Efficient Sub-1 Minute Analysis of Selected Biomarker Catecholamines by Core-Shell Hydrophilic Interaction Liquid Chromatography (HILIC) with Nanomolar Detection at a Boron-Doped Diamond (BDD) Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cyclic Voltammetry (CV)
2.3. HPLC Instrumentation
2.4. Chromatographic Conditions
2.5. Validation Procedures
3. Results and Discussion
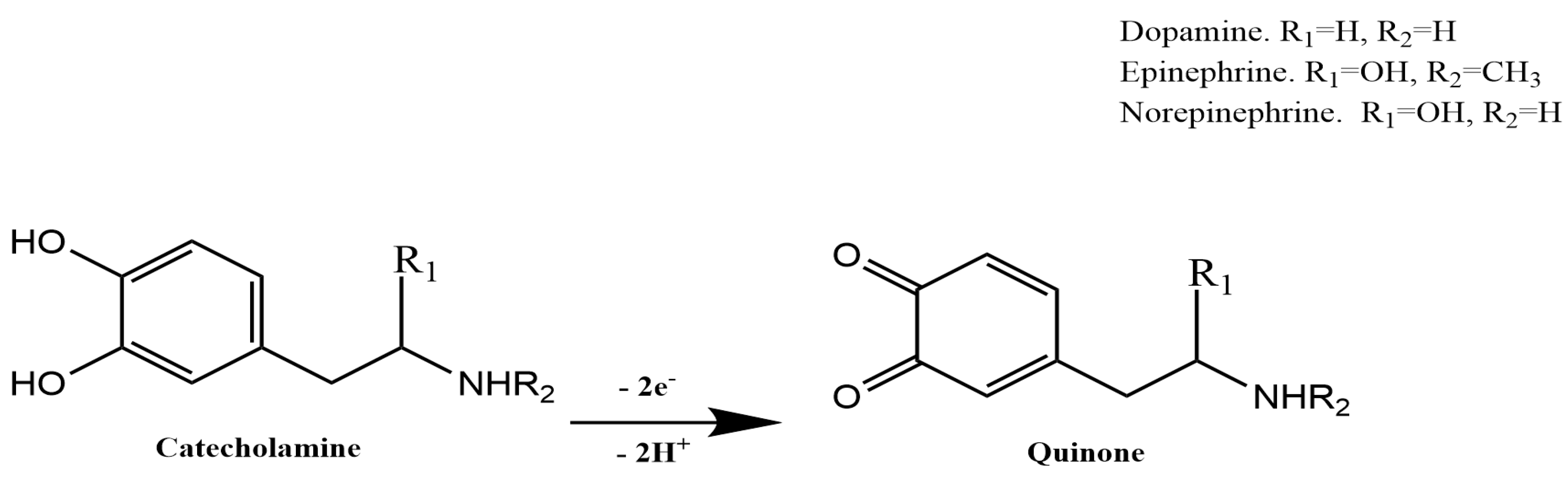
3.1. Electrochemical Behavior of the CAs Using Cyclic Voltammetry (CV)
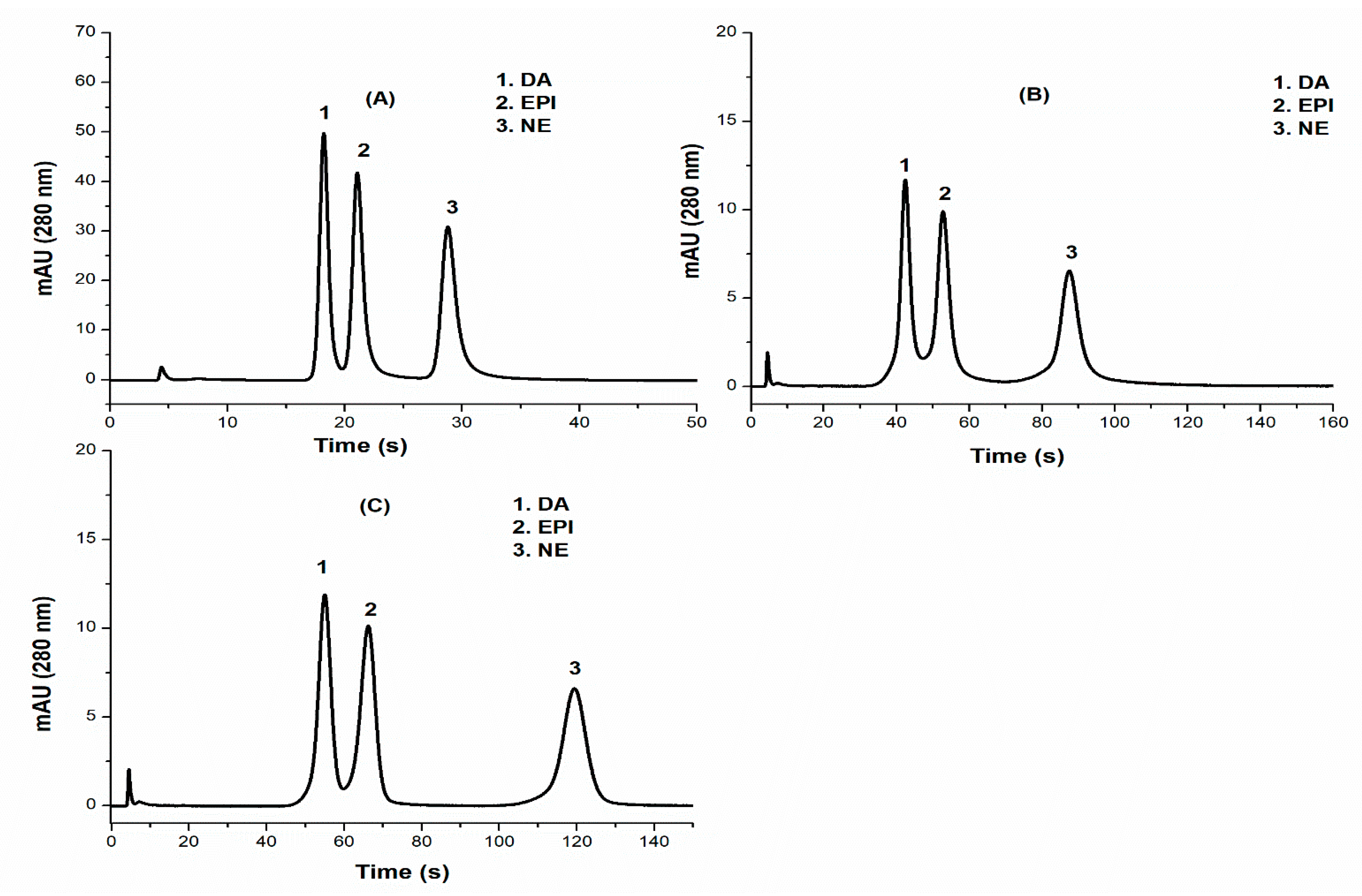
3.2. Comparison of Zwitterionic HILIC Columns
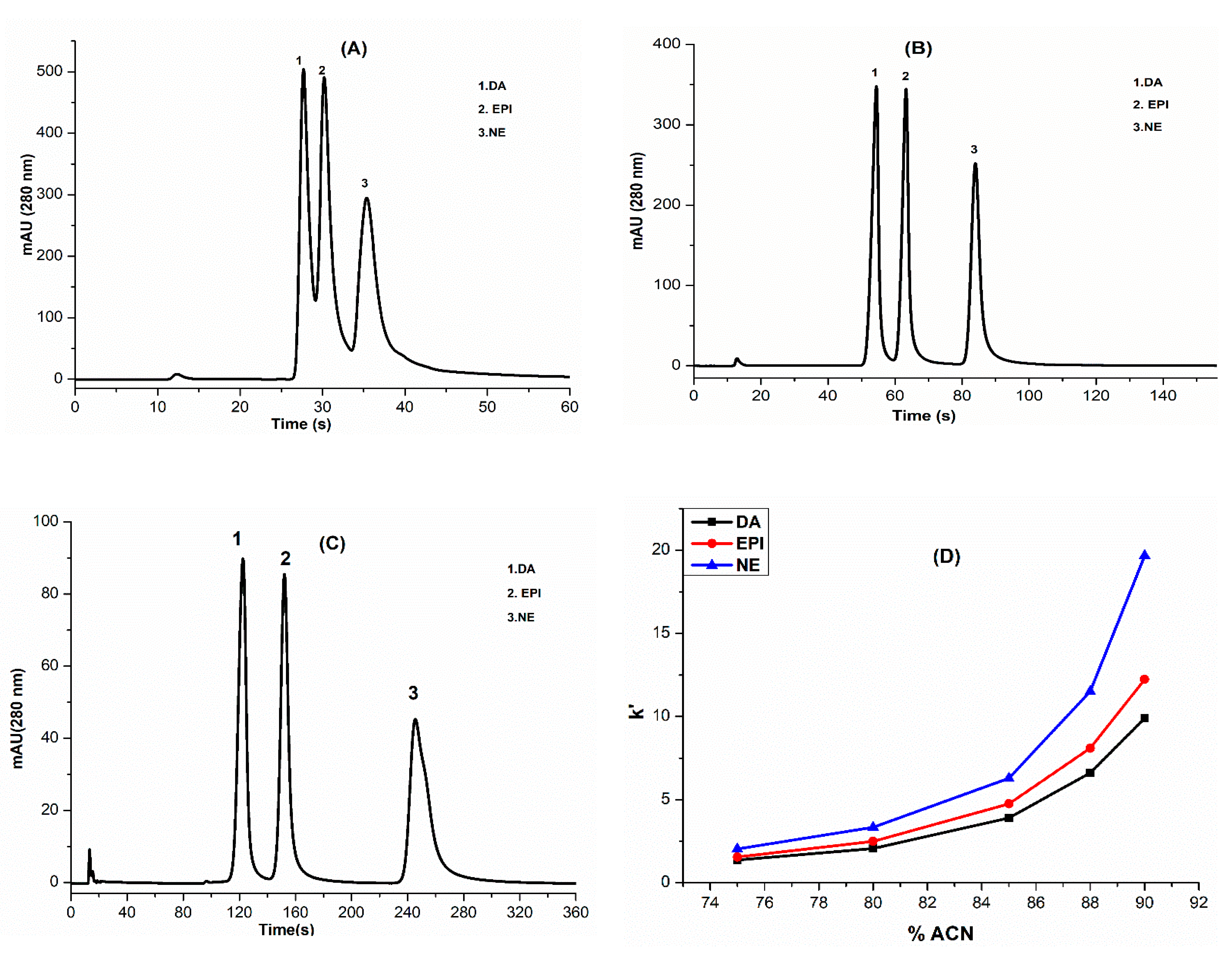
3.3. Optimization of HILIC Separation Conditions
3.4. Optimization of Detection Potential for LC-ECD
3.5. Method Validation
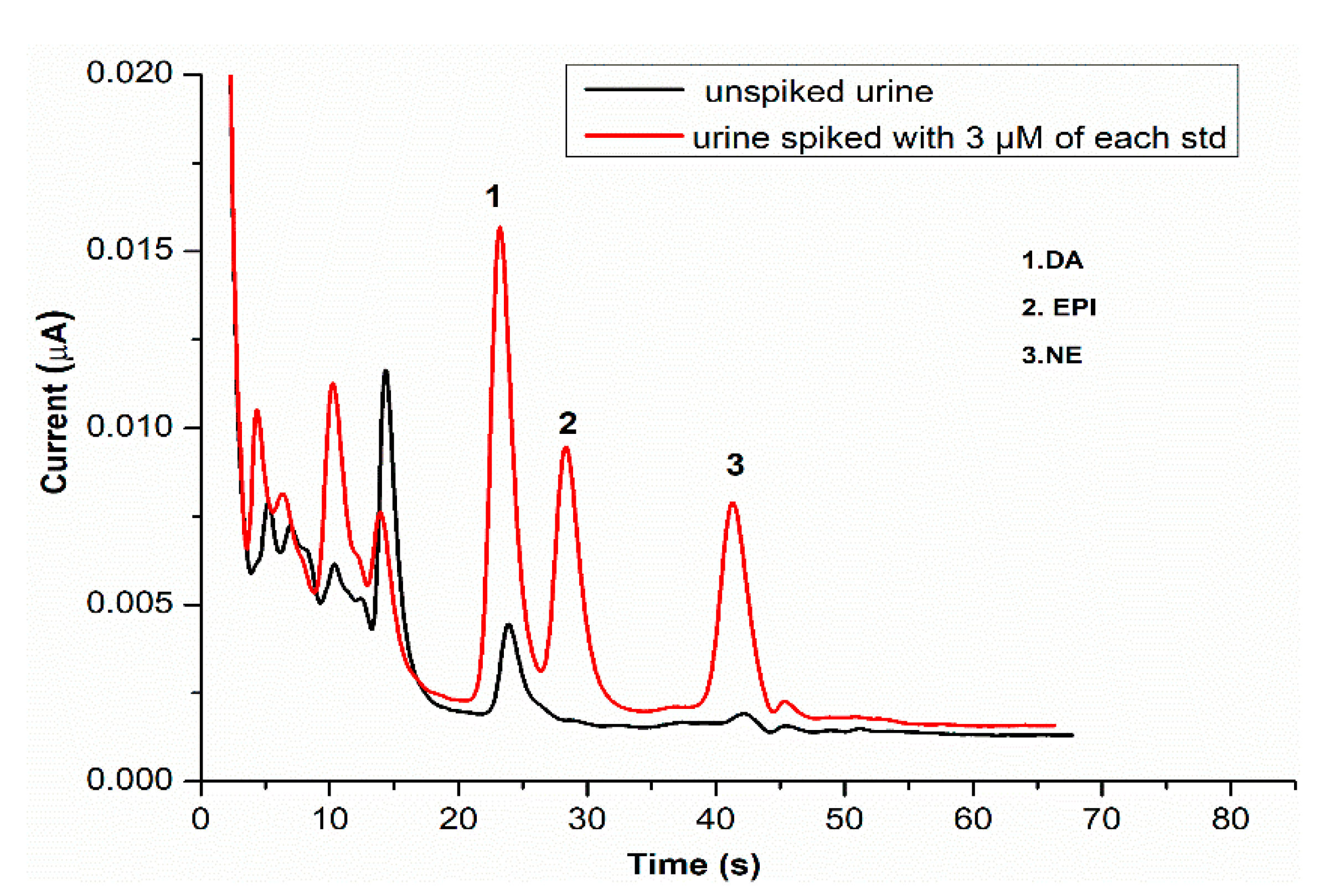
3.6. Urine Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, D.S.; Eisenhofer, G.; Kopin, I.J. Sources and Significance of Plasma Levels of Catechols and Their Metabolites in Humans. J. Pharmacol. Exp. Ther. 2003, 305, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverty, R. Catecholamines: Role in Health and Disease. Drugs 1978, 16, 418–440. [Google Scholar] [CrossRef] [PubMed]
- Focke, P.J.; Wang, X.; Larsson, H.P. Neurotransmitter transporters: Structure meets function. Structure 2013, 21, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Kaminga, A.C.; Jia, P.; Wen, S.W.; Acheampong, K.; Liu, A. Catecholamines in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 184. [Google Scholar] [CrossRef]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Hernández, A.; Figuero-Pérez, L.; Cruz-Hernandez, J.J.; González Sarmiento, R.; Usategui-Martin, R.; Miramontes-González, J.P. Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications. Biomolecules 2021, 11, 254. [Google Scholar] [CrossRef]
- Adameova, A.; Abdellatif, Y.; Dhalla, N.S. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can. J. Physiol. Pharmacol. 2009, 87, 493–514. [Google Scholar] [CrossRef]
- Fung, M.M.; Viveros, O.H.; O’Connor, D.T. Diseases of the adrenal medulla. Acta Physiol. 2008, 192, 325–335. [Google Scholar] [CrossRef]
- Barron, J. Phaeochromocytoma: Diagnostic challenges for biochemical screening and diagnosis. J. Clin. Pathol. 2010, 63, 669–674. [Google Scholar] [CrossRef]
- Rizoli, S.B.; Jaja, B.N.R.; Di Battista, A.P.; Rhind, S.G.; Neto, A.C.; da Costa, L.; Inaba, K.; da Luz, L.T.; Nascimento, B.; Perez, A.; et al. Catecholamines as outcome markers in isolated traumatic brain injury: The COMA-TBI study. Crit. Care 2017, 21, 37. [Google Scholar] [CrossRef] [Green Version]
- Hamill, R.W.; Woolf, P.D.; McDonald, J.V.; Lee, L.A.; Kelly, M. Catecholamines predict outcome in traumatic brain injury. Ann. Neurol. 1987, 21, 438–443. [Google Scholar] [CrossRef]
- Clifton, G.L.; Ziegler, M.G.; Grossman, R.G. Circulating catecholamines and sympathetic activity after head injury. Neurosurgery 1981, 8, 10–14. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Urazgildeeva, G.R.; Abaimov, D.A.; Fedotova, E.Y.; Poleschuk, V.V.; Illarioshkin, S.N.; Lokhov, P.G. Parkinson’s Disease: Available Clinical and Promising Omics Tests for Diagnostics, Disease Risk Assessment, and Pharmacotherapy Personalization. Diagnostics 2020, 10, 339. [Google Scholar] [CrossRef]
- Sawada, H.; Oeda, T.; Yamamoto, K. Catecholamines and Neurodegeneration in Parkinson’s Disease-From Diagnostic Marker to Aggregations of α-Synuclein. Diagnostics 2013, 3, 210–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y. Cerebrospinal fluid biomarkers of central catecholamine deficiency in Parkinson’s disease and other synucleinopathies. Brain 2012, 135, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, Y.; Hirschberg, Y.; Mertens, I.; De Deyn, P.P. Biofluid Markers for Prodromal Parkinson’s Disease: Evidence from a Catecholaminergic Perspective. Front. Neurol. 2020, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Nodel, M.R.; Pavlenko, T.A.; Chesnokova, N.B.; Yakhno, N.N.; Ugrumov, M.V. Tear Fluid Catecholamines as Biomarkers of the Parkinson’s Disease: A Clinical and Experimental Study. Acta Nat. 2019, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, J. Degeneration of Dopaminergic Neurons due to Metabolic Alterations and Parkinson’s Disease. Front. Aging Neurosci. 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Chekhonin, V.P.; Baklaushev, V.P.; Kogan, B.M.; Savchenko, E.A.; Lebedev, S.V.; Man’kovskaya, I.V.; Filatova, T.S.; Yusupova, I.U.; Dmitrieva, T.B. Catecholamines and their metabolites in the brain and urine of rats with experimental Parkinson’s disease. Bull. Exp. Biol. Med. 2000, 130, 805–809. [Google Scholar]
- van Faassen, M.; Bischoff, R.; Eijkelenkamp, K.; de Jong, W.H.A.; van der Ley, C.P.; Kema, I.P. In Matrix Derivatization Combined with LC-MS/MS Results in Ultrasensitive Quantification of Plasma Free Metanephrines and Catecholamines. Anal. Chem. 2020, 92, 9072–9078. [Google Scholar] [CrossRef]
- Chung, H.; Tajiri, S.; Hyoguchi, M.; Koyanagi, R.; Shimura, A.; Takata, F.; Dohgu, S.; Matsui, T. Analysis of Catecholamine and Their Metabolites in Mice Brain by Liquid Chromatography–Mass Spectrometry Using Sulfonated Mixed-mode Copolymer Column. Anal. Sci. 2019, 35, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Peitzsch, M.; Pelzel, D.; Glöckner, S.; Prejbisz, A.; Fassnacht, M.; Beuschlein, F.; Januszewicz, A.; Siegert, G.; Eisenhofer, G. Simultaneous liquid chromatography tandem mass spectrometric determination of urinary free metanephrines and catecholamines, with comparisons of free and deconjugated metabolites. Clin. Chim. Acta 2013, 418, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Sakaguchi, A.; Kamada, S.; Fujimoto, T.; Takechi, S.; Kakinoki, S.; Nomura, A. Simultaneous analysis of human plasma catecholamines by high-performance liquid chromatography with a reversed-phase triacontylsilyl silica column. J. Chromatogr. B Biomed. Sci. Appl. 2006, 830, 249–254. [Google Scholar] [CrossRef]
- Kumarathasan, P.; Vincent, R. New approach to the simultaneous analysis of catecholamines and tyrosines in biological fluids. J. Chromatogr. A 2003, 987, 349–358. [Google Scholar] [CrossRef]
- Ehrenström, F.; Johansson, P. A method for very rapid determinations of catechols using ion-pairing reverse phase HPLC with electrochemical detection: Effects of L-DOPA treatment on the catechol content in various rat brain structures. Life Sci. 1985, 36, 867–879. [Google Scholar] [CrossRef]
- Speek, A.J.; Odink, J.; Schrijver, J.; Schreurs, W.H.P. High-performance liquid chromatographic determination of urinary free catecholamines with electrochemical detection after prepurification on immobilized boric acid. Clin. Chim. Acta 1983, 128, 103–113. [Google Scholar] [CrossRef]
- Birbeck, J.A.; Mathews, T.A. Simultaneous Detection of Monoamine and Purine Molecules Using High-Performance Liquid Chromatography with a Boron-Doped Diamond Electrode. Anal. Chem. 2013, 85, 7398–7404. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, X.-F.; Wang, H.; Zhang, H.-S. Analysis of catecholamines and related compounds in one whole metabolic pathway with high performance liquid chromatography based on derivatization. Arab. J. Chem 2019, 12, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Konieczna, L.; Roszkowska, A.; Niedźwiecki, M.; Bączek, T. Hydrophilic interaction chromatography combined with dispersive liquid–liquid microextraction as a preconcentration tool for the simultaneous determination of the panel of underivatized neurotransmitters in human urine samples. J. Chromatogr. A 2016, 1431, 111–121. [Google Scholar] [CrossRef]
- Miękus, N.; Konieczna, L.; Kowiański, P.; Moryś, J.; Bączek, T. HILIC-MS Rat Brain Analysis, A New Approach for the Study of Ischemic Attack. Transl. Neurosci. 2017, 8, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Hart, J.P.; McCalley, D.V. Determination of catecholamines in urine using hydrophilic interaction chromatography with electrochemical detection. J. Chromatogr. A 2011, 1218, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.V. Study of the selectivity, retention mechanisms and performance of alternative silica-based stationary phases for separation of ionised solutes in hydrophilic interaction chromatography. J. Chromatogr. A 2010, 1217, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ikegami, T.; Kimura, H.; Hara, T.; Tokuda, D.; Tanaka, N. Properties of monolithic silica columns for HPLC. Anal. Sci. 2006, 22, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, K. Applications of silica-based monolithic HPLC columns. J. Sep. Sci 2004, 27, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core–shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. J. Chromatogr. A 2014, 1357, 36–52. [Google Scholar] [CrossRef] [Green Version]
- Gosetti, F. Benefits and Drawbacks of Fully Porous sub-2 µm and Core-Shell Particles. Comparative UHPLC Applications in Food, Environmental, Forensic, Biopharmaceutical and Natural Products Analyses. Curr. Chromatogr 2018, 5, 18–32. [Google Scholar] [CrossRef]
- DeStefano, J.J.; Schuster, S.A.; Lawhorn, J.M.; Kirkland, J.J. Performance characteristics of new superficially porous particles. J. Chromatogr. A 2012, 1258, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, J.J.; Schuster, S.A.; Johnson, W.L.; Boyes, B.E. Fused-core particle technology in high-performance liquid chromatography: An overview. J. Pharm. Anal. 2013, 3, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Wagner, B.M.; Schuster, S.A.; Boyes, B.E.; Kirkland, J.J. Superficially porous silica particles with wide pores for biomacromolecular separations. J. Chromatogr. A 2012, 1264, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Langsi, V.K.; Ashu-Arrah, B.A.; Ward, N.; Glennon, J.D. Synthesis and characterisation of non-bonded 1.7μm thin-shell (TS1.7-100nm) silica particles for the rapid separation and analysis of uric acid and creatinine in human urine by hydrophilic interaction chromatography. J. Chromatogr. A 2017, 1506, 37–44. [Google Scholar] [CrossRef]
- Ruta, J.; Guillarme, D.; Rudaz, S.; Veuthey, J.-L. Comparison of columns packed with porous sub-2 μm particles and superficially porous sub-3 μm particles for peptide analysis at ambient and high temperature. J. Sep. Sci. 2010, 33, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Barman, K.; Jasimuddin, S. Simultaneous electrochemical detection of dopamine and epinephrine in the presence of ascorbic acid and uric acid using a AgNPs–penicillamine–Au electrode. RSC Adv. 2016, 6, 99983–99988. [Google Scholar] [CrossRef]
- Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical oxidation of histamine and serotonin at highly boron- doped diamond electrodes. Anal. Chem. 2000, 72, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.M.; Anderson, A.B.; Angus, J.C. Applications of Diamond Thin Films in Electrochemistry. MRS Bull. 1998, 23, 56–60. [Google Scholar] [CrossRef]
- Bouamrane, F.; Tadjeddine, A.; Butler, J.E.; Tenne, R.; Lévy-Clément, C. Electrochemical study of diamond thin films in neutral and basic solutions of nitrate. J. Electroanal. Chem. 1996, 405, 95–99. [Google Scholar] [CrossRef]
- DeClements, R.; Swain, G.M. The formation and electrochemical activity of microporous diamond thin film electrodes in concentrated KOH. J. Electrochem. Soc. 1997, 144, 856–866. [Google Scholar] [CrossRef]
- Rao, T.N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Electroanalytical study of sulfa drugs at diamond electrodes and their determination by HPLC with amperometric detection. J. Electroanal. Chem. 2000, 491, 175–181. [Google Scholar] [CrossRef]
- Terashima, C.; Rao, T.N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Electrochemical Oxidation of Chlorophenols at a Boron-Doped Diamond Electrode and Their Determination by High-Performance Liquid Chromatography with Amperometric Detection. Anal. Chem. 2002, 74, 895–902. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Sato, R.; Makide, Y.; Fujishima, A.; Einaga, Y. Pt-implanted boron-doped diamond electrodes and the application for electrochemical detection of hydrogen peroxide. Diam. Relat. Mater. 2005, 14, 2133–2138. [Google Scholar] [CrossRef]
- Hayes, P.E.; Luong, J.H.T.; Gilchrist, E.S.; Buzid, A.; Glennon, J.D. Profiling of phenolic flavorings using core-shell reversed-phase liquid chromatography with electrochemical detection at a boron-doped diamond electrode. J. Chromatogr. A 2020, 1612, 460649. [Google Scholar] [CrossRef] [PubMed]
- Hawley, M.D.; Tatawawadi, S.V.; Piekarski, S.; Adams, R.N. Electrochemical Studies of the Oxidation Pathways of Catecholamines. J. Am. Chem. Soc. 1967, 89, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Sochr, J.; Švorc, Ľ.; Rievaj, M.; Bustin, D. Electrochemical determination of adrenaline in human urine using a boron-doped diamond film electrode. Diam. Relat. Mater. 2014, 43, 5–11. [Google Scholar] [CrossRef]
- Wei, M.; Sun, L.-G.; Xie, Z.-Y.; Zhii, J.-F.; Fujishima, A.; Einaga, Y.; Fu, D.-G.; Wang, X.-M.; Gu, Z.-Z. Selective Determination of Dopamine on a Boron-Doped Diamond Electrode Modified with Gold Nanoparticle/Polyelectrolyte-coated Polystyrene Colloids. Adv. Funct. Mater. 2008, 18, 1414–1421. [Google Scholar] [CrossRef]
- Brycht, M.; Baluchová, S.; Taylor, A.; Mortet, V.; Sedláková, S.; Klimša, L.; Kopeček, J.; Schwarzová-Pecková, K. Comparison of electrochemical performance of various boron-doped diamond electrodes: Dopamine sensing in biomimicking media used for cell cultivation. Bioelectrochemistry 2021, 137, 107646. [Google Scholar] [CrossRef]
- Colleran, J.J.; Breslin, C.B. Simultaneous electrochemical detection of the catecholamines and ascorbic acid at PEDOT/S-β-CD modified gold electrodes. J. Electroanal. Chem. 2012, 667, 30–37. [Google Scholar] [CrossRef]
- Ferry, Y.; Leech, D. Amperometric Detection of Catecholamine Neurotransmitters Using Electrocatalytic Substrate Recycling at a Laccase Electrode. Electroanalysis 2005, 17, 113–119. [Google Scholar] [CrossRef]
- Benická, E.; Krupcík, J.; Lehotay, J.; Sandra, P.; Armstrong, D.W. Selectivity Tuning in an HPLC Multicomponent Separation. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1453–1471. [Google Scholar] [CrossRef]
- McCalley, D.V. Is hydrophilic interaction chromatography with silica columns a viable alternative to reversed-phase liquid chromatography for the analysis of ionisable compounds? J. Chromatogr. A 2007, 1171, 46–55. [Google Scholar] [CrossRef]
- Alvarez-Segura, T.; Subirats, X.; Rosés, M. Retention-pH profiles of acids and bases in hydrophilic interaction liquid chromatography. Anal. Chim. Acta 2019, 1050, 176–184. [Google Scholar] [CrossRef]
- Bicker, W.; Wu, J.; Yeman, H.; Albert, K.; Lindner, W. Retention and selectivity effects caused by bonding of a polar urea-type ligand to silica: A study on mixed-mode retention mechanisms and the pivotal role of solute-silanol interactions in the hydrophilic interaction chromatography elution mode. J. Chromatogr. A 2011, 1218, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Shi, H.; Ma, C.; Cong, B.; Kang, W. Determination of three major catecholamines in human urine by capillary zone electrophoresis with chemiluminescence detection. Anal. Biochem. 2012, 427, 10–17. [Google Scholar] [CrossRef]
- Bouri, M.; Lerma-García, M.J.; Salghi, R.; Zougagh, M.; Ríos, A. Selective extraction and determination of catecholamines in urine samples by using a dopamine magnetic molecularly imprinted polymer and capillary electrophoresis. Talanta 2012, 99, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.J. Simultaneous measurement of urinary metanephrines and catecholamines by liquid chromatography with tandem mass spectrometric detection. Ann. Clin. Biochem. 2009, 46, 129–136. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.-q.; Luo, Y.; Shang, J.-c.; Jiang, X.-h. Simultaneous determination of eleven compounds related to metabolism of bioamines in rat cortex and hippocampus by HPLC-ECD with boron-doped diamond working electrode. J. Pharm. Biomed. Anal. 2016, 118, 41–51. [Google Scholar] [CrossRef]
- Nikolajsen, R.P.H.; Hansen, Å.M. Analytical methods for determining urinary catecholamines in healthy subjects. Anal. Chim. Acta 2001, 449, 1–15. [Google Scholar] [CrossRef]
- Thomas, D.H.; Taylor, J.D.; Barnaby, O.S.; Hage, D.S. Determination of free catecholamines in urine by tandem affinity/ion-pair chromatography and flow injection analysis. Clin. Chim. Acta 2008, 398, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.H.; Gornet, T.G. Preparation of urine samples for liquid-chromatographic determination of catecholamines: Bonded-phase phenylboronic acid, cation-exchange resin, and alumina adsorbents compared. Clin. Chem. 1985, 31, 298–302. [Google Scholar] [CrossRef]
- Bicker, J.; Fortuna, A.; Alves, G.; Falcão, A. Liquid chromatographic methods for the quantification of catecholamines and their metabolites in several biological samples—A review. Anal. Chim. Acta 2013, 768, 12–34. [Google Scholar] [CrossRef]
- Chou, D. Clinical Guide to Laboratory Tests. JAMA 1984, 251, 2587–2588. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, L. Biochemical Diagnosis of Pheochromocytoma and Paraganglioma. In Paraganglioma: A Multidisciplinary Approach; Mariani-Costantini, R., Ed.; Codon Publications: Brisbane, Australia, 2019. [Google Scholar]
- Patel, D.; Phay, J.E.; Yen, T.W.F.; Dickson, P.V.; Wang, T.S.; Garcia, R.; Yang, A.D.; Solórzano, C.C.; Kim, L.T. Update on Pheochromocytoma and Paraganglioma from the SSO Endocrine/Head and Neck Disease-Site Work Group. Part 1 of 2: Advances in Pathogenesis and Diagnosis of Pheochromocytoma and Paraganglioma. Ann. Surg. Oncol. 2020, 27, 1329–1337. [Google Scholar] [CrossRef] [PubMed]


| HILIC Columns | Analyte | N/m | RSD (%) |
|---|---|---|---|
| Poro-Shell Z-HILIC | DA | 49,040 | 0.98 |
| EPI | 43,132 | 0.91 | |
| NE | 44,172 | 1.86 | |
| Fully Porous Z-cHILIC | DA | 23,019 | 0.72 |
| EPI | 23,887 | 1.04 | |
| NE | 31,247 | 1.36 | |
| Fully Porous Z-HILIC | DA | 18,395 | 0.69 |
| EPI | 16,973 | 2.01 | |
| NE | 22,598 | 2.27 |
| HILIC Columns | Analyte | α | RSD (%) |
|---|---|---|---|
| Poro-Shell Z-HILIC | DA& EPI | 1.30 | 0.572 |
| EPI& NE | 1.39 | 0.187 | |
| Fully Porous Z-cHILIC | DA& EPI | 1.22 | 0.417 |
| EPI& NE | 1.84 | 0.132 | |
| Fully Porous Z-HILIC | DA& EPI | 1.26 | 0.231 |
| EPI& NE | 1.71 | 0.214 |
| Analyte | Linear Range (µM) | Linear Regression Equation | Correlation Coefficient (R2) | LOD a HPLC-ECD (nM) | LOD b HPLC-UV (µM) |
|---|---|---|---|---|---|
| DA | 0.1–25 | y = 6 × 10−8 C − 4 × 10−9 | 0.994 | 40 | 0.5 |
| EPI | 0.1–25 | y = 5 × 10−8 C − 3 × 10−9 | 0.993 | 50 | 0.7 |
| NE | 0.1–25 | y = 4 × 10−8 C − 4 × 10−9 | 0.995 | 50 | 1 |
| Technique | Analytes | Separation Time | LOD | Ref. |
|---|---|---|---|---|
| CE-CL | DA EPI NE | 6 min | 69 nM 79 nM 100 nM | [62] |
| MMIP-CE | DA EPI NE | 8 min | 60 nM 60 nM 50 nM | [63] |
| HPLC- fluorescence detection | DA EPI NE | 40 min | 0.1 nM 0.4 nM 0.4 nM | [29] |
| RP-Amperometric detection (BDD electrode) | DA EPI NE | 9 min | 4 × 10−3 µg/mL (26 nM) 2 × 10−3 µg/mL (11 nM) 4 × 10−3 µg/mL (23 nM) | [65] |
| HPLC-MS-MS | DA EPI NE | 6 min | 5 nM 10 nM 10 nM | [64] |
| HILIC- Amperometric detection (BDD electrode) | DA EPI NE | less than 1 min | 40 nM (7.5 × 10−3 µg/mL) 50 nM (10.6 × 10−3 µg/mL) 50 nM (10.2 × 10−3 µg/mL) | This work |
| Analyte | RSD a (%) Intra-Day | RSD b (%) Inter-Day |
|---|---|---|
| DA | 0.42 | 0.65 |
| EPI | 0.35 | 0.40 |
| NE | 0.36 | 0.49 |
| Analytes | Concentration (nM) in Unspiked Urine | Concentration (nM) Added | Concentration (nM) Found | Recovery % | RSD % |
|---|---|---|---|---|---|
| DA | 1440 | 600 1500 3000 | 1990 2800 4170 | 91.6 93 90.9 | 4.6 2.7 2.3 |
| EPI | ND a | 250 500 1000 | 231 471 915 | 92.4 94 91.5 | 2.3 6 4 |
| NE | 112 | 400 800 1600 | 473 840 1550 | 90.3 91 90 | 1.4 2.7 2.4 |
| Sample | DA (nM) ± SD | EPI (nM) ± SD | NE (nM) ± SD |
|---|---|---|---|
| 1 | 1440 ± 1.44 | ND a | 112 ± 1.67 |
| 2 | 1793 ± 1.34 | ND a | 158 ± 2.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaeedi, M.; Alghamdi, H.; Hayes, P.E.; Hogan, A.M.; Glennon, J.D. Efficient Sub-1 Minute Analysis of Selected Biomarker Catecholamines by Core-Shell Hydrophilic Interaction Liquid Chromatography (HILIC) with Nanomolar Detection at a Boron-Doped Diamond (BDD) Electrode. Separations 2021, 8, 124. https://doi.org/10.3390/separations8080124
Alsaeedi M, Alghamdi H, Hayes PE, Hogan AM, Glennon JD. Efficient Sub-1 Minute Analysis of Selected Biomarker Catecholamines by Core-Shell Hydrophilic Interaction Liquid Chromatography (HILIC) with Nanomolar Detection at a Boron-Doped Diamond (BDD) Electrode. Separations. 2021; 8(8):124. https://doi.org/10.3390/separations8080124
Chicago/Turabian StyleAlsaeedi, Majidah, Huda Alghamdi, Phyllis E. Hayes, Anna M. Hogan, and Jeremy D. Glennon. 2021. "Efficient Sub-1 Minute Analysis of Selected Biomarker Catecholamines by Core-Shell Hydrophilic Interaction Liquid Chromatography (HILIC) with Nanomolar Detection at a Boron-Doped Diamond (BDD) Electrode" Separations 8, no. 8: 124. https://doi.org/10.3390/separations8080124
APA StyleAlsaeedi, M., Alghamdi, H., Hayes, P. E., Hogan, A. M., & Glennon, J. D. (2021). Efficient Sub-1 Minute Analysis of Selected Biomarker Catecholamines by Core-Shell Hydrophilic Interaction Liquid Chromatography (HILIC) with Nanomolar Detection at a Boron-Doped Diamond (BDD) Electrode. Separations, 8(8), 124. https://doi.org/10.3390/separations8080124





