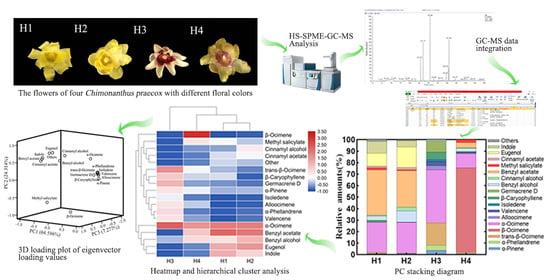
Analysis of Floral Fragrance Compounds of Chimonanthus praecox with Different Floral Colors in Yunnan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Collection
2.3. HS-SPME Analysis
2.4. GC-MS Analysis
2.5. Data Analysis
3. Results
3.1. Analysis on Types of Floral Fragrance Compounds of C. praecox with Different Floral Colors
3.2. Analysis of Floral Fragrance Compounds of C. praecox with Different Floral Colors
3.3. Principal Component (PC) Stacking Diagram of Floral Fragrance Compounds of C. praecox with Different Floral Colors
3.4. Hierarchical Cluster Analysis of Floral Fragrance Compounds of C. praecox with Different Floral Colors
3.5. PC Analysis (PCA) of Floral Fragrance Compounds of C. praecox with Different Floral Colors
4. Discussion
4.1. Comparison of Floral Fragrance Compounds of C. praecox with Different Floral Colors
4.2. Comparison of Floral Fragrance Compounds of C. praecox in Different Geographical Areas
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.Q. Research Advances on Calycanthaceae. Chin. Landsc. Archit. 2012, 28, 49–53. [Google Scholar] [CrossRef]
- Lin, S.Q.; Chen, R.D. Landscape Application and Industry Progress of Wintersweet. Chin. Landsc. Archit. 2020, 36, 104–108. [Google Scholar] [CrossRef]
- Tang, G.L.; Wang, M.B. On Aesthetic Value and Landscape Application of Wintersweet. J. Nanjing For. Univ. Humanit. Soc. Sci. Ed. 2017, 17, 146–152. [Google Scholar] [CrossRef]
- Lu, A.X.; Zhou, X.R.; Ye, Y.L.; Li, X.L.; Xie, G.H.; Wang, B.; Tong, H.R. Changes of Sensory Characteristic and Volatiles of Harvested Flowers of Chimonanthus praecox During Spreading Process. Acta Hortic. Sin. 2020, 47, 73–84. [Google Scholar] [CrossRef]
- Shen, Z.G.; Sun, M.; Yuan, D.Y.; Cheng, J.M.; Ding, X.; Shang, Z.H. HS-SPME-GC-MS Analysis of Volatile Components in Tender Shoots from Six Plants of Calycanthaceae. Acta Hortic. Sin. 2020, 47, 2349–2361. [Google Scholar] [CrossRef]
- Li, S.L.; Zhou, Z.R. Research Progress on Flavonoids and Coumarins from Chimonanthus Plants and its Pharmacological Activities. Chin. Tradit. Herb. Drugs 2018, 49, 3425–3431. [Google Scholar]
- Xu, J.B.; Pan, J.J.; Lv, Q.D.; Cheng, K.J. Research Advances on Chemical Constituents from Calycanthaceae Plants and their Pharmacological Activities. China J. Chin. Mater. Med. 2018, 43, 1957–1968. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The Formation and Function of Plant Volatiles: Perfumes for Pollinator Attraction and Defense. Physiol. Metab. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective Perfumes: The Role of Vegetative Volatiles in Plant Defense Against Herbivores. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J. Herbivore-Induced Defense Response in a Model Legume. Two-Spotted Spider Mites Induce Emission of (E)-β-Ocimene and Transcript Accumulation of (E)-β-Ocimene Synthase in Lotus Japonicas. Plant Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Y.; Wan, Y.M.; Sun, Z.H.; Li, T.Q.; Liu, X.F.; Ma, H.; Liu, X.X.; He, R.; Ma, Y.; Li, Z. Floral Scent Chemistry of Luculia yunnanensis (Rubiaceae), a Species Endemic to China with Sweetly Fragrant Flowers. Molecules 2017, 22, 879. [Google Scholar] [CrossRef] [Green Version]
- Natalia, D.; Eran, P.; Jonathan, G. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Schiestl, F.P. The Evolution of Floral Scent and Insect Chemical Communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Wake Up and Smell the Roses: The Ecology and Evolution of Floral Scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, H.H.; Lei, P.S.; Zhang, H.X.; Cheng, F.Y. Fragrance Composition in Six Tree Peony Cultivars. Hortic. Sci. Technol. 2012, 30, 617–625. [Google Scholar] [CrossRef]
- Vega, C.D.; Herrera, C.M.; Dötterl, S. Floral Volatiles Play a Key Role in Specialized Ant Pollination. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.Q.; Xiang, L.; Chen, L.Q. Reliminary Studies on the Components of Volatile Floral Flavor and Flower Pigments of Chimonanthus praecox. Beijing For. Univ. 2007, 29, 22–25. [Google Scholar]
- Zhou, J.R.; Ni, D.J. Changes in Flower Aroma Compounds of Cultivars of Chimonanthus praecox (L.) Link and at Different Stages Relative to Chimonanthus Tea Quality. Acta Hortic. Sin. 2010, 37, 1621–1628. [Google Scholar] [CrossRef]
- Yu, L. The Study of Violate Compounds and Flower Pigment in Chimonanthus praecox L. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2013. [Google Scholar]
- Li, Y.Y. Study of Floral Volatile Components and Lins Promoter Structure in Wintersweet. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar] [CrossRef]
- Li, Z.G.; Liu, M.C.; Deng, W.; Wang, X.Y.; Yang, Y.W. Comparative Analysis of the Essential Oil from Two Chimonanthus praecox Cultivars. Fine Chem. 2008, 25, 985–988, 992. [Google Scholar] [CrossRef]
- Feng, N. Determination of Floral Volatile Components and Preliminary Study on Function of Two Terpene Synthases in Wintersweet. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2017. [Google Scholar]
- Lin, X. Research Pogresson Volatile Components of Chimonanthus Lindl. Fujian Agric. Sci. Technol. 2019, 7, 57–64. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.W.; Wu, L.S.; Liu, J.J.; Si, J.P.; Zhang, X.F. Determination of Volatile Components from Chimonanthus Flowers by HS-SPME-GC-MS. Sci. Silvae Sin. 2016, 52, 58–65. [Google Scholar] [CrossRef]
- Xu, N.J.; Bai, H.B.; Yan, X.J.; Xu, J.L. Analysis of Volatile Components in Essential Oil of Chimonanthus nitens by Capillary Gas Chromatography-Mass Spectrometry. Instrum. Anal. 2006, 25, 90–93. [Google Scholar] [CrossRef]
- Pan, C.F. Studies on the Chemical Constituents from Chimonanthus praecox Flower and Its Extractions Obtained by Supercritical CO2. Master’s Thesis, Southwest University, Chongqing, China, 2017. [Google Scholar]
- Pichersky, E.; Dudareva, N. Scent Engineering: Toward the Goal of Controlling How Flowers Smell. Trends Biotechnol. 2007, 25, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.Q.; Zhu, Y.; Zhang, R.Y.; Sun, Y.L.; Wu, Z.P.; Liu, M.X. Study on the Aroma Components of Chimonanthus praecox. Acta Sci. Nat. Univ. Pekin. 1990, 36, 667–673. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.H.; Huang, Y.; Si, H.Q. Identification and Analysis on Aroma Components of Chimonanthus praecox. Sci. Technol. Food Ind. 2016, 37, 54–59. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y. Floral Scent Chemistry and Stamen Movement of Chimonanthus praecox (L.) Link (Calycanthaceae). Acta Phytotax. Geobot. 2005, 56, 197–201. [Google Scholar] [CrossRef]
- Cai, B.G.; Jiang, X.W.; Chen, Y. Analysis of Perfumed Constituent in Chimonanthus praecox by SPME-GC-MS. J. Technol. 2016, 16, 257–261. [Google Scholar] [CrossRef]
- Azuma, H.; Thien, L.B.; Kawano, S. Molecular Phylogeny of Magnolia (Magnoliaceae) Inferred from cpDNA Sequences and Evolutionary Divergence of the Floral Scents. J. Plant Res. 1999, 112, 291–306. [Google Scholar] [CrossRef]
- Raguso, R.A.; Schlumpberger, B.O.; Kaczorowski, R.L.; Holtsford, T.P. Phylogenetic fragrance patterns in Nicotiana sections Alatae and Suaveolentes. Phytochemistry 2006, 67, 1932–1942. [Google Scholar] [CrossRef]
- Deng, C.H.; Song, G.X.; Hu, Y.M. Rapid Determination of Volatile Compounds Emitted from Chimonanthus praecox Flowers by HS-SPME-GC-MS. Z. Für Nat. C 2004, 59, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Cao, H.; Lee, M.R.; Shen, D.L. Analysis of Volatile Compounds Emitted from Chimonanthus praecox (L.) Link in Different Florescence and QSRR Study of GC Retention Indices. Chromatographia 2009, 70, 1153–1162. [Google Scholar] [CrossRef]
- Jiang, T.; Yuan, J.P.; Cheng, C.G.; Li, S.E.; Wang, X.; Chen, L.Z. Analysis of the Essential Oil from Chimonanthus praecox. Chin. J. Spectrosc. Lab. 2005, 22, 211–214. [Google Scholar] [CrossRef]
- Wang, Y.G.; Huang, Y.H.; Zhang, C.; Fu, J.X.; Zhao, H.B. Analysis of Aroma Compounds from Calycanthus floridus var. glaucus Flowers. In Proceedings of the Ornamental Horticulture Symposium in China 2015, Xiamen, China, 18 August 2015. [Google Scholar]
- Ding, X.; Shen, X.H.; Guo, W.; Tang, Z.H. Breeding of a New Cultivar Chimonanthus praecox ‘Yuqiao No.1′ and Its Key Cultivation Techniques. North. Hortic. 2020, 13, 178–180. [Google Scholar] [CrossRef]
- Song, X.R.; Yuan, P.Y.; He, X.D. A New Cultivar of Chimonanthus praecox ‘Juanbei Jinpan’. Acta Hortic. Sin. 2020, 47, 3108–3109. [Google Scholar] [CrossRef]




| Compound Category | Relative Content (%) ± SD and Type Number | |||
|---|---|---|---|---|
| H1 | H2 | H3 | H4 | |
| Terpenes | 28.48 ± 0.37 (9) 1 | 26.04 ± 1.18 (7) | 96.65 ± 2.98 (9) | 88.15 ± 2.28 (12) |
| Alcohols | 4.23 ± 0.46 (2) | 11.85 ± 0.65 (2) | 0.84 ± 0.03 (1) | 3.12 ± 0.14 (2) |
| Esters | 39.88 ± 1.27 (3) | 31.90 ± 1.46 (5) | - 2 | 6.89 ± 0.54 (2) |
| Phenols | 10.63 ± 0.38 (1) | 16.44 ± 0.30 (1) | - | 0.06 ± 0.02 (1) |
| Aldehydes | 0.42 ± 0.03 (2) | 0.64 ± 0.13 (1) | - | - |
| Aromatic hydrocarbons | - | - | 0.43 ± 0.09 (1) | 0.56 ± 0.10 (2) |
| Heterocyclic | 9.88 ± 0.46 (1) | 4.86 ± 0.63 (1) | - | - |
| Others | 1.22 ± 0.08 (1) | 0.87 ± 0.20 (1) | - | 0.02 ± 0.01 (1) |
| Total | 94.74 ± 3.05 (19) | 92.60 ± 4.55 (18) | 97.92 ± 3.10 (11) | 98.8 ± 3.09 (20) |
| Classification | Compound Name | RI 1 | Relative Content (%) ± SD | |||
|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | |||
| Terpenes | Cyclooctatetraene | 850 | - 2 | 0.07 ± 0.02 | - | 0.10 ± 0.02 |
| α-Thujene | 929 | 0.19 ± 0.04 | - | - | - | |
| α-Pinene | 937 | 0.78 ± 0.10 | 0.80 ± 0.16 | 3.60 ± 0.27 | 1.02 ± 0.16 | |
| Sabinene | 974 | - | - | - | 0.33 ± 0.06 | |
| α-Phellandrene | 1005 | 0.04 ± 0.01 | 0.12 ± 0.02 | 4.45 ± 0.38 | - | |
| β-Ocimene | 1037 | - | - | - | 71.51 ± 0.60 | |
| α-Ocimene | 1047 | 24.59 ± 0.05 | 24.52 ± 0.88 | 45.09 ± 0.87 | 11.92 ± 0.94 | |
| trans-β-Ocimene | 1049 | 0.85 ± 0.00 | 0.25 ± 0.01 | 18.75 ± 0.23 | 1.09 ± 0.14 | |
| γ-Terpinene | 1060 | 0.04 ± 0.00 | - | - | 0.11 ± 0.03 | |
| Terpinolene | 1088 | 0.15 ± 0.00 | - | - | - | |
| Alloocimene | 1131 | - | - | 3.57 ± 0.18 | 0.63 ± 0.10 | |
| Isoledene | 1375 | - | - | 1.61 ± 0.38 | - | |
| β-Caryophyllene | 1419 | 1.30 ± 0.13 | - | 6.25 ± 0.12 | 0.71 ± 0.08 | |
| Aromandendrene | 1440 | - | - | - | 0.24 ± 0.04 | |
| Germacrene D | 1481 | 0.54 ± 0.04 | 0.15 ± 0.06 | 9.70 ± 0.28 | 0.25 ± 0.07 | |
| Valencene | 1492 | - | 0.13 ± 0.03 | 3.63 ± 0.27 | 0.24 ± 0.04 | |
| Alcohols | Benzyl alcohol | 1036 | 3.62 ± 0.33 | 8.89 ± 0.64 | 0.84 ± 0.03 | 2.95 ± 0.10 |
| Cinnamyl alcohol | 1313 | 0.61 ± 0.13 | 2.96 ± 0.01 | - | 0.17 ± 0.04 | |
| Esters | Benzyl acetate | 1164 | 36.85 ± 0.97 | 28.97 ± 0.97 | - | 4.49 ± 0.24 |
| Methyl salicylate | 1192 | 1.72 ± 0.14 | 0.96 ± 0.14 | - | 2.40 ± 0.30 | |
| Bornyl acetate | 1285 | - | 0.28 ± 0.07 | - | - | |
| Methyl cinnamate | 1379 | - | 0.05 ± 0.01 | - | - | |
| Cinnamyl acetate | 1445 | 1.31 ± 0.16 | 1.64 ± 0.27 | - | - | |
| Phenols | Eugenol | 1357 | 10.63 ± 0.38 | 16.44 ± 0.30 | - | 0.06 ± 0.02 |
| Aldehydes | Benzyaldehyde | 962 | 0.24 ± 0.00 | - | - | - |
| Cinnamal dehyde | 1274 | 0.18 ± 0.03 | 0.64 ± 0.13 | - | - | |
| Aromatic | p-Xylene | 865 | - | - | - | 0.40 ± 0.07 |
| hydrocarbons | o-Cymene | 1022 | - | - | - | 0.16 ± 0.03 |
| m-Cymene | 1023 | - | - | 0.43 ± 0.09 | - | |
| Heterocyclic | Indole | 1295 | 9.88 ± 0.46 | 4.86 ± 0.63 | - | - |
| Others | Phenyl-pentamethyl-disiloxane | 1157 | 1.22 ± 0.08 | 0.87 ± 0.20 | - | 0.02 ± 0.01 |
| Total | 31 | 19 | 19 | 11 | 19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Shi, R.; Wang, Q.; Wang, S. Analysis of Floral Fragrance Compounds of Chimonanthus praecox with Different Floral Colors in Yunnan, China. Separations 2021, 8, 122. https://doi.org/10.3390/separations8080122
Meng L, Shi R, Wang Q, Wang S. Analysis of Floral Fragrance Compounds of Chimonanthus praecox with Different Floral Colors in Yunnan, China. Separations. 2021; 8(8):122. https://doi.org/10.3390/separations8080122
Chicago/Turabian StyleMeng, Liubei, Rui Shi, Qiong Wang, and Shu Wang. 2021. "Analysis of Floral Fragrance Compounds of Chimonanthus praecox with Different Floral Colors in Yunnan, China" Separations 8, no. 8: 122. https://doi.org/10.3390/separations8080122
APA StyleMeng, L., Shi, R., Wang, Q., & Wang, S. (2021). Analysis of Floral Fragrance Compounds of Chimonanthus praecox with Different Floral Colors in Yunnan, China. Separations, 8(8), 122. https://doi.org/10.3390/separations8080122