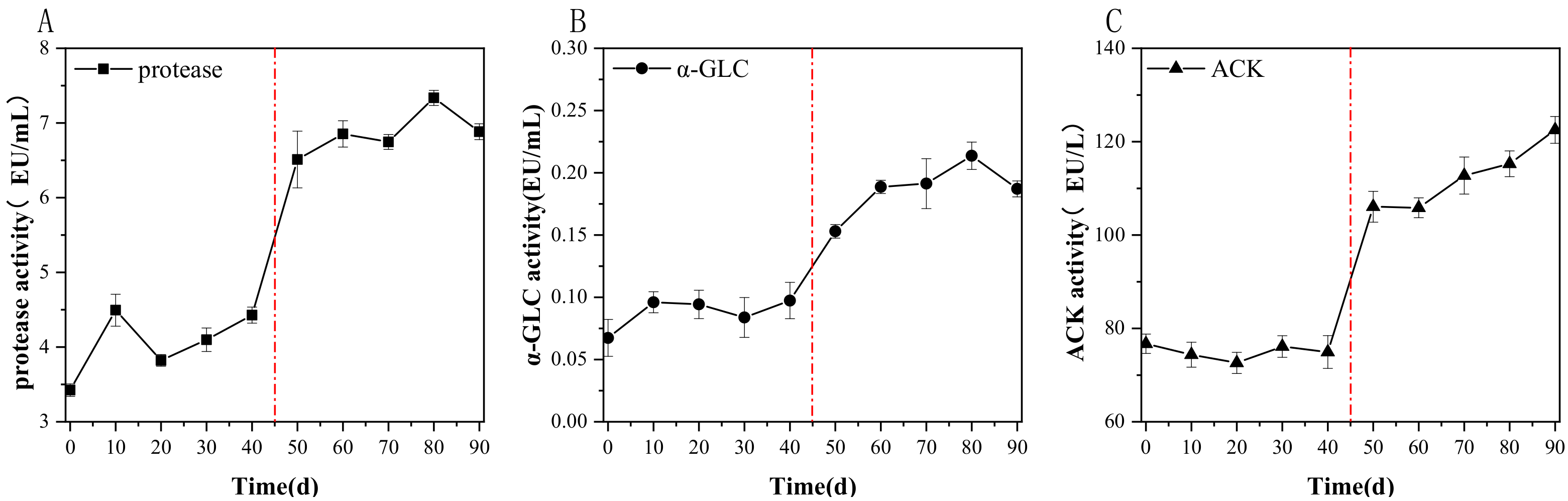
3.1.3. Effects of the AnMBR on the Key Enzyme Activities and Microorganisms
3.2. Preparation of Organic Deicing Salt
3.3. Melting Performance and Environmental Impact Evaluation of ODS
3.3.1. Melting Ice Performance of Different Deicing Salts
3.3.2. Corrosion of Deicing Salts on Concrete and Carbon Steel
3.3.3. Environmentally-Friendly Evaluation of ODS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramakrishna, D.M.; Viraraghavan, T. Environmental Impact of Chemical Deicers—A Review. Water Air Soil Pollut. 2005, 166, 49–63. [Google Scholar] [CrossRef]
- Ganjyal, G.; Fang, Q.; Hanna, M.A. Freezing points and small-scale deicing tests for salts of levulinic acid made from grain sorghum. Bioresour. Technol. 2007, 98, 2814–2818. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Li, Z.; Liang, Y.; Tao, W.; Du, M. Impacts of chloride de-icing salt on bulk soils, fungi, and bacterial populations surrounding the plant rhizosphere. Appl. Soil Ecol. 2013, 72, 69–78. [Google Scholar] [CrossRef]
- Jamshidi, A.; Goodarzi, A.R.; Razmara, P. Long-term impacts of road salt application on the groundwater contamination in urban environments. Environ. Sci. Pollut. Res. Int. 2020, 27, 30162–30177. [Google Scholar] [CrossRef]
- Fu, W.; Mathews, A.P. Two-stage fermentation process for the production of calcium magnesium acetate and propionate road deicers. Enzym. Microb. Technol. 2005, 36, 953–959. [Google Scholar] [CrossRef]
- Wu, Y.H. Study on Environmentally-Friendly Snow-Melting Agents Application. Adv. Mater. Res. 2013, 709, 923–927. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Jiang, F.Y.; Liu, R.C. Preparation of Environment-Friendly Deicing Salt of Calcium-Magnesium Acetate from Apple Sticks. Appl. Mech. Mater. 2012, 178, 954–957. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Zhao, Y.; Liu, Y. Preparation of Calcium Magnesium Acetate Snow Melting Agent Using Raw Calcium Acetate-Rich Made from Eggshells. Waste Biomass Valorizat. 2019, 11, 6757–6767. [Google Scholar] [CrossRef]
- Kolesar, K.R.; Mattson, C.N.; Peterson, P.K.; May, N.W.; Prendergast, R.K.; Pratt, K.A. Increases in wintertime PM2.5 sodium and chloride linked to snowfall and road salt application. Atmos. Environ. 2018, 177, 195–202. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Tsianou, M.; Botsaris, G.D. Investigation of the Conditions for the Production of Calcium Magnesium Acetate (CMA) Road Deicer in an Extractive Crystallization Process. Crystal Res. Technol. 2000, 35, 1035–1049. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Liu, M.; Ogunmoroti, A.; Liu, W.; Li, M.; Bi, M.; Liu, W.; Cui, Z. Assessment and projection of environmental impacts of food waste treatment in China from life cycle perspectives. Sci. Total Environ. 2021, 807, 150751. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Luo, J.; Huang, W.; Guo, W.; Ge, R.; Zhang, Q.; Fang, F.; Feng, Q.; Cao, J.; Wu, Y. Novel strategy to stimulate the food wastes anaerobic fermentation performance by eggshell wastes conditioning and the underlying mechanisms. Chem. Eng. J. 2020, 398, 125560. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, H.; Yin, B.; Zu, Y.; Liu, H.; Fu, B.; Ma, H. Enhanced volatile fatty acid production by a modified biological pretreatment in anaerobic fermentation of waste activated sludge. Chem. Eng. J. 2016, 284, 194–201. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Techno.l 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Parchami, M.; Wainaina, S.; Mahboubi, A.; I’Ons, D.; Taherzadeh, M.J. MBR-Assisted VFAs Production from Excess Sewage Sludge and Food Waste Slurry for Sustainable Wastewater Treatment. Appl. Sci. 2020, 10, 2921. [Google Scholar] [CrossRef] [Green Version]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yin, B.; Zhu, Y.; Fu, B.; Liu, H. Improving volatile fatty acid yield from sludge anaerobic fermentation through self-forming dynamic membrane separation. Bioresour. Technol. 2016, 218, 92–100. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chung, K.H.; Jung, S.C.; Park, B.G. Eco-friendly deicer prepared from waste oyster shells and its deicing properties with metal corrosion. Environ. Technol. 2021, 42, 3360–3368. [Google Scholar] [CrossRef]
- Goel, R.; Mino, T.; Satoh, H.; Matsuo, T. Enzyme activities under anaerobic and aerobic conditions in activated sludge sequencing batch reactor. Water Res. 1998, 32, 2081–2088. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A toolkit for illustrating heatmaps. PLoS ONE 2014, 9, e111988. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, Q.; Liu, C.; Xing, Z. Preparation of Ca-Al-Fe deicing salt and modified with sodium methyl silicate for reducing the influence of concrete structure. Constr. Build. Mater. 2018, 172, 263–271. [Google Scholar] [CrossRef]
- Huang, W.; Huang, W.; Yuan, T.; Zhao, Z.; Cai, W.; Zhang, Z.; Lei, Z.; Feng, C. Volatile fatty acids (VFAs) production from swine manure through short-term dry anaerobic digestion and its separation from nitrogen and phosphorus resources in the digestate. Water Res. 2016, 90, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seedorf, H.; Fricke, W.F.; Veith, B.; Bruggemann, H.; Liesegang, H.; Strittmatter, A.; Miethke, M.; Buckel, W.; Hinderberger, J.; Li, F.; et al. The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc. Natl. Acad. Sci. USA 2008, 105, 2128–2133. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Ortiz, R.; Steele, T.W.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Drake, H.L.; Küsel, K.; Matthies, C. Acetogenic Prokaryotes. In The Prokaryotes; Springer: Cham, Switzerland, 2006; pp. 354–420. [Google Scholar]
- Aino, K.; Hirota, K.; Okamoto, T.; Tu, Z.; Matsuyama, H.; Yumoto, I. Microbial Communities Associated With Indigo Fermentation That Thrive in Anaerobic Alkaline Environments. Front. Microbiol. 2018, 9, 2196. [Google Scholar] [CrossRef]
- Xiao-Ran, J.; Jin, Y.; Xiangbin, C.; Guo-Qiang, C. Halomonas and Pathway Engineering for Bioplastics Production. Methods Enzymol. 2018, 608, 309–328. [Google Scholar] [CrossRef]
- Tomazetto, G.; Hahnke, S.; Maus, I.; Wibberg, D.; Puhler, A.; Schluter, A.; Klocke, M. Complete genome sequence of Peptoniphilus sp. strain ING2-D1G isolated from a mesophilic lab-scale completely stirred tank reactor utilizing maize silage in co-digestion with pig and cattle manure for biomethanation. J. Biotechnol. 2014, 192, 59–61. [Google Scholar] [CrossRef]
- Blandin, G.; Rosselló, B.; Monsalvo, V.M.; Batlle-Vilanova, P.; Viñas, J.M.; Rogalla, F.; Comas, J. Volatile fatty acids concentration in real wastewater by forward osmosis. J. Membr. Sci. 2019, 575, 60–70. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R.; Qi, X.; Bajwa, D.S.; Battocchi, D. Employing corn derived products to reduce the corrosivity of pavement deicing materials. Constr. Build. Mater. 2020, 263, 120662. [Google Scholar] [CrossRef]
- Liu, Z.; Hansen, W. Pore damage in cementitious binders caused by deicer salt frost exposure. Constr. Build. Mater. 2015, 98, 204–216. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]




| Parameters | Food Waste Slurry | Sludge |
|---|---|---|
| pH | 4.20 ± 0.30 | 7.70 ± 0.04 |
| TSS (g/L) | 27.70 ± 2.80 | 12.11 ± 1.17 |
| VSS (g/L) | 24.20 ± 2.17 | 10.02 ± 0.45 |
| Total COD (g/L) | 128.16 ± 10.00 | 16 ± 1.20 |
| Soluble COD (g/L) | 59.33 ± 5.80 | 10 ± 0.95 |
| Protein (g/L) | 10.25 ± 2.20 | / |
| Polysaccharide (g/L) | 51.87 ± 3.40 | / |
| TP (mg/L) | 136.75 ± 10.75 | / |
| TN (mg/L) | 1250.72 ± 125 | / |
| NH4+-N (mg/L) | 130.73 ± 14.70 | / |
| Parameter | Acetate | Propionate | Iso-Butyrate | n-Butyrate | Valerate | VFAs |
|---|---|---|---|---|---|---|
| Initial concentration (g/L) | 38.48 | 4.47 | 1.48 | 7.70 | 1.27 | 53.41 |
| Final concentration (g/L) | 175.87 | 20.67 | 6.61 | 36.36 | 5.29 | 244.80 |
| Concentration factor | 4.57 | 4.62 | 4.48 | 4.72 | 4.17 | 4.58 |
| loss VFA (g) | / | / | / | / | / | 4.55 |
| Recovery rate (%) | / | / | / | / | / | 95.74 |
| Parameter | CK | NaCl | CaCl2 | CMA | ODS | |
|---|---|---|---|---|---|---|
| Initial mass of test block (g) | 394.25 | 403.34 | 375.02 | 416.18 | 406.74 | |
| Concrete | Loss mass of test block (g) | 3.62 | 17.22 | 27.62 | 13.66 | 11.65 |
| ΔWn (kg/m2) | 0.13 | 0.60 | 0.95 | 0.47 | 0.40 | |
| Initial mass of test piece (g) | 24.07 | 23.94 | 24.00 | 23.87 | 24.09 | |
| Carbon steel | Mass of test piece after corrosion (g) | 24.03 | 23.89 | 23.94 | 23.85 | 24.08 |
| V (mm/a) | 0.12 | 0.16 | 0.19 | 0.06 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Yang, K.; Chen, Z.; Li, M.; Zhang, Y.; Wang, X.; Jiang, Y.; Gu, P.; Miao, H. A Novel and Green Method for Turning Food Waste into Environmentally-Friendly Organic Deicing Salts: Enhanced VFA Production through AnMBR. Separations 2022, 9, 11. https://doi.org/10.3390/separations9010011
Xiang Q, Yang K, Chen Z, Li M, Zhang Y, Wang X, Jiang Y, Gu P, Miao H. A Novel and Green Method for Turning Food Waste into Environmentally-Friendly Organic Deicing Salts: Enhanced VFA Production through AnMBR. Separations. 2022; 9(1):11. https://doi.org/10.3390/separations9010011
Chicago/Turabian StyleXiang, Qiuhong, Kunlun Yang, Ziwen Chen, Manman Li, Yuanqi Zhang, Xiaorui Wang, Yingying Jiang, Peng Gu, and Hengfeng Miao. 2022. "A Novel and Green Method for Turning Food Waste into Environmentally-Friendly Organic Deicing Salts: Enhanced VFA Production through AnMBR" Separations 9, no. 1: 11. https://doi.org/10.3390/separations9010011
APA StyleXiang, Q., Yang, K., Chen, Z., Li, M., Zhang, Y., Wang, X., Jiang, Y., Gu, P., & Miao, H. (2022). A Novel and Green Method for Turning Food Waste into Environmentally-Friendly Organic Deicing Salts: Enhanced VFA Production through AnMBR. Separations, 9(1), 11. https://doi.org/10.3390/separations9010011