An Evaluation of the Antioxidant Activity of a Methanolic Extract of Cucumis melo L. Fruit (F1 Hybrid)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Procurement of the Research Raw Materials
2.3. Fruit Extract Preparation
2.4. Preparation of MECM
2.5. Phytochemical Screening
2.5.1. Tests for Carbohydrates
- Molisch’s test: Filtrate (2 mL) was added with two drops of alcoholic solution of α-naphthol. The mixture was shaken well and 1 mL of concentrated sulphuric acid was added slowly along the sides of the test tube. It was allowed to stand for a few seconds. The colour of the mixture was noted.
- Iodine test: Filtrate (2 mL) was added with a few drops of dilute iodine solution and observed for the formation of a blue, orange or red colour.
- Fehling’s test: Filtrate (1 mL) was boiled in a water bath with 2 mL of a mixed Fehling’s solution (1 mL each of Fehling’s solutions A and B). The change in the colour of the solution was noted.
- Benedict’s test: Filtrate (0.5 mL) was added with 0.5 mL of Benedict’s reagent and heated. The mixture was kept on a boiling water bath for 2 min. The change in the colour of the solution was noted.
- Barfoed’s test: Filtrate (1 mL) was added with 1 mL of Barfoed’s reagent. It was heated in a boiling water bath for 2 min. The change in the colour of the solution was noted.
2.5.2. Tests for Glycosides
- Borntrager’s test: Filtered hydrolysate (2 mL) was added with 3 mL chloroform and shaken. The chloroform layer was separated and a 10% ammonia solution was added to it. The change in the colour of the solution was noted.
- Legal’s test: MECM (50 mg) was dissolved in pyridine. A sodium nitroprusside solution was added and made alkaline by using a 10% sodium hydroxide solution. The change in the colour of the solution was noted.
2.5.3. Tests for Alkaloids
- Mayer’s test: Filtrate (2 mL) was taken in a test tube. Two drops of Mayer’s reagent were added along the sides and the change in the colour was noted.
- Dragendorff’s test: Filtrate (2 mL) was added with 1 mL of Dragendorff’s reagent and the colour change and formation of any precipitate were noted.
- Hager’s test: Filtrate (2 mL) was added with 1 mL of Hager’s reagent and the change in the colour of the solution was noted.
- Wagner’s test: Filtrate (2 mL) was added with 1 mL of Wagner’s reagent and the change in the colour of the solution was noted.
2.5.4. Tests for Amino acids
- Biuret test: Filtrate (2 mL) was treated with one drop of a 2% copper sulphate solution. A total of 1 mL of (95%) ethanol was added to the solution followed by an excess of potassium hydroxide pellets and the change in the colour was observed.
- Ninhydrin test: Ninhydrin solution of 1% was added (2 drops) to the aqueous filtrate (2 mL). The mixture was heated and the change in the colour was observed.
- Million’s test: A few drops of Million’s reagent were added to the filtrate (2 mL) and the colour change was noted.
- Xanthoprotein test: The filtrate (3 mL) was added with 1 mL of concentrated nitric acid. The solution was cooled and made alkaline with 10% sodium hydroxide. The change in the colour of the solution was noted.
2.5.5. Tests for Sterols/Steroids
- Liebermann’s sterol test: MECM (500 mg) was mixed with 2 mL of glacial acetic acid and one drop of concentrated sulphuric acid was added. The change in the colour of the solution was noted.
- Liebermann–Burchard test: MECM (500 mg) was mixed with 5 mL chloroform and a few mL of acetic anhydride was added. The mixture was added with one drop of concentrated sulphuric acid. This was then shaken well and the change in the colour was observed.
- Salkowski’s test: MECM (500 mg) was shaken vigorously with 3 mL of chloroform and concentrated sulphuric acid was added through the sides of the test tube. The colour change of the solution was observed.
2.5.6. Tests for Fixed Oils and Fats
- Grease spot test: MECM (a small quantity) was pressed between two filter papers.
- Saponification test: MECM (a small quantity) was added with a few drops of a 0.5 N alcoholic potassium hydroxide solution along with a drop of phenolphthalein and the mixture was heated on a water bath for 2 h. The solution was observed for the formation of soap or any colour change indicating the neutralisation of potassium hydroxide on saponification.
2.5.7. Tests for Phenolic Compounds and Tannins
- Ferric chloride test: MECM (50 mg) was boiled with 5 mL distilled water and filtered. A few drops of a neutral, freshly prepared 5% ferric chloride solution were added to the filtrate and the colour change was noted (brownish green or blue-black colouration indicated the presence of tannins and phenolic compounds).
- Test for phlobatannins: MECM (1 g) was boiled with 1% HCl in a boiling tube and observed for the deposition of a red precipitate.
- Lead acetate test: MECM (50 mg) was boiled with distilled water and filtered. The filtrate was added with 3 mL of a 10% lead acetate solution. The formation of any precipitate was observed.
- Gelatine test: MECM (50 mg) was boiled with distilled water and 2 mL of a 1% solution of gelatine was added containing 10% sodium chloride. The solution was observed for the formation of a precipitate.
2.5.8. Tests for Terpenes/Terpenoids
- Tin and Thionyl chloride test: MECM (1 g) was boiled with 5 mL distilled water and filtered and the filtrate was treated with tin and thionyl chloride. Any change in the colour that occurred in the solution was noted.
2.5.9. Tests for Saponins
- Foam test: MECM (50 mg) was dissolved in distilled water and was diluted to 20 mL. The suspension was shaken vigorously in a graduated cylinder for 15 min. It was observed for the formation of foam.
- MECM (1 g) was boiled with 20 mL distilled water for 5 min and then cooled and filtered. The filtrate (10 mL) was shaken vigorously after adding 5 mL of distilled water. The frothing solution was mixed with a few drops of arachis oil and again shaken vigorously and observed for the formation of an emulsion.
2.5.10. Tests for Gums and Mucilage
- Precipitation test: MECM (100 mg) was shaken vigorously with 10 mL of distilled water and 25 mL of absolute alcohol was then added with constant stirring.
- Ruthenium red test: MECM (50 mg) was allowed to swell in water and then a few drops of Ruthenium red were added.
2.5.11. Tests for Flavones and Flavonoids
- Aqueous sodium hydroxide: An MECM solution of 2 mL was added with an aqueous solution of sodium hydroxide and mixed well. The change in the colour was observed.
- Concentrated sulphuric acid: An MECM solution (50 mg dissolved in 10 mL of water) was added with a concentrated sulphuric acid solution.
- Shinoda test: MECM (50 mg) was dissolved in alcohol. A few magnesium turnings and concentrated hydrochloric acid (dropwise) were added and the change in the colour was noted.
2.6. HPTLC Analysis
Sample Application
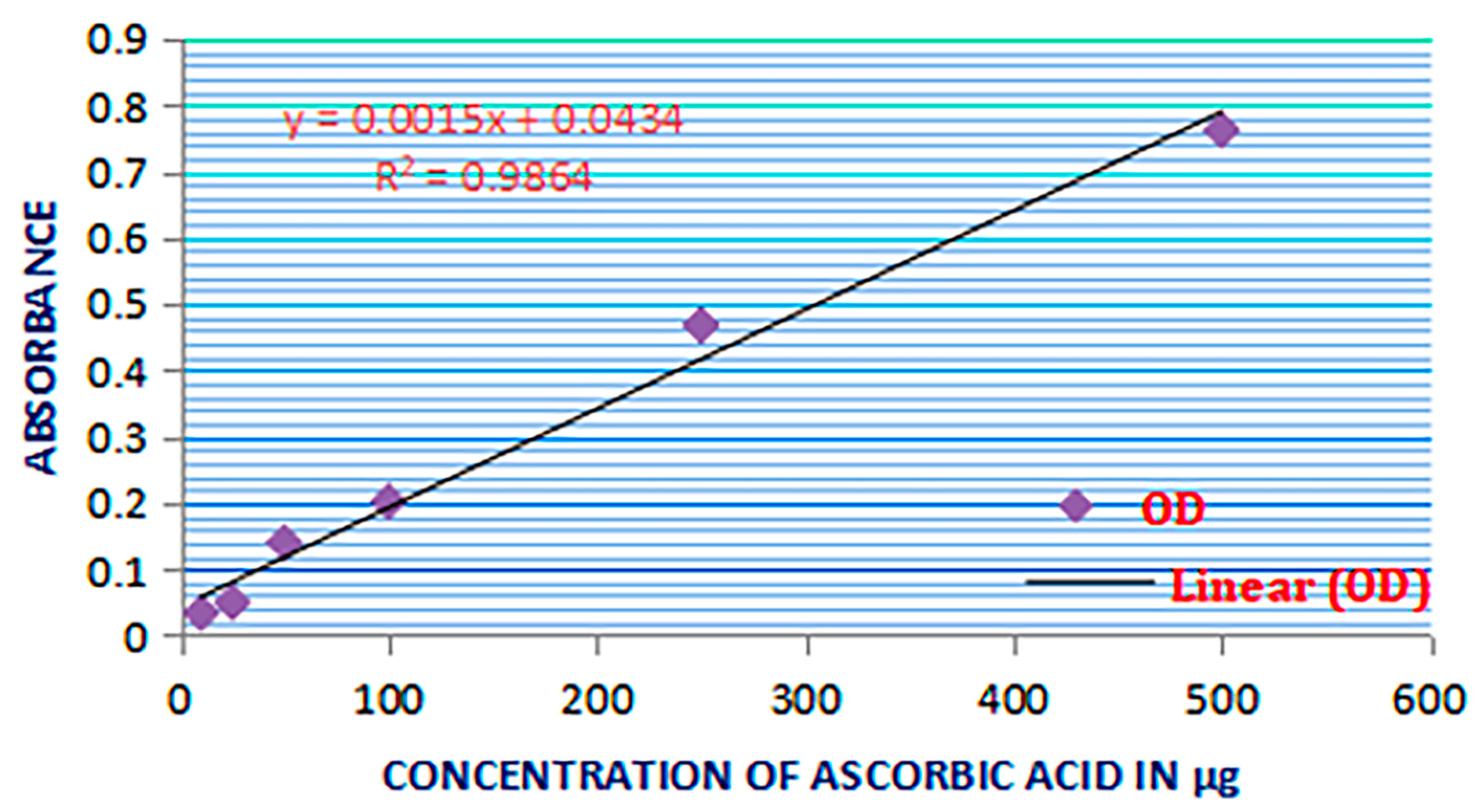
2.7. Total Antioxidant Activity Estimation
2.8. DPPH Radical Scavenging Assay
2.9. Hydroxyl Radical Scavenging Activity
2.10. Nitric Oxide Generation and the Assay of Nitric Oxide Scavenging
2.11. Evaluation of the Reducing Power Activity
3. Results
3.1. Qualitative Screening Tests of the Methanolic Extract of Muskmelon
3.2. Estimation of the Total Antioxidant Activity
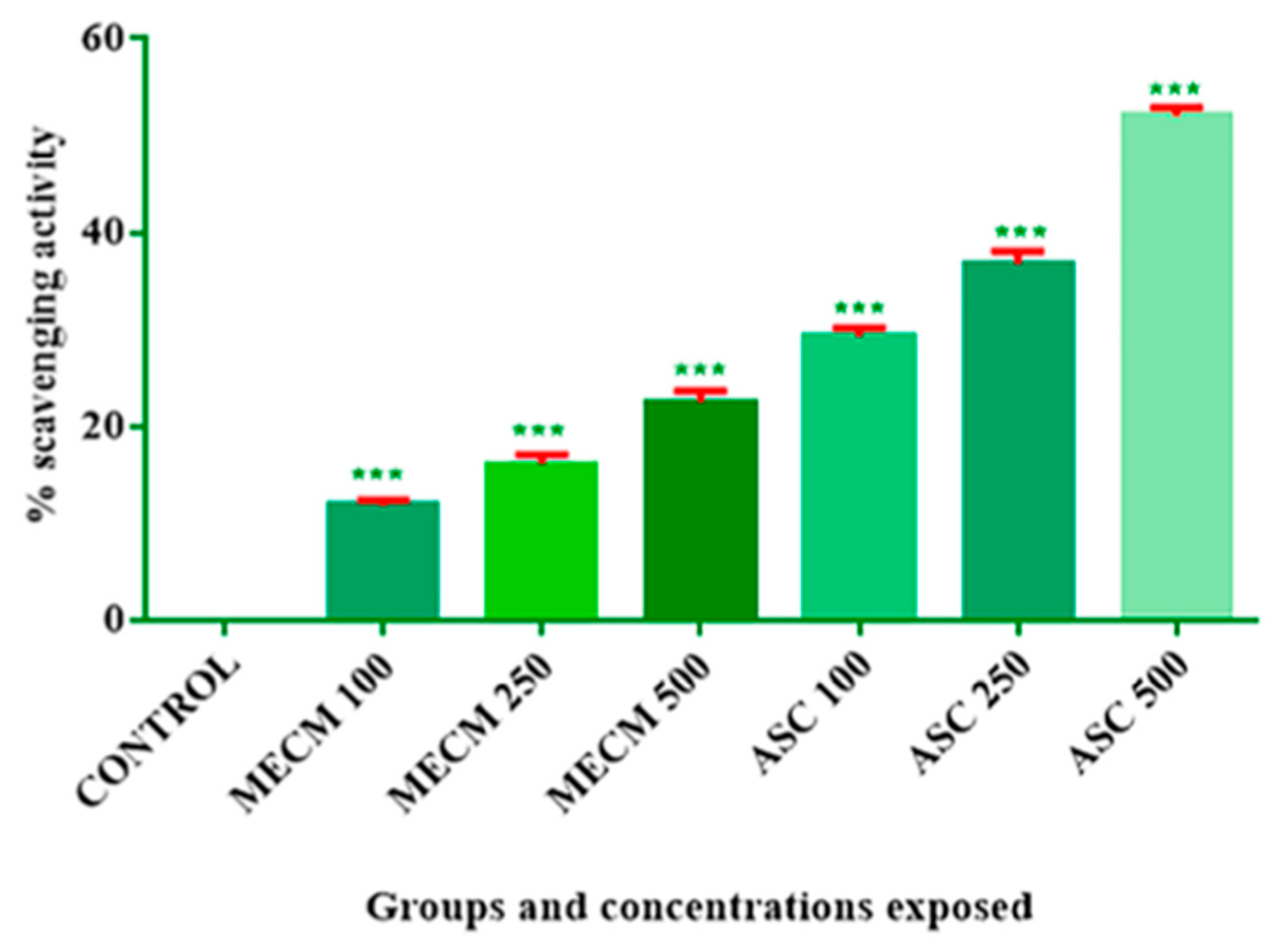
3.3. DPPH Scavenging Activity
3.4. Nitric Oxide Scavenging Activity
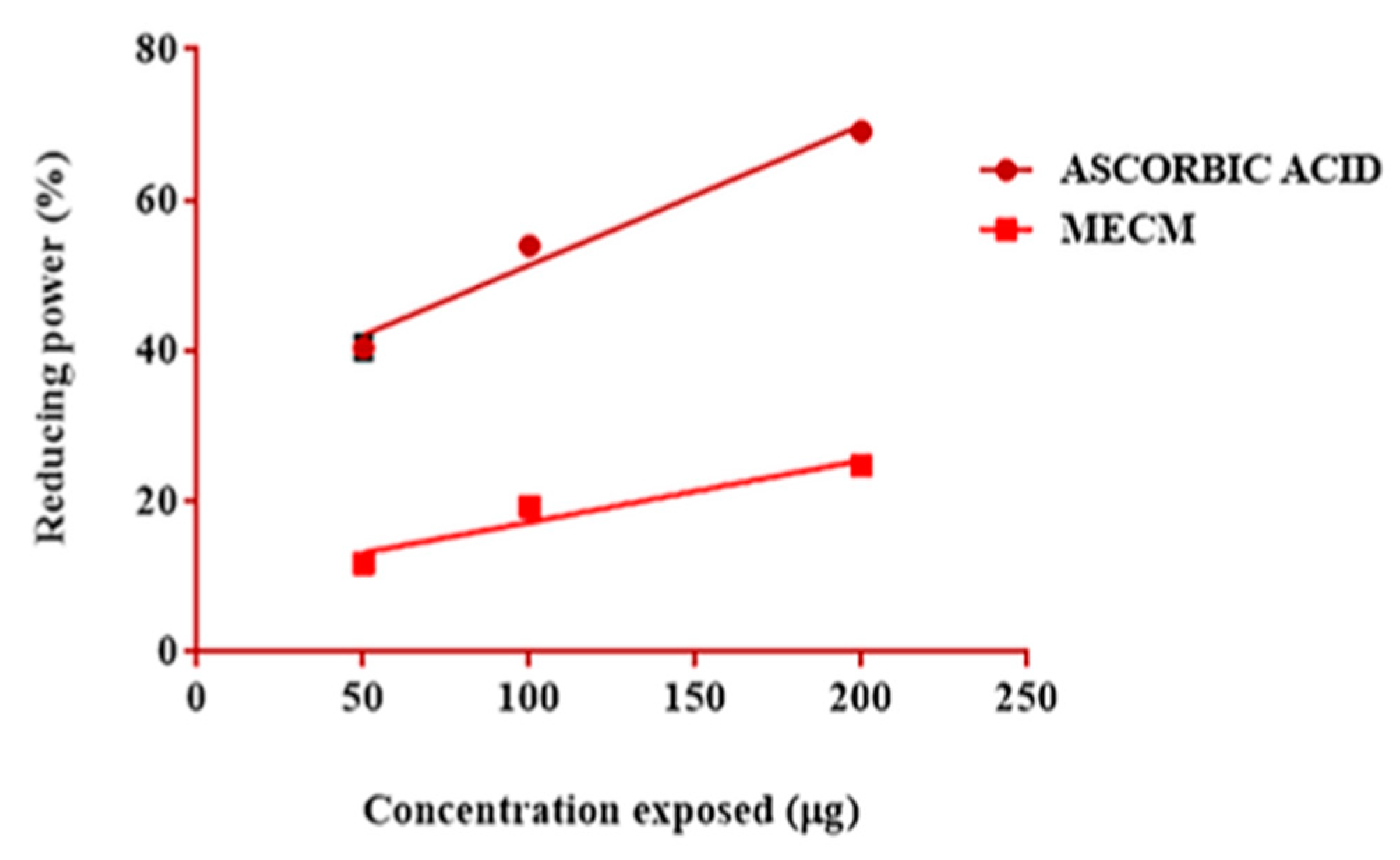
3.5. Evaluation of the Reducing Power Activity
4. Discussion
4.1. DPPH Radical Scavenging Activity of MECM
4.2. Hydroxyl Radical Scavenging Potential of MECM
4.3. Nitric Oxide Scavenging Potential of MECM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Adwas, A.A.; Elsayed, A.S.I.; Azab, A.E. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Kasole, R.; Martin, H.D.; Kimiywe, J. Traditional medicine and its role in the management of diabetes mellitus: “patients’ and herbalists’ perspectives. Evid.-Based Complement. Altern. Med. 2019, 2019, 2835691. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic acids and derivatives formulations for skin damages and disorders: A review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Contardi, M.; Kossyvaki, D.; Picone, P.; Cristaldi, L.; Galizzi, G.; Bosco, G.; Scoglio, S.; Athanassiou, A.; Di Carlo, M. Heat-resistant Aphanizomenon flos-aquae (AFA) Extract (Klamin®) as a functional ingredient in food strategy for prevention of oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 9481390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. Biomed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Larayetan, R.; Ololade, Z.S.; Ogunmola, O.O.; Ladokun, A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid.-Based Complement. Altern. Med. 2019, 2019, 5410923. [Google Scholar] [CrossRef] [Green Version]
- Rajasree, R.S.; Sibi, P.I.; Francis, F.; William, H. Phytochemicals of Cucurbitaceae family—A review. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 113–123. [Google Scholar]
- Marwat, S.K.; Rehman, F.U. Medicinal folk recipes used as traditional phytotherapies in district Dera Ismail Khan, KPK, Pakistan. Pak. J. Bot. 2011, 43, 1453–1462. [Google Scholar]
- Ittiyavirah, S.P.; George, A.; Santhosh, A.M.; Kurian, S.T.; Pappachan, P.; Jacob, G. Studies of cytotoxic potential of Cucumismelo. Linn fruit aqueous extract in prostate cancer cell lines PC-3 using MTT and neutral red assay. Iran. J. Pharmacol. Ther. 2013, 12, 24–30. [Google Scholar]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chem. 2017, 221, 1691–1697. [Google Scholar] [CrossRef]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Portela, S.I.; Cantwell, M.I. Cutting blade sharpness affects appearance and other quality attributes of fresh-cut cantaloupe melon. J. Food Sci. 2001, 66, 1265–1270. [Google Scholar] [CrossRef]
- Gokhale, S.B.; Kokate, C.K.; Purohit, A.P. A Textbook of Pharmacognosy, 28th ed.; Niraliprakashan: New Delhi, India, 2007; pp. 1–20. [Google Scholar]
- Becket, A.H.; Stenlake, J.B. Practical Pharmaceutical Chemistry, 2nd ed.; C.B.S. Publishers and Distributors: New Delhi, India, 1986; pp. 333–337. [Google Scholar]
- Vouldoukis, I.; Lacan, D.; Kamate, C.; Coste, P.; Calenda, A.; Mazier, D.; Conti, M.; Dugas, B. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J. Ethnopharmacol. 2004, 94, 67–75. [Google Scholar] [CrossRef]
- Reddy, G.M.; Muralikrishna, A.; Padmavathi, V.; Padmaja, A.; Tilak, T.K.; Rao, C.A. Synthesis and antioxidant activity of styryl sulfonyl methyl 1, 3, 4-oxadiazoles, pyrazolyl/isoxazolyl-1, 3, 4-oxadiazoles. Chem. Pharm. Bull. 2013, 61, 1291–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, P.R.; Jothi, T.A. Multisource five level inverter using an improved PWM scheme. Int. J. Sci. Res. 2013, 2, 279–282. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. The utility of superoxide dismutase in studying free radical reactions: I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969, 244, 6056–6063. [Google Scholar] [CrossRef]
- Umamaheswari, M.; Chatterjee, T.K. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Elizebeth, K.; Rao, M.W.A. Oxygen radical scavenging activity of Curcumin. Int. J. Pharm. 1991, 58, 237–240. [Google Scholar]
- Enrol, D.; Mechmet, U.; Ferda, C.; Dimitra, D.; Gulhan, V.U.; Mosschos, P.; Atalay, S. Antimicrobial and antioxidant activities of essential oils and methanol extract of Saliva cryptantha (Montbret et AucherexBenth) and Saliva multicaulis (Vahl). J. Food Chem. 2013, 84, 519–525. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods Hum. Nutr. 2006, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D.; Tsai, C.L. Relationship between antioxidant activity and maturity of peanut hulls. J. Agric. Food Chem. 1993, 41, 67–70. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Da Porto, C.; Calligaris, S.; Celotti, E.; Nicoli, M.C. Antiradical properties of commercial cognacs assessed by the DPPH test. J. Agric. Food Chem. 2000, 48, 4241–4245. [Google Scholar] [CrossRef]
- Soare, J.R.; Dinis, T.C.; Cunha, A.P.; Almeida, L. Antioxidant activities of some extracts of Thymus Zygis. Free Radic. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]
- Badarinath, A.V.; Rao, K.M.; Chetty, C.M.; Ramkanth, S.T.; Rajan, T.V.; Gnanaprakash, K. A review on in-vitro antioxidant methods: Comparisons, correlations and considerations. Int. J. Pharmtech. Res. 2010, 2, 1276–1285. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Garratt, D.C. The Quantitative Analysis of Drugs, Japan; Chapman and Hall: Tokyo, Japan, 1964; Volume 3, pp. 456–458. [Google Scholar]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Tamir, S.; Tannenbaum, S.R. The role of nitric oxide (NO) in the carcinogenic process. Biochim. Biophys. Acta Rev. Cancer 1996, 1288, 31–36. [Google Scholar] [CrossRef]
- Sreejayan, N.; Rao, M.N.; Priyadarsini, K.I.; Devasagayam, T.P. Inhibition of radiation-induced lipid peroxidation by curcumin. Int. J. Pharm. 1997, 151, 127–130. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef]




| Sl No | Phytochemicals | Test/Reagent Used | Result |
|---|---|---|---|
| 01 | Carbohydrates | Molisch’s test | + |
| Iodine test | − | ||
| Fehling’s test | − | ||
| Benedict’s test | − | ||
| Barfoed’s test | − | ||
| Million’s test | − | ||
| 02 | Proteins | Biuret test | − |
| Xanthoproteic test | − | ||
| Ninhydrin test | − | ||
| Grease spot test | − | ||
| 03 | Fats/oil | Saponification test | − |
| Bontrager’s test | − | ||
| 04 | Alkaloids | Hager’s test | + |
| Dragendorff’s test | + | ||
| Liebermann’s sterol test | + | ||
| 05 | Sterols/steroids | Liebermann–Burchard test | + |
| Salkowski’s test | + | ||
| Ferric chloride test | + | ||
| 06 | Phenolics | Lead acetate test | + |
| Gelatine test | − | ||
| 07 | Terpenes/terpenoids | Tin and thionyl chloride test | + |
| 08 | Saponin glycosides | Form test | − |
| Haemolysis test | − | ||
| 09 | Gums/mucilage | Precipitation test | − |
| Ruthenium red test | − | ||
| Alkali (aqueous NaOH) | + | ||
| 10 | Flavones/flavonoids | Conc. H2SO4 | + |
| Shinoda test | + |
| Sl No. | Concentrations Exposed (µg/mL) | % Scavenging | ||
|---|---|---|---|---|
| Control | MECM | Ascorbic Acid | ||
| 1 | 100 | 0.00 | 11.79 ± 0.5469 *** | 29.24 ± 0.8712 *** |
| 2 | 250 | 16.50 ± 1.065 *** | 36.76 ± 1.3 *** | |
| 3 | 500 | 22.45 ± 1.131 *** | 52.06 ± 0.7963 *** | |
| Group Treated | Concentration Exposed (µg) | % Inhibition (Mean ± SEM) | Groups Compared and Significance |
|---|---|---|---|
| Control (A) | -- | 00 | -- |
| Standard (B) ascorbic acid | 50 (B1) | 36.09 ± 0.296 | A and B1 *** |
| 100 (B2) | 52.4 ± 0.387 | A and B2 *** | |
| 200 (B3) | 65.98 ± 0.589 | A and B3 *** | |
| Test (C) MECM | 50 (C1) | 19.56 ± 0.194 | A and C1 *** |
| 100 (C2) | 24.92 ± 0.194 | A and C2 *** | |
| 200 (C3) | 33.3 ± 0.194 | A and C3 *** |
| Sl No | Group | % Inhibition (Mean ± SEM) | Level of Significance and Groups Compared | ||
|---|---|---|---|---|---|
| 50 µg (A) | 100 µg (B) | 200 µg (C) | |||
| 1 | Control (I) | 00.00 | 00.00 | 00.00 | --- |
| 2 | Std (II) (Gallic acid) | 51.8 ± 0.744 | 68.8 ± 0.562 | 85.7 ± 0.342 | I(A) and II(A), II(B), II(C) *** |
| 3 | MECM (III) | 10.1 ± 1.39 | 20.13 ± 0.281 | 36.5 ± 1.55 | I(A) and III(A), III(B), III(C) *** |
| Sl NO | Concentration (µg /mL) | Reducing Power as % (Mean ± SEM) | Statistics | ||
|---|---|---|---|---|---|
| Control (A) | MECM (B) | Ascorbic Acid (C) | |||
| 1 | 50 (i) | 0.0 | 11.8 ± 0.6132 | 40.42 ± 1.35. | Bi and Ci *** Bi and A *** Ci and A *** |
| 2 | 100 (ii) | 19.37 ± 0.8192 | 53.98 ± 0.2405 | Bii and Cii ** Bii and A *** Cii and A *** | |
| 3 | 200 (iii) | 24.78 ± 0.8110 | 69.14 ± 0.2309 | Biii and Ciii *** Biii and A *** Ciii and A *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajasree, R.S.; Ittiyavirah, S.P.; Naseef, P.P.; Kuruniyan, M.S.; Anisree, G.S.; Elayadeth-Meethal, M. An Evaluation of the Antioxidant Activity of a Methanolic Extract of Cucumis melo L. Fruit (F1 Hybrid). Separations 2021, 8, 123. https://doi.org/10.3390/separations8080123
Rajasree RS, Ittiyavirah SP, Naseef PP, Kuruniyan MS, Anisree GS, Elayadeth-Meethal M. An Evaluation of the Antioxidant Activity of a Methanolic Extract of Cucumis melo L. Fruit (F1 Hybrid). Separations. 2021; 8(8):123. https://doi.org/10.3390/separations8080123
Chicago/Turabian StyleRajasree, R. S., Sibi P. Ittiyavirah, Punnoth Poonkuzhi Naseef, Mohamed Saheer Kuruniyan, G. S. Anisree, and Muhammed Elayadeth-Meethal. 2021. "An Evaluation of the Antioxidant Activity of a Methanolic Extract of Cucumis melo L. Fruit (F1 Hybrid)" Separations 8, no. 8: 123. https://doi.org/10.3390/separations8080123
APA StyleRajasree, R. S., Ittiyavirah, S. P., Naseef, P. P., Kuruniyan, M. S., Anisree, G. S., & Elayadeth-Meethal, M. (2021). An Evaluation of the Antioxidant Activity of a Methanolic Extract of Cucumis melo L. Fruit (F1 Hybrid). Separations, 8(8), 123. https://doi.org/10.3390/separations8080123






