Immunomodulatory Activity of Phyllanthus maderaspatensis in LPS-Stimulated Mouse Macrophage RAW 264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Collection of Plant Material
2.3. Extraction of Plant Materials
2.3.1. Preparation of Aqueous Ethanolic Extract
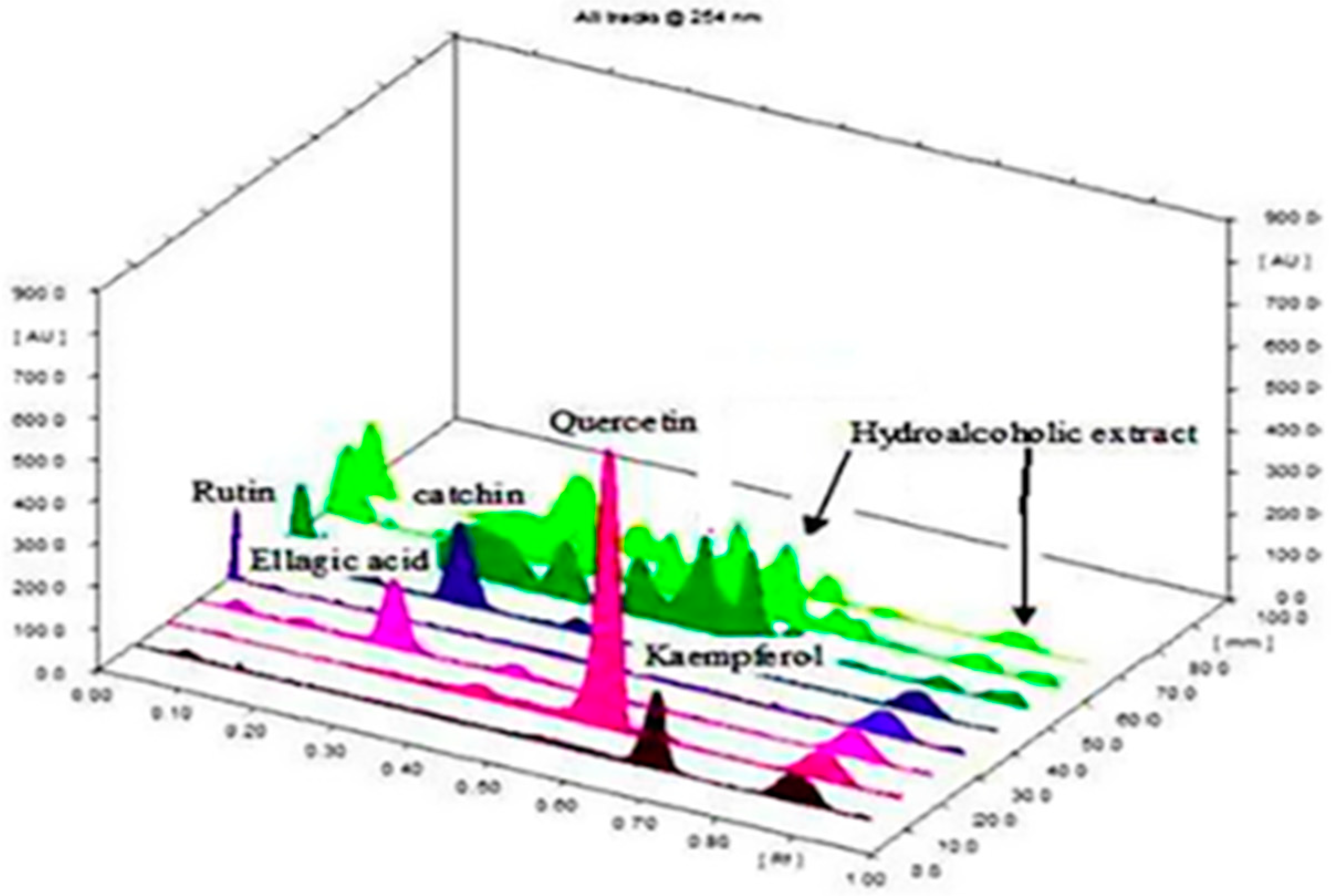
2.3.2. Standardization of Polyphenols in P. maderaspatensis by HPTLC
2.3.3. Estimation of Different Markers
2.3.4. Fractionation of Potent Aqueous Ethanolic (50% v/v) Extracts of P. maderaspatensis
2.3.5. Nitric Oxide Estimation of Various Fractions of P. maderaspatensis
2.3.6. Isolation and Purification of Potent Fractions by Vacuum Liquid Chromatography
2.3.7. Statistical Analysis
2.3.8. Maintenance of Cell Lines
2.3.9. Method for Passaging the Cells
2.3.10. Calibration Curves for Standard Markers
3. Results
3.1. Percentage Yield of the Different Crude Extracts of P. maderaspatensis
3.2. HPTLC Fingerprint Profile of Aqueous Ethanolic Extracts of P. maderaspatensis
Development of Optimum Mobile Phase
3.3. Fractionation of Aqueous Ethanolic Extract of P. maderaspatensis
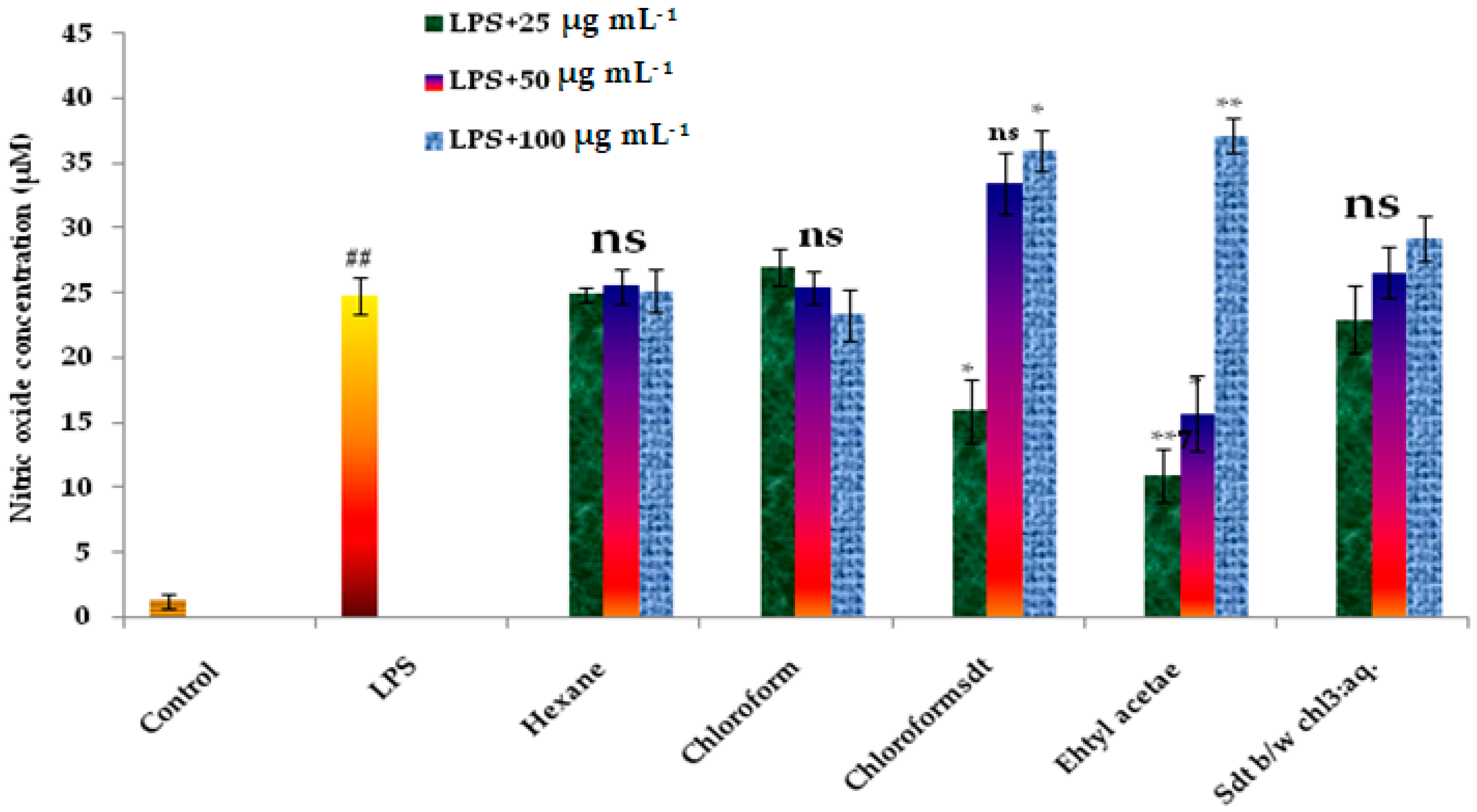
3.4. Stimulation of Inducible NO Synthesis by the Different Fractions of P. maderaspatensis
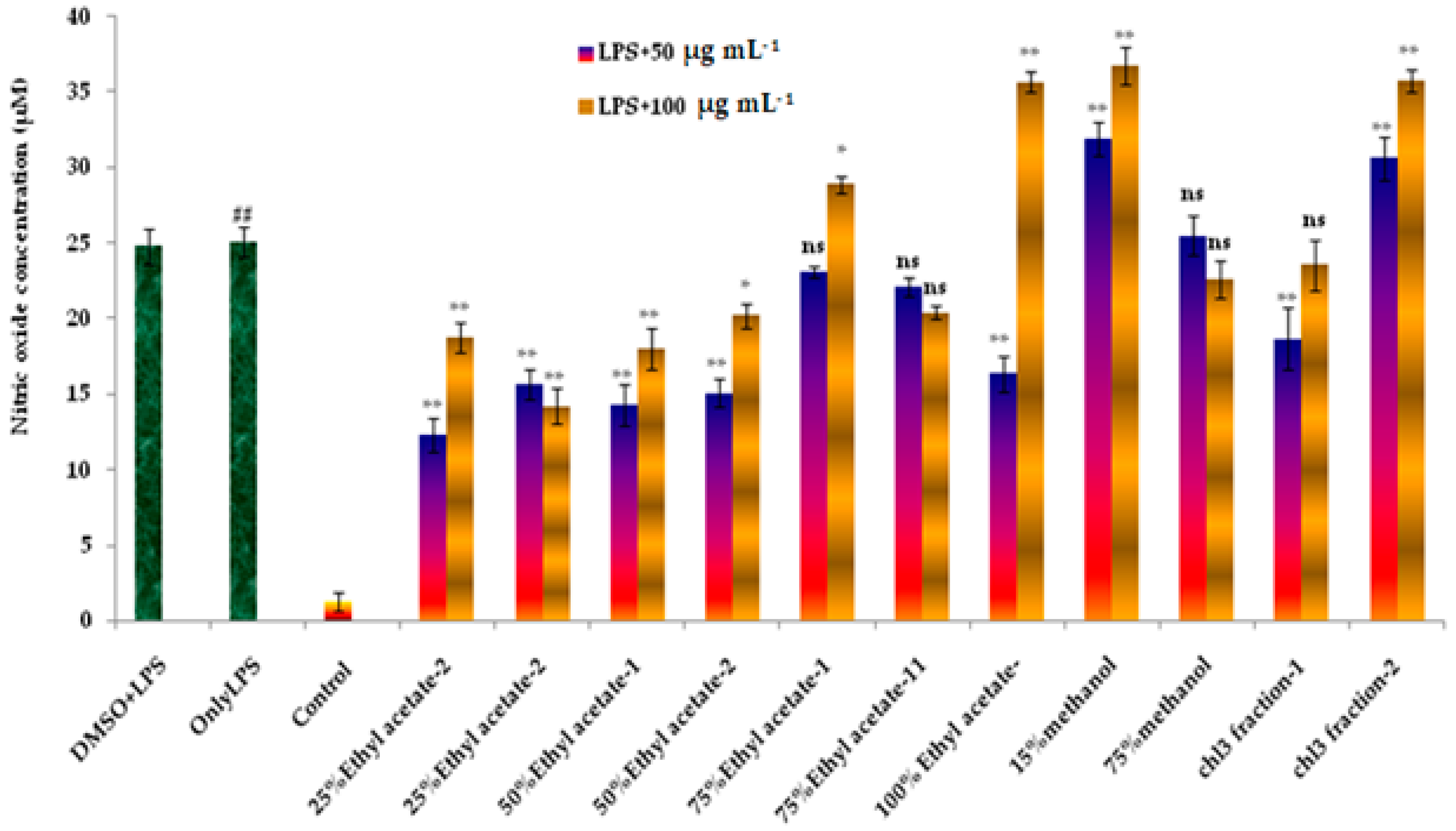
3.5. Vacuum Liquid Chromatography and Selected Activity of Column Eluents of Ethyl Acetate Fraction
3.6. Effect of Column Eluents of Ethyl Acetate Fraction on LPS-Stimulated NO Production in RAW 264.7 Cells
3.7. Stimulation of Inducible NO Synthesis by Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Munshi, A.; Mehrotra, R.; Ramesh, R.; Panda, S.K. Evaluation of anti-hepadnavirus activity of Phyllanthus amarus and Phyllanthus maderaspatensis in duck hepatitis B virus carrier Pekin ducks. J. Med. Virol. 1993, 41, 275–281. [Google Scholar] [CrossRef]
- Asha, V.V.; Akhila, S.; Wills, P.J.; Subramoniam, A. Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis Linn. J. Ethnopharmacol. 2004, 92, 67–70. [Google Scholar] [CrossRef]
- Bagavan, A.; Kamaraj, C.; Elango, G.; Abduz Zahir, A.; Abdul Rahuman, A. Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Vet. Parasitol. 2009, 166, 286–292. [Google Scholar] [CrossRef]
- Prashanth, D.; Padmaja, R.; Samiulla, D.S. Effect of certain plant extracts on alpha-amylase activity. Fitoterapia 2001, 72, 179–181. [Google Scholar] [CrossRef]
- Srirama, R.; Deepak, H.B.; Senthilkumar, U.; Ravikanth, G.; Gurumurthy, B.R.; Shivanna, M.B.; Chandrasekaran, C.V.; Agarwal, A.; Shaanker, R.U. Hepatoprotective activity of Indian Phyllanthus. Pharm. Biol. 2012, 50, 948–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xia, Q.; Yang, G.; Zhu, D.; Shao, Y.; Zhang, J.; Cui, Y.; Wang, R.; Zhang, L. The anti-HIV-1 activity of polyphenols from Phyllanthus urinaria and the pharmacokinetics and tissue distribution of its marker compound, gallic acid. J. Tradit. Chin. Med. Sci. 2017, 4, 158–166. [Google Scholar] [CrossRef]
- Tan, W.C.; Jaganath, I.B.; Manikam, R.; Sekaran, S.D. Evaluation of antiviral activities of four local Malaysian Phyllanthus species against herpes simplex viruses and possible antiviral target. Int. J. Med. Sci. 2013, 10, 1817–1829. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. Acetone, ethanol and methanol extracts of Phyllanthus urinaria inhibit HSV-2 infection in vitro. Antiviral. Res. 2005, 67, 24–30. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Kaur, R.; Bhat, Z.A.; Gupta, V.K.; Kumar, V. A review of immunomodulators in the Indian traditional health care system. J. Microbiol. Immunol. Infect. 2012, 45, 165–184. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 18, 286–313. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Misra, H.; Verma, R.K.; Gupta, M.M. Chemical fingerprinting of Andrographis paniculata using HPLC, HPTLC and densitometry. Phytochem. Anal. 2004, 15, 280–285. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajuguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. Ca. Cancer J. Clin. 2006, 56, 69–83. [Google Scholar] [CrossRef]
- Ilyas, U.; Katare, D.P.; Aeri, V.; Naseef, P.P. A Review on hepatoprotective and immunomodulatory herbal plants. Pharmacogn. Rev. 2016, 10, 66–70. [Google Scholar]
- Tomar, N.; De, R.K. A brief outline of the immune system. In Immunoinformatics; Humana Press: New York, NY, USA, 2014; pp. 3–12. [Google Scholar]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.K. Nutrition and the immune system: An introduction. Am. J. Clin. Nutr. 1997, 66, 460S–463S. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, M.; Corsini, E.; Racchi, M.; Lanni, C. Immunomodulatory inspired by nature: A review on curcumin and echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattler, S. The role of the immune system beyond the fight against infection. Adv. Exp. Med. Biol. 2017, 1003, 3–14. [Google Scholar]
- Schaller, M. The behavioral immune system and the psychology of human sociality. Philos. Trans. R Soc. Lond. B Boil. Sci. 2011, 366, 3418–3426. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorder. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed. Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef]
- Green, S.J.; Mellouk, S.; Hoffman, S.L.; Meltzer, M.S.; Nacy, C.A. Cellular mechanisms of nonspecific immunity to intracellular infection: Cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol. Lett. 1990, 25, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Yue, G.G.L.; Lau, C.B.S.; Leung, P.C. Medicinal Plants and Mushrooms with Immunomodulatory and Anticancer Properties—A Review on Hong Kong’s Experience. Molecules 2021, 26, 2173. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.J.; De Zoysa, M.; Whang, I.; Ryu, B. Octominin inhibits LPS-induced chemokine and pro-inflammatory cytokine secretion from RAW 264.7 macrophages via blocking TLRs/NF-κB signal transduction. Biomolecules 2020, 10, 511. [Google Scholar] [CrossRef]
- Arango, D.G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Shivaprasad, H.N.; Kharya, M.D.; Rana, A.C.; Mohan, S. Preliminary immunomodulatory activities of the aqueous extract of Terminalia chebula. Pharm. Biol. 2016, 44, 32–34. [Google Scholar] [CrossRef] [Green Version]
- Bayan, L.; Koulivand, P.H.; Gorji, A. A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar]
- Muhammad, R.; Najm, U.-R.; Muhammad, Z.-U.-H.; Hawa, Z.E.J.; Rosana, M. Ginseng: A dietary supplement as immune-modulator in various diseases. Trends Food Sci. Tech. 2019, 83, 12–30. [Google Scholar]
- Smit, H.F.; Kroes, B.H.; van den Berg, A.J.; Van der Wal, D.; Van den Worm, E.; Beukelman, C.J.; Van Dij, K.H.; Labadie, R.P. Immunomodulatory and anti-inflammatory activity of Picrorhiza scrophulariiflora. J. Ethnopharmacol. 2000, 73, 101–109. [Google Scholar] [CrossRef]
- Punturee, K.; Wild, C.P.; Kasinrerk, W.; Vinitketkumnuen, U. Immunomodulatory activities of Centella asiatica and Rhinacanthusnasutus extracts. Asian Pac. J. Cancer Prev. 2005, 6, 396–400. [Google Scholar]
- Sharma, U.; Bala, M.; Kumar, N.; Singh, B.; Munshi, R.K.; Bhalerao, S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012, 14, 918–926. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Ilangkovan, M.; Arshad, L. An insight in to the modulatory effects and mechanisms of action of Phyllanthus species and their bioactive metabolites on the immune system. Front. Pharmacol. 2019, 10, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bin-Hafeez, B.; Haque, R.; Parvez, S.; Pandey, S.; Sayeed, I.; Raisuddin, S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003, 3, 257–265. [Google Scholar] [CrossRef]
- Sudam, V.S.; Potnuri, A.G.; Subhashini, N.J.P. Syk—GTP RAC-1 mediated immune-stimulatory effect of Cuscutaepithymum, Ipomoea batata and Euphorbia hirta plant extracts. Biomed. Pharmacother. 2017, 96, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.V.; Padmavathi, K. Assessment of immunomodulatory activity of Euphorbia hirta L. Indian J. Pharm. Sci. 2010, 72, 621–625. [Google Scholar] [PubMed] [Green Version]
- Zhou, Y.; Tang, Q.; Du, H.; Tu, Y.; Wu, S.; Wang, W.; Xu, M. Antiviral effect of ovotransferrin in mouse peritoneal macrophages by up-regulating type I interferon expression. Food Agric. Immunol. 2018, 29, 600–614. [Google Scholar] [CrossRef] [Green Version]

| Sl. No. | Solvent System | Visualizing Agents | Inference |
|---|---|---|---|
| 01 | Hexane/ethyl acetate (7:3) | Anisaldehyde sulphuric acid | Poor separation |
| 02 | Ethyl acetate/chloroform (40:60) | Anisaldehyde sulphuric acid | Poor separation |
| 03 | Ethyl acetate/methanol/water (100:13.5:10) | Anisaldehyde sulphuric acid | Poor separation |
| 04 | Ethyl acetate/formic acid/acetic acid/water (100:11:11:27) | Anisaldehyde sulphuric acid | Tailing |
| 05 | n-propanol/ethyl acetate/water (40:40:30) | Anisaldehyde sulphuric acid | Tailing |
| 06 | Toluene/ethyl acetate/formic acid (5:4:1) | NP reagents | Good separation |
| 07 | Toluene/ethyl acetate/formic acid/methanol (3:3:0.8:0.02) | NP reagents | Best separation |
| Dose | Control | LPS | Hexane | Chloroform | Chloroform Std. | Ethyl Acetate | Std b/w chl3: aq. |
|---|---|---|---|---|---|---|---|
| - | 1.3 ± 0.6 | 25.77 ± 1.4 | |||||
| LPS + 25 μg mL−1 | - | - | 25.9 ± 0.6 | 27.03 ± 1.4 | 15.96 ± 2.5 | 10.97 ± 2.1 | 22.99 ± 2.6 |
| LPS + 50 μg mL−1 | - | - | 25.56 ± 1.4 | 25.43 ± 1.3 | 33.45 ± 2.34 | 15.76 ± 2.9 | 26.62 ± 1.9 |
| LPS + 100 μg mL−1 | - | - | 25.19 ± 1.6 | 23.33 ± 2.0 | 35.92 ± 1.6 | 37.17 ± 1.3 | 29.25 ± 1.74 |
| Tracks | Solvent Combination | Rf Values | Tracks | Solvent Combination | Rf Values |
|---|---|---|---|---|---|
| 01 | Ellagic acid | 0.55 | 11 | Toluene + 50% ethyl acetate-1 | 0.48, 0.48, 0.52, 0.72, 0.78 |
| 02 | eupalitin | 0.23 | 12 | Toluene + 50% ethyl acetate-2 | 0.27, 0.48 |
| 03 | Rutin | 0.08 | 13 | Toluene + 50% ethyl acetate-3 | 0.08, 0.12, 0.18, 0.26, 0.36, 0.42, 0.51, 0.60, 0.67 |
| 04 | Kaempferol | 0.81 | 14 | Toluene + 50% ethyl acetate-4 | 0.08, 0.16, 0.26, 0.56, 0.62, 0.70, 0.87 |
| 05 | Epicatechin | 0.50 | 15 | Toluene + 75% ethyl acetate-1 | 0.17, 0.27, 0.34, 0.50, 0.54, 0.75 |
| 06 | Catechin | 0.54 | 16 | Toluene + 75% ethyl acetate-2 | 0.17, 0.27, 0.35, 0.46, 0.54 |
| 07 | Toluene + 25% ethyl acetate-1 | 0.76, 0.81 | 17 | Toluene + 75% ethyl acetate-3 | 0.17, 0.27, 0.35, 0.46, 0.55, 0.68, 0.75 |
| 08 | Toluene + 25% ethyl acetate-2 | 0.25, 0.34, 0.48, 0.59, 0.64, 0.7, 0.81, 0.90 | 18 | Toluene + 75% ethyl acetate-4 | 0.05, 0.17, 0.27, 0.35, 0.46, 0.55 |
| 09 | Toluene + 25% ethyl acetate-3 | 0.58, 0.68, 0.72 | 19 | 100% ethyl acetate | 0.08, 0.55 |
| 10 | Toluene + 25% ethyl acetate-4 | 0.48 | 20 | 50% ethyl acetate remain | 0.25, 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, U.; Katare, D.P.; Naseef, P.P.; Kuruniyan, M.S.; Elayadeth-Meethal, M.; Aeri, V. Immunomodulatory Activity of Phyllanthus maderaspatensis in LPS-Stimulated Mouse Macrophage RAW 264.7 Cells. Separations 2021, 8, 129. https://doi.org/10.3390/separations8090129
Ilyas U, Katare DP, Naseef PP, Kuruniyan MS, Elayadeth-Meethal M, Aeri V. Immunomodulatory Activity of Phyllanthus maderaspatensis in LPS-Stimulated Mouse Macrophage RAW 264.7 Cells. Separations. 2021; 8(9):129. https://doi.org/10.3390/separations8090129
Chicago/Turabian StyleIlyas, Uoorakkottil, Deepshikha P. Katare, Punnoth Poonkuzhi Naseef, Mohamed Saheer Kuruniyan, Muhammed Elayadeth-Meethal, and Vidhu Aeri. 2021. "Immunomodulatory Activity of Phyllanthus maderaspatensis in LPS-Stimulated Mouse Macrophage RAW 264.7 Cells" Separations 8, no. 9: 129. https://doi.org/10.3390/separations8090129
APA StyleIlyas, U., Katare, D. P., Naseef, P. P., Kuruniyan, M. S., Elayadeth-Meethal, M., & Aeri, V. (2021). Immunomodulatory Activity of Phyllanthus maderaspatensis in LPS-Stimulated Mouse Macrophage RAW 264.7 Cells. Separations, 8(9), 129. https://doi.org/10.3390/separations8090129






