New Concept for the Study of the Fluid Dynamics of Lithium Extraction Using Calix[4]arene Derivatives in T-Type Microreactor Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of the Microreactor System
2.3. Study of Fluid Dynamics of Li(I) Extraction in a T-Type Microreactor System
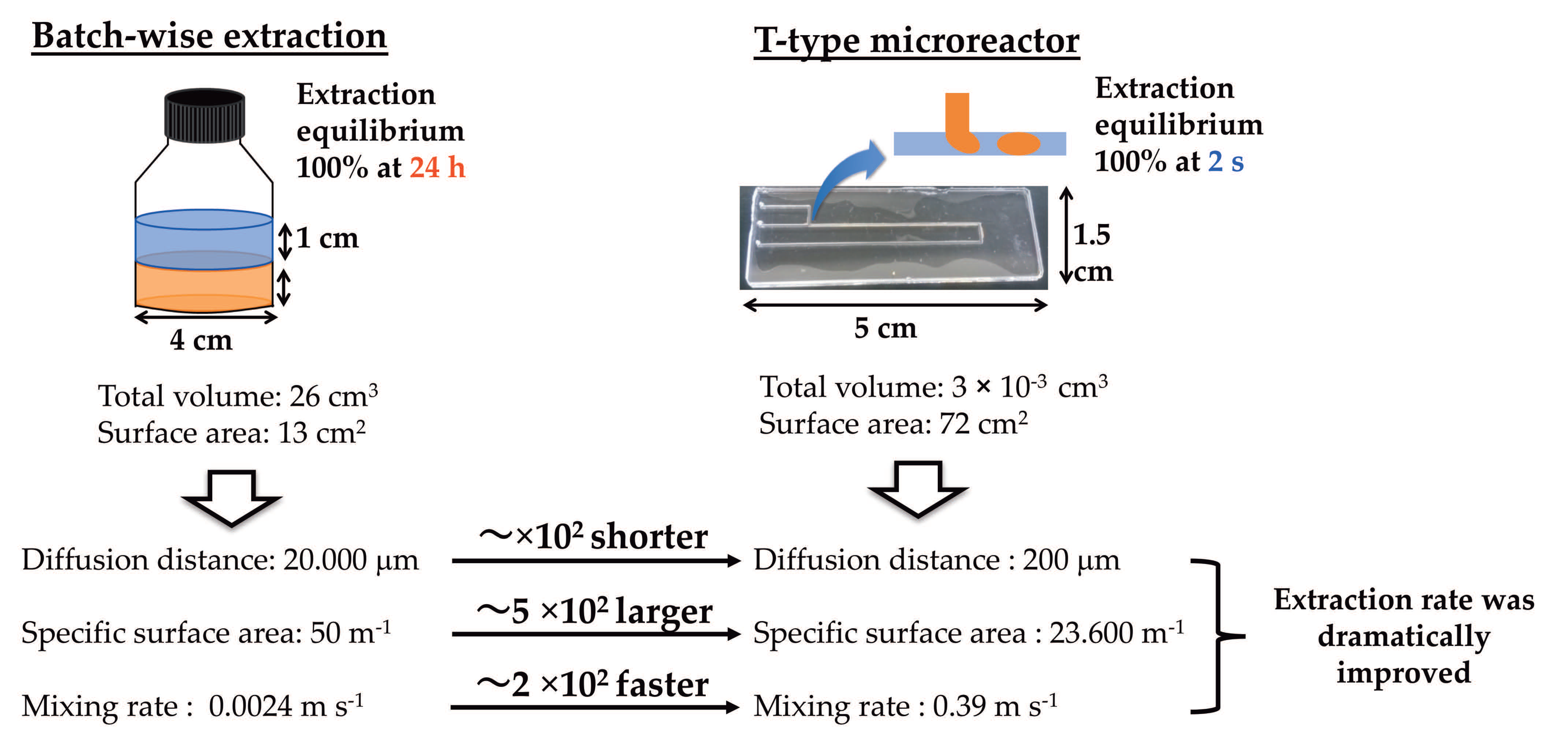
3. Results and Discussion
3.1. Fluid Flow Pattern in a T-Type Microreactor System
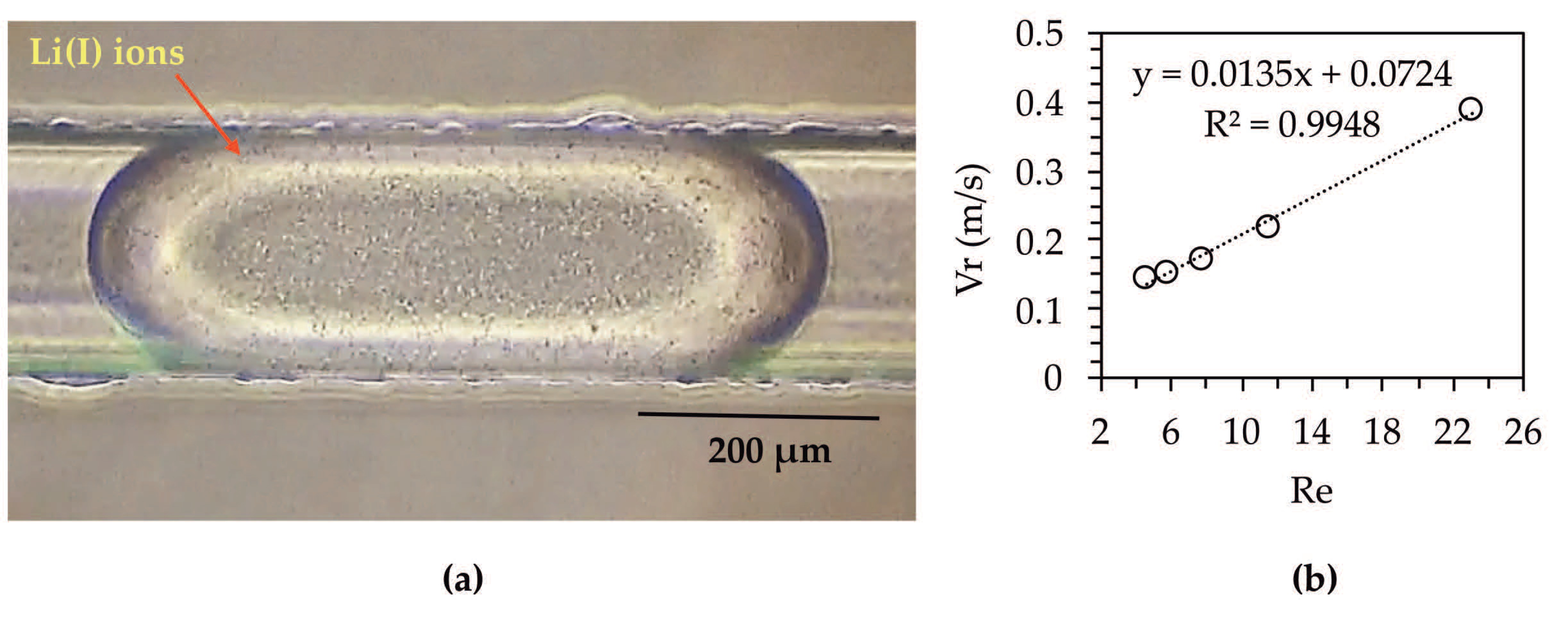
3.2. Organic Droplet Formation in a T-Type Microreactor System
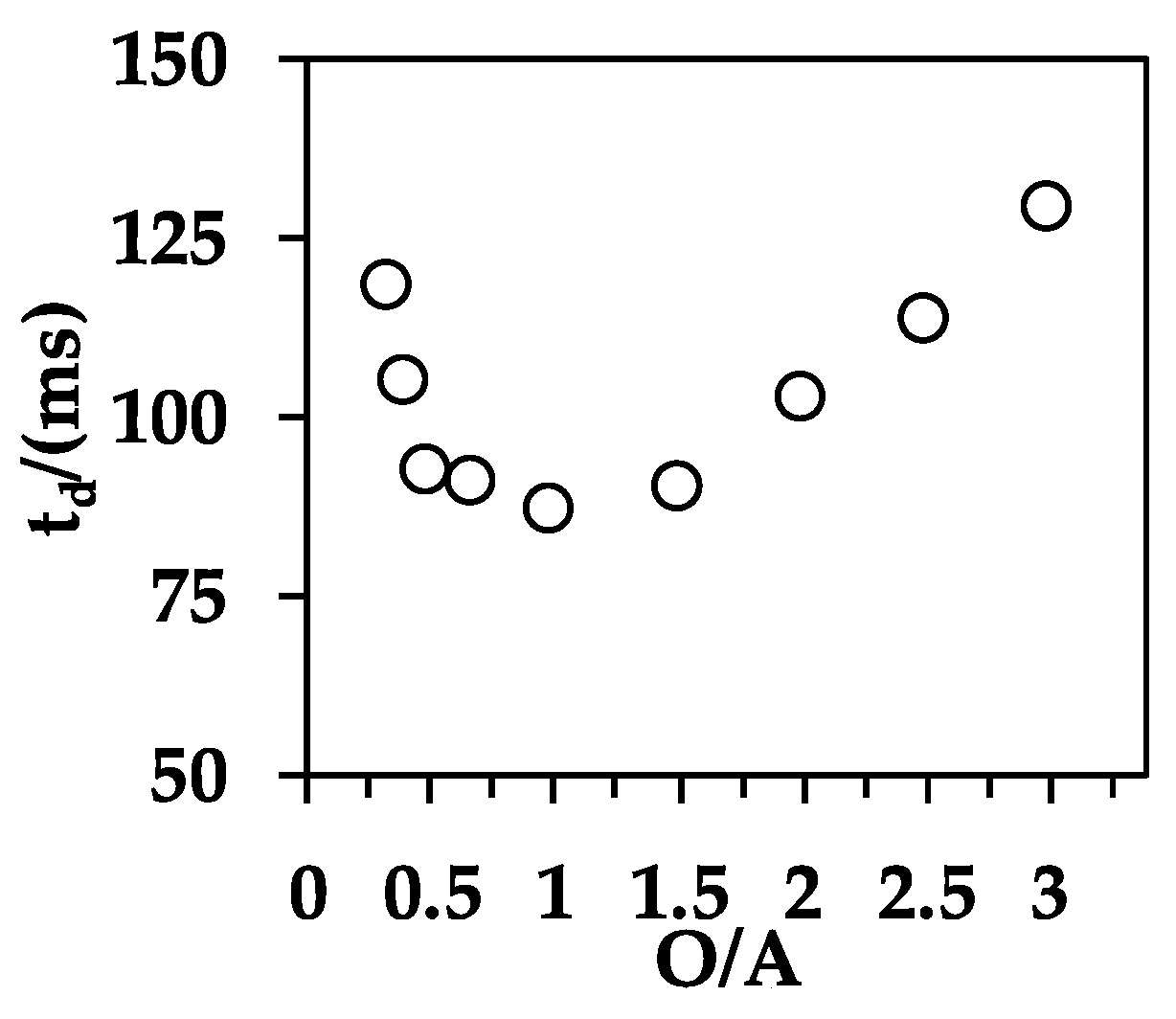
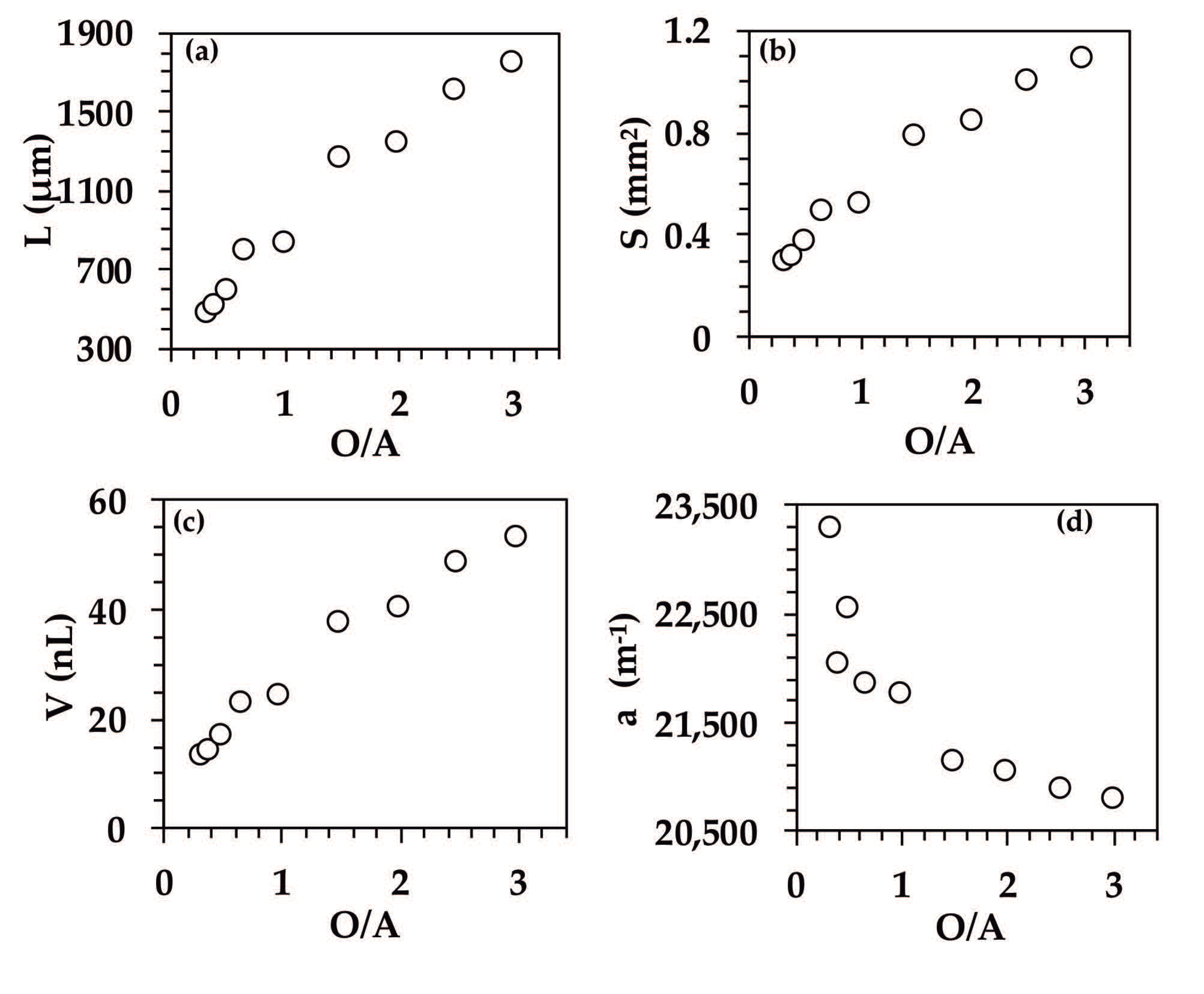
3.3. Effect of O/A Ratio on the Fluid Dynamics of Organic Droplets
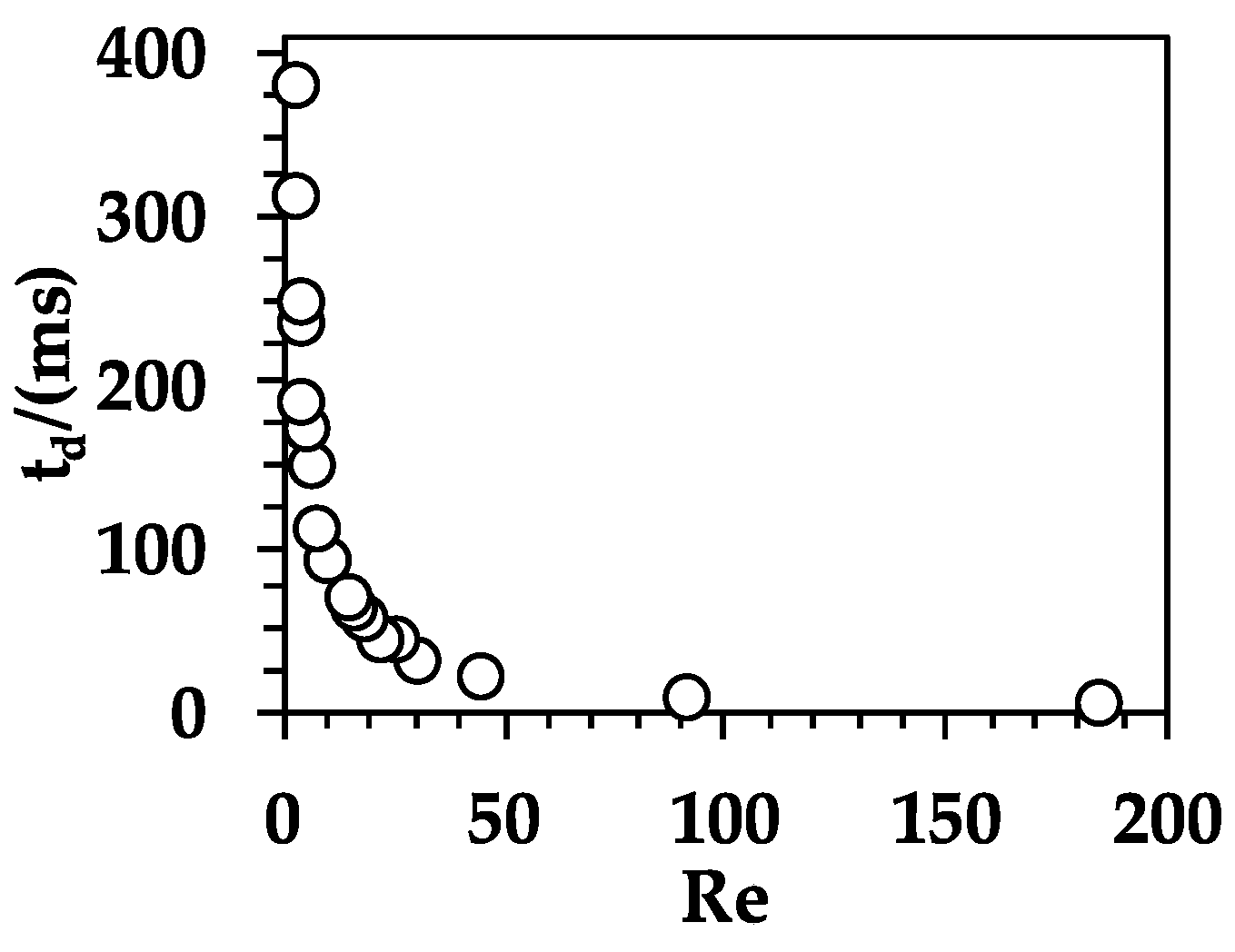
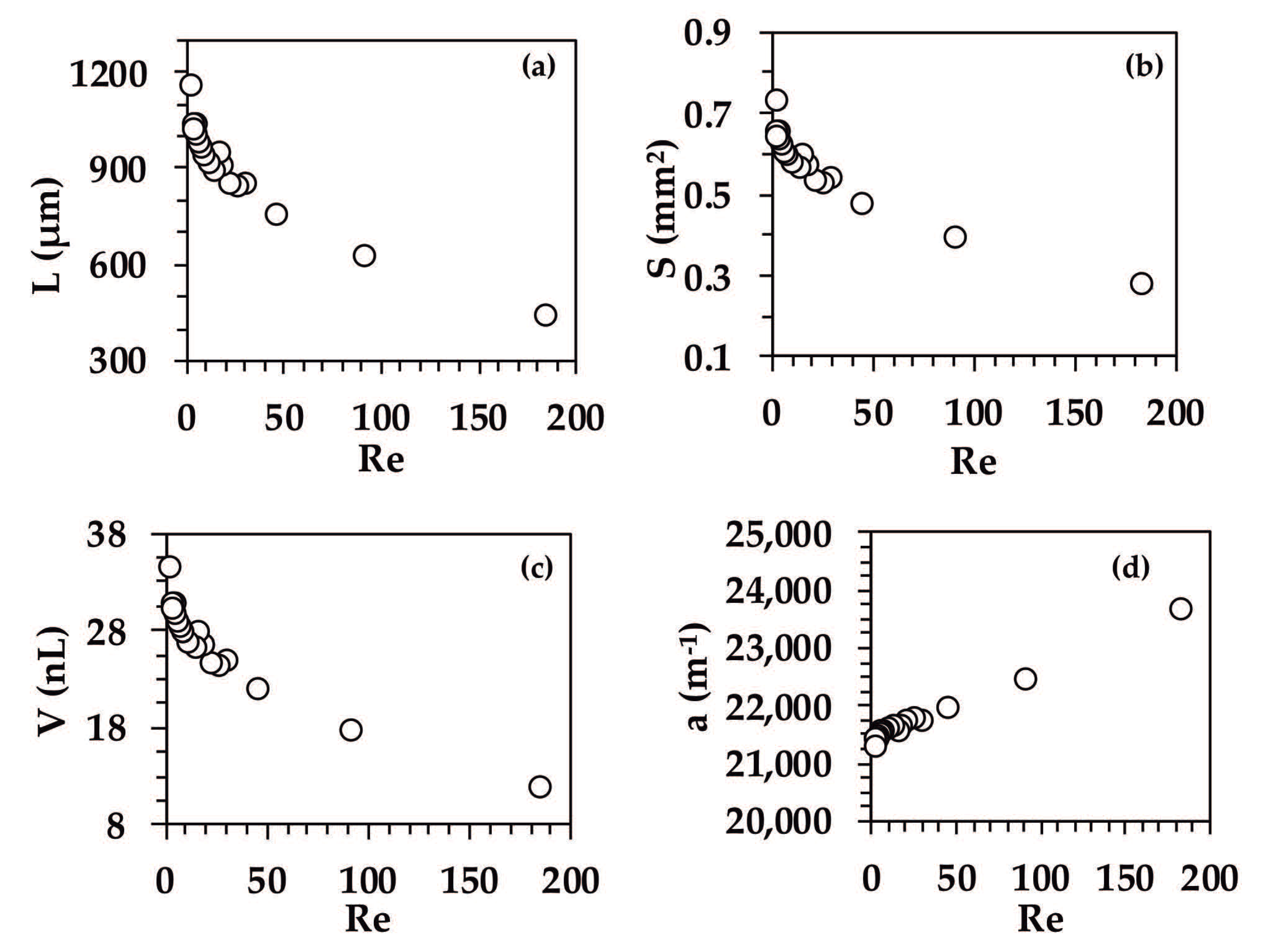
3.4. Effect of Reynolds Number on the Fluid Dynamics of Organic Droplets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Chu, D.Y.; Zhuo, Z. Cycle stability of dual-phase lithium titanate (LTO)/TiO2 nanowires as lithium battery anode. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 54–61. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Muto, A.; Hirayama, Y.; Tokumoto, H.; Matsuoka, A.; Noishiki, K. Liquid-liquid extraction of lithium ions using a slug flow microreactor: Effect of extraction reagent and microtube material. Solvent Extr. Ion. Exch. 2016, 35, 61–73. [Google Scholar] [CrossRef]
- Hirayama, Y.; Hinoue, M.; Tokumoto, H.; Matsuoka, A.; Noishiki, K.; Muto, A. Liquid-liquid extraction and separation of cobalt and lithium ions using a slug flow microreactor. J. Chem. Eng. Jpn. 2018, 51, 222–228. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Sathuluri, R.R.; Ohto, K.; Iwasaki, W.; Kawakita, H.; Morisada, S.; Miyazaki, M.; Jumina, J. A rapid and efficient lithium-ion recovery from seawater with tripropyl-monoacetic acid calix[4]arene derivative employing droplet-based microfluidic reactor system. Sep. Purif. Technol. 2019, 211, 925–934. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Sathuluri, R.R.; Ohto, K. Droplet microfluidic device for rapid and efficient metal separation using host-guest chemistry. In Advances in Microfluidic Technologies for Energy and Environmental Applications; Ren, Y., Ed.; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Islam, M.M.; Georghiou, P.E.; Rahman, S.; Yamato, T. Calix[3]arene-analogous metacyclophanes: Synthesis, structures and properties with infinite potential. Molecules 2020, 25, 4202. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Kassim, A.; Rezayi, M.; Rounaghi, G.H. Thermodynamic study of the complexation of p-isopropylcalix[6]arene with Cs+ cation in dimethylsulfoxide-acetonitrile binary media. Molecules 2011, 16, 8130–8142. [Google Scholar] [CrossRef]
- Bhatt, M.; Maity, D.; Hingu, V.; Suresh, E.; Ganguly, B.; Paul, P. Functionalized calix[4]arene as a colorimetric dual sensor for Cu(II) and cysteine in aqueous media: Experimental and computational study. New J. Chem. 2017, 41, 12541–12553. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Ryu, M.; Sathuluri, R.R.; Iwasaki, W.; Morisada, S.; Kawakita, H.; Ohto, K.; Maeki, M.; Miyazaki, M.; Jumina, J. Separation of Pb(II) ion with tetraacetic acid derivative of calix[4]arene by using droplet-based microreactor system. Indones. J. Chem. 2019, 19, 368–375. [Google Scholar] [CrossRef]
- Zadmard, R.; Hokmabadi, F.; Jalali, M.R.; Akbarzadeh, A. Recent progress to construct calixarene-based polymers using covalent bonds: Synthesis and applications. RSC Adv. 2020, 10, 32690–32722. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Sathuluri, R.R.; Iwasaki, W.; Morisada, S.; Kawakita, H.; Ohto, K.; Miyazaki, M.; Jumina, J. Microfluidic reactor for Pb(II) ion extraction and removal with amide derivative of calix[4]arene supported by spectroscopic studies. Microchem. J. 2018, 142, 377–384. [Google Scholar] [CrossRef]
- Amr, A.E.G.; Al-Omar, M.A.; Kamel, A.H.; Elsayed, E.A. Single-piece solid contact Cu2+-selective electrodes based on a synthesized macrocyclic calix[4]arene derivatives as a neutral carrier ionophore. Molecules 2019, 24, 920. [Google Scholar] [CrossRef]
- Sadamatsu, H.; Hanada, T.; Morisada, S.; Kawakita, H.; Ohto, K. Comprehensive comparison of alkali metal extraction with a series of calix[4]arene derivatives with propyl and/or acetic acid groups. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 87–97. [Google Scholar] [CrossRef]
- Sathuluri, R.R.; Kurniawan, Y.S.; Kim, J.Y.; Maeki, M.; Iwasaki, W.; Morisada, S.; Kawakita, H.; Miyazaki, M.; Ohto, K. Droplet-based microreactor system for stepwise recovery of precious metal ions from real metal waste with calix[4]arene derivatives. Sep. Sci. Technol. 2018, 53, 1261–1272. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Su, Y.; Chen, G. An experimental study of copper extraction characteristics in a T-junction microchannel. Chem. Eng. Technol. 2013, 36, 985–992. [Google Scholar] [CrossRef]
- Jiang, F.; Yin, S.; Srinivaskannan, C.; Li, S.; Peng, J. Separation of lanthanum and cerium from chloride medium in presence of complexing agent along with EHEHPA (P507) in a serpentine microreactor. Chem. Eng. J. 2018, 334, 2208–2214. [Google Scholar] [CrossRef]
- Jafari, O.; Rahimi, M.; Kakavandi, F.H. Liquid-liquid extraction in twisted micromixers. Chem. Eng. Proc. 2016, 101, 33–40. [Google Scholar] [CrossRef]
- Guan, Q.; Urness, K.N.; Ormond, T.K.; David, D.E.; Ellison, G.B.; Daily, J.W. The properties of a microreactor for the study of the unimolecular decomposition of large molecules. Int. Rev. Phys. Chem. 2014, 33, 447–487. [Google Scholar] [CrossRef]
- Bao, B.; Wang, Z.; Thushara, D.; Liyanage, A.; Gunawardena, S.; Yang, Z.; Zhao, S. Recent advances in microfluidics-based chromatography—A mini review. Separations 2021, 8, 3. [Google Scholar] [CrossRef]
- Gutierrez-Serpa, A.; Pacheco-Fernandez, I.; Pasan, J.; Pino, V. Metal-organic frameworks for solid-phase microextraction devices—A review. Separations 2019, 6, 47. [Google Scholar] [CrossRef]
- Ahmed, I.; Akram, Z.; Bule, M.H.; Iqbal, H.M.N. Advancements and potential applications of microfluidic approaches—A review. Chemosensors 2018, 6, 46. [Google Scholar] [CrossRef]
- Hake, V.; Sonawane, S.; Anandan, S.; Sonawane, S.; Ashokkumar, M. Process intensification approach using microreactors for synthesizing nanomaterials—A critical review. Nanomaterials 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, R. Effect of geometry on droplet formation in the squeezing regime in a microfluidic T-junction. Microfluid. Nanofluid. 2010, 8, 799–812. [Google Scholar] [CrossRef]
- Singh, K.K.; Renjith, A.U.; Shenoy, K.T. Liquid-liquid extraction in microchannels and conventional stage-wise extractors: A comparative study. Chem. Eng. Proc. 2015, 98, 95–105. [Google Scholar] [CrossRef]
- Sahu, A.; Vir, A.B.; Molleti, L.N.S.; Ramji, S.; Pushpavanam, S. Comparison of liquid-liquid extraction in batch systems and micro-channels. Chem. Eng. Proc. 2016, 104, 190–200. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Ciceri, D.; Mason, L.R.; Harvie, D.J.E.; Perera, J.M.; Stevens, G.W. Modelling of interfacial mass transfer in microfluidic solvent extraction: Part II. Heterogeneous transport with chemical reaction. Microfluid. Nanofluid. 2013, 14, 213–224. [Google Scholar] [CrossRef]
- Ciceri, D.; Peera, J.M.; Stevens, G.W. The use of microfluidic devices in solvent extraction. J. Chem. Technol. Biotechnol. 2013, 89, 771–786. [Google Scholar] [CrossRef]
- Ghaini, A.; Kashid, M.N.; Agar, D.W. Effective interfacial area for mass transfer in the liquid-liquid slug flow capillary microreactors. Chem. Eng. Proc. 2010, 49, 356–366. [Google Scholar] [CrossRef]
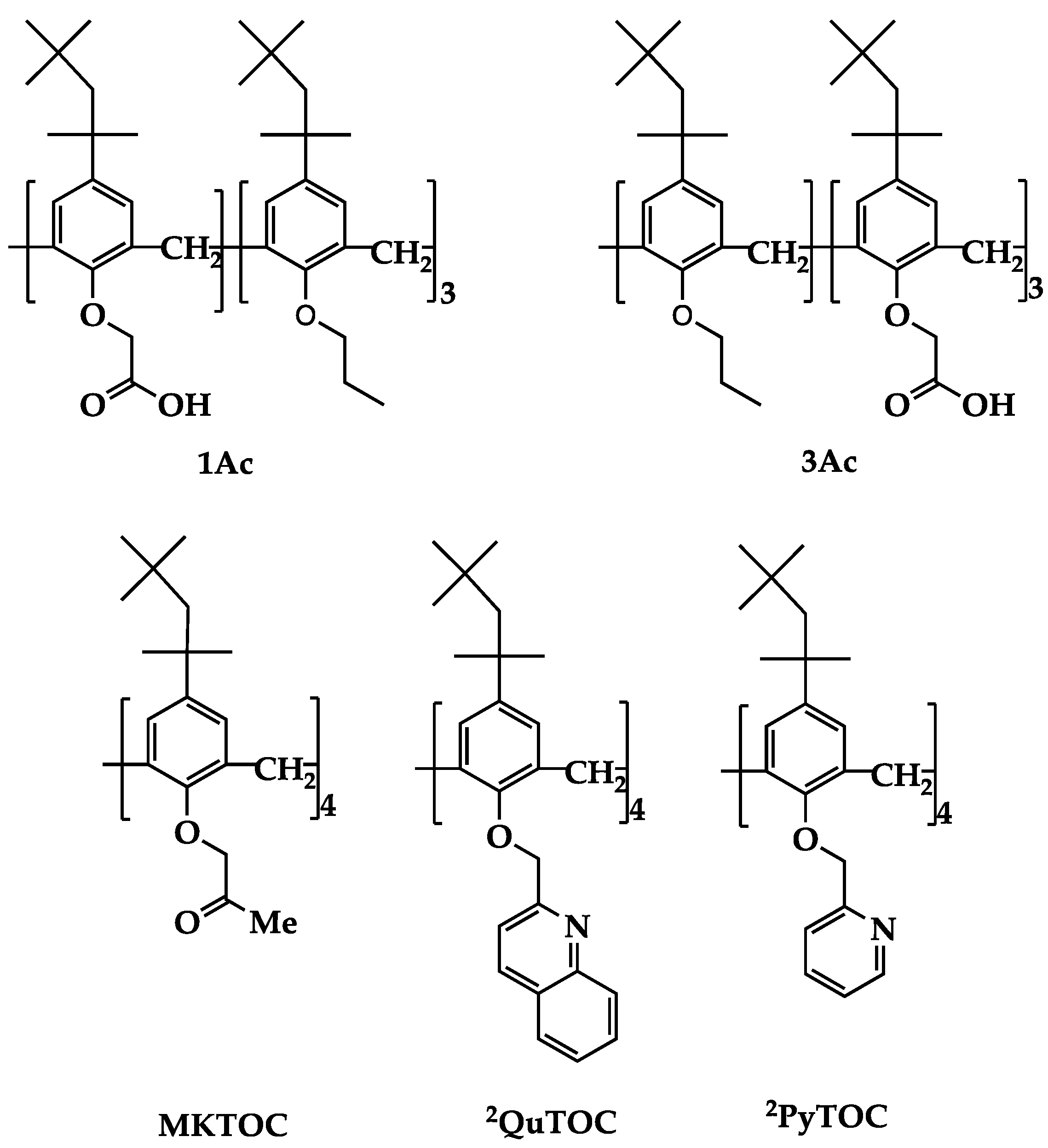



| Metal Ion | Host Compound a | Time to Reach Equilibrium in a Batch System | Extraction Rate Enhancement Factor b | Reference |
|---|---|---|---|---|
| Li(I) | 1Ac | 1 d | 4.3 × 104 | Kurniawan et al. (2019) [7] |
| Na(I) | 3Ac | 1 d | 4.3 × 104 | Kurniawan et al. (2019) [7] |
| Ag(I) | MKTOC | 3 d | 1.3 × 105 | Sathuluri et al. (2018) [18] |
| Pd(II) | 2QuTOC | 1 d | 4.3 × 104 | Sathuluri et al. (2018) [18] |
| Pt(IV) | 2PyTOC | 1 d | 4.3 × 104 | Sathuluri et al. (2018) [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, Y.S.; Sathuluri, R.R.; Ohto, K.; Iwasaki, W.; Kawakita, H.; Morisada, S.; Miyazaki, M.; Jumina, J. New Concept for the Study of the Fluid Dynamics of Lithium Extraction Using Calix[4]arene Derivatives in T-Type Microreactor Systems. Separations 2021, 8, 70. https://doi.org/10.3390/separations8050070
Kurniawan YS, Sathuluri RR, Ohto K, Iwasaki W, Kawakita H, Morisada S, Miyazaki M, Jumina J. New Concept for the Study of the Fluid Dynamics of Lithium Extraction Using Calix[4]arene Derivatives in T-Type Microreactor Systems. Separations. 2021; 8(5):70. https://doi.org/10.3390/separations8050070
Chicago/Turabian StyleKurniawan, Yehezkiel Steven, Ramachandra Rao Sathuluri, Keisuke Ohto, Wataru Iwasaki, Hidetaka Kawakita, Shintaro Morisada, Masaya Miyazaki, and Jumina Jumina. 2021. "New Concept for the Study of the Fluid Dynamics of Lithium Extraction Using Calix[4]arene Derivatives in T-Type Microreactor Systems" Separations 8, no. 5: 70. https://doi.org/10.3390/separations8050070
APA StyleKurniawan, Y. S., Sathuluri, R. R., Ohto, K., Iwasaki, W., Kawakita, H., Morisada, S., Miyazaki, M., & Jumina, J. (2021). New Concept for the Study of the Fluid Dynamics of Lithium Extraction Using Calix[4]arene Derivatives in T-Type Microreactor Systems. Separations, 8(5), 70. https://doi.org/10.3390/separations8050070









