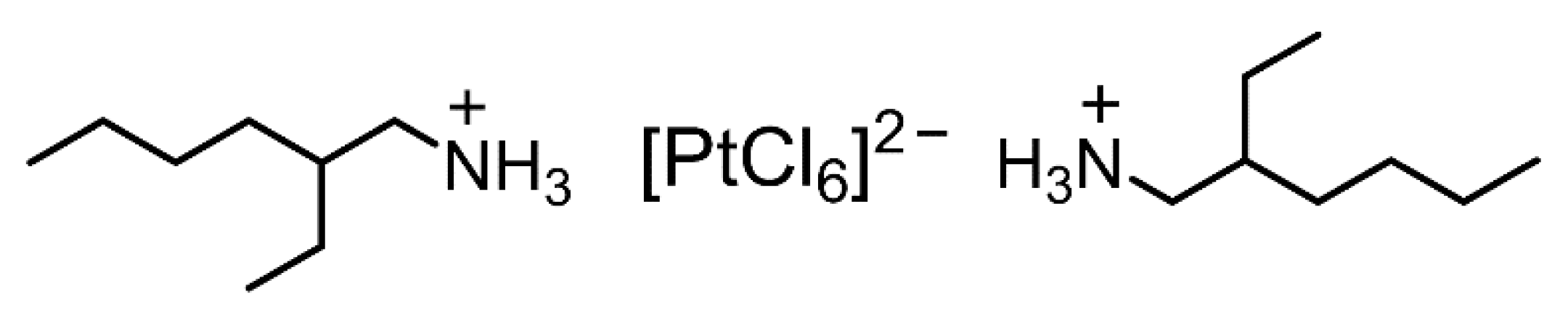Abstract
The selective separation and recovery of specific platinum-group metals (PGMs) from metal mixtures is a significant challenge owing to the similarity of these metals in terms of chemical and physical properties. Among the typical PGMs (Pd, Pt, and Rh), the selective recovery of Pt prior to the recovery of Pd and Rh is in high demand. In this study, we attempted the selective precipitation of Pt(IV) from mixed-metal HCl solutions using 2-ethylhexylamine (2EHA) as a precipitant and achieved the selective precipitation of Pt(IV) from Pd(II) and Rh(III) over a wide range of HCl concentrations. Selective precipitation of Pt(IV) was also achieved from HCl solutions with high levels of base metals, such as Al, Cu, Fe, and Zn. High yields of undegraded 2EHA remaining in the HCl solution after Pt(IV) precipitation were recovered using hydrophobic porous resins. X-ray photoelectron spectroscopy and thermogravimetric measurements revealed that the Pt(IV)-containing precipitate was an ion-pair comprising one [PtCl6]2− and two ammonium cations of 2EHA. The steric hindrance and high hydrophilicity of 2EHA suppressed the formation of Rh(III)- and Pd(II)-containing precipitates, respectively, resulting in the selective precipitation of Pt(IV).
1. Introduction
Platinum-group metals (PGMs) play an essential role in modern economies owing to their wide range of applications, including in catalysts and electrical devices [1,2,3,4,5]. Palladium (Pd), platinum (Pt), and rhodium (Rh), which are typical PGMs, are mainly used as catalysts to clean automobile exhaust gases. The scarcity and expense of these materials have led to a high demand for PGM recycling from spent catalysts and electronic waste. However, the selective separation and recovery of specific PGMs from metal mixtures remains a significant challenge owing to the chemical and physical similarities of these metals.
In general, PGMs are separated individually via solvent extraction using organic extractants and solvents from metal-containing acid solutions [6,7,8,9,10,11,12]. Thus, Pd can be recovered selectively prior to the recovery of other PGMs, such as Pt and Rh [6,7,8]. On the other hand, the preferential recovery of Pt or Rh over Pd is challenging, as the ease of Pt(IV) and Rh(III) extraction is generally equal to or much lower than that of Pd(II) [13,14,15]. Therefore, Pt or Rh recovery is typically performed after Pd extraction [16]. However, since the prices of PGMs fluctuate in response to socioeconomic factors, the development of selective and preferential recovery methods for Pt and Rh is an important step in achieving economically efficient recycling.
Recently, precipitation-based selective PGM recovery methods have been developed using primary amines as precipitants in metal-containing hydrochloric acid (HCl) solutions [17,18,19,20,21]. Selective Rh(III) precipitation using 4-alkylanilines from HCl solutions containing Pd(II) and Pt(IV) was successfully achieved via the formation of unique ion-pairs comprising [RhCl6]3−, anilinium cations, and chloride anions in a 1:6:3 ratio (Rh(III) hexa-ammonium) [18]. Aromatic diamines also act as selective Rh(III) precipitants, and their Rh(III) selectivity is derived from the formation of unique ionic crystals composed of [RhCl6]3− and ammonium [19,20]. Aliphatic primary amines with linear alkyl chains can precipitate Pt(IV), whereas Pd(II) cannot be precipitated using amines shorter than nonyl [21]. However, Rh(III) co-precipitated with Pt(IV) using aliphatic primary amines at high HCl concentrations by forming Rh(III) hexa-ammonium [21]. Based on the PGM precipitation results, selective Pt(IV) precipitation is expected to be achieved using aliphatic primary amines if the formation of Rh(III) hexa-ammonium is effectively inhibited.
In this paper, we report the selective recovery of Pt(IV) from metal-mixed HCl solutions using 2-ethylhexylamine (2EHA) as a precipitant. The selective separation of Pt(IV) from Pd(II) and Rh(III) was achieved via the formation of Pt(IV)-containing precipitates over a wide range of HCl concentrations. Furthermore, high Pt(IV) selectivity was preserved even with the use of HCl solutions with high levels of base metals. Steric hindrance and the high hydrophilicity of 2EHA suppressed the formation of Rh(III) hexa-ammonium and Pd(II)-containing precipitates, respectively, resulting in the selective precipitation of Pt(IV).
2. Materials and Methods
2.1. Materials
2EHA, AlCl3, and ZnCl2 were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan); Pd(II) and Pt(IV) standard solutions were purchased from FUJIFILM Wako Pure Chemical Industries, Ltd. (Osaka, Japan); and Rh(III) standard solution, CuCl, and FeCl3 were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan); and all were used as received. Styrene-divinyl benzene resin (Sepabeads SP850) was generously provided by Mitsubishi Chemical Co., Ltd. (Tokyo, Japan). HCl solutions containing base metals were prepared by dissolving metal chlorides in HCl and mixing them with metal standard solutions.
2.2. Metal Precipitation Experiments Using 2EHA
First, 2EHA (0.05 mmol) was added to HCl solutions (1 mL) containing Pd(II), Pt(IV), and Rh(III) (1.0 mmol/L each), and the resulting mixtures were shaken vigorously for 30 min. The formed precipitates were spun down by centrifugation (7200× g, 10 min), and the metal concentrations in the resulting supernatants were evaluated using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The HCl concentration, loading amount of 2EHA, and shaking time were varied in the metal precipitation experiments.
2.3. Metal Precipitation from Base Metal-Containing Solutions
First, 2EHA (0.15 mmol) was added to 6 M HCl solutions (3 mL) containing base metals (Pd(II), Pt(IV), Rh(III): 1.0 mmol/L each; Al, Cu, Fe, Zn: 1000 or 10,000 ppm each), and the resulting mixtures were shaken vigorously for 30 min. The formed precipitates were recovered by filtration and washed with 6 M HCl solution (3 mL) containing 2EHA (0.08 M). The metal precipitation rates were evaluated from water solutions of the resulting precipitates using ICP-AES.
2.4. 2EHA Recovery by Porous Resins
Sepabeads SP850 (2.0 g) was added to 30 mL of 6 M HCl solution containing 2EHA (0.08 M), and the mixture was shaken for 1 h. The beads were recovered by filtration and then added to 30 mL of methanol. The resulting mixture was shaken for 30 min and then filtered. This methanol washing process was repeated three times, and the combined methanol filtrate was evaporated and dried under vacuum conditions at room temperature for 24 h. The hydrochloride form of 2EHA was recovered with a 94.3% yield.
2.5. Metal Precipitation and 2EHA Recovery from Metal-Containing Solution
First, 2EHA (1.5 mmol) was added to a 6 M HCl solution (30 mL) containing Pd(II), Pt(IV), and Rh(III) (1.0 mmol/L each), and the resulting mixture was shaken vigorously for 30 min. The formed precipitates were recovered by filtration and washed with 6 M HCl solution (10 mL) containing 2EHA (0.08 M). The metal precipitation rates in the water solutions evaluated using ICP-AES (90% for Pt(IV), 4% for Pd(II), and 0% for Rh(III)). Sepabeads SP850 (2.0 g) was added to the resulting filtrate, and the mixture was shaken for 30 min. The recovery of 2EHA-adsorbed beads and the stripping of 2EHA using methanol were performed as described above. The recovered 2EHA hydrochloride amounted to 85.6% of the total 2EHA feed. Five percent of Pt(IV), 92% of Pd(II), and 99% of Rh(III) remained in the HCl filtrate after 2EHA adsorption.
2.6. Preparation of 2EHA-Containing Precipitate
First, 2EHA (0.75 mmol) was added to a 6 M HCl solution (10 mL) containing Pt(IV) (2.5 mmol/L), and the resulting mixture was shaken vigorously for 30 min. The formed precipitates were recovered by filtration and washed with a 6 M HCl solution (0.5 mL). The obtained powder was dried under vacuum conditions at room temperature for 24 h.
2.7. Measurements
1H NMR spectra in DMSO-d6 at 25 °C were recorded using a JEOL JNM-ECX 500 NMR spectrometer (Jeol Co., Tokyo, Japan). An ICP-AES instrument (SPS5510, Hitachi High-Tech Science Corp., Tokyo, Japan) was used to evaluate metal concentrations. ICP samples were prepared by diluting metal-containing solutions with water or HCl solution, and the concentrations of analysis target metals in ICP samples were set below 20 ppm. X-ray photoelectron spectroscopy (XPS) measurements were performed using an AXIS-ULTRA X-ray photoelectron spectrometer (Kratos Analytical Ltd., Manchester, UK). Elemental quantification was performed using the relative sensitivity factors supplied with the instrument control software (N 1s: 0.477, Cl 2s: 0.493, Pt 4d: 4.637). Thermogravimetric (TG) measurements were performed using an STA7300 (Hitachi High-Tech Science Corp., Tokyo, Japan) at a heating rate of 10 °C/min under an airflow of 200 mL/min.
3. Results
3.1. Metal Precipitation Experiments
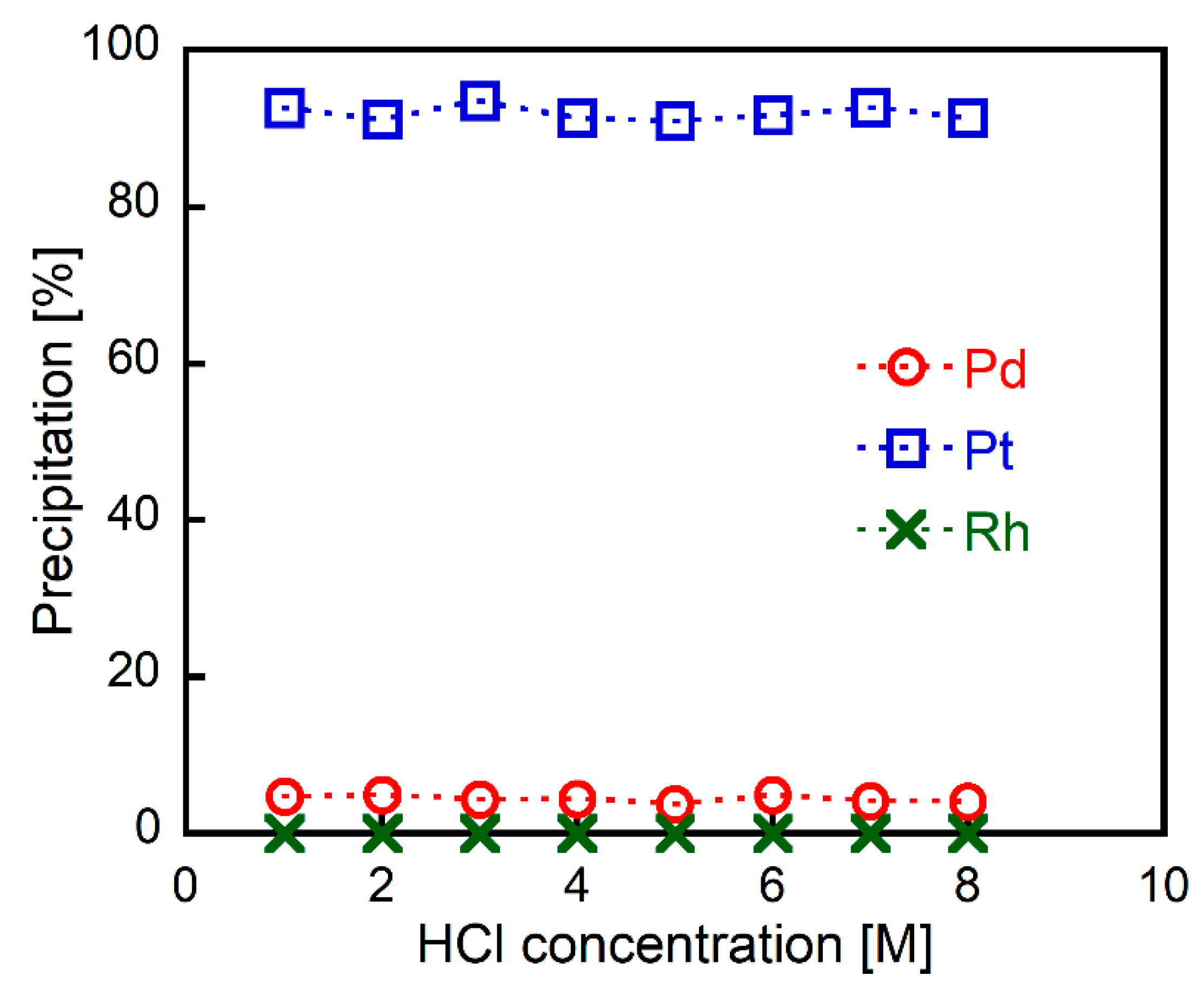
Metal precipitation experiments were performed as follows: 2EHA was added to HCl solutions containing Pd(II), Pt(IV), and Rh(III) (1.0 mmol/L each), and the mixtures were shaken vigorously. The metal content in the formed precipitates was evaluated from the supernatants of the mixtures using ICP-AES. Figure 1 shows the relationship between the HCl and precipitate metal concentrations. High yields of Pt(IV) were precipitated (over 90%) over a wide range of HCl concentrations (1–8 M). On the other hand, very little Pd(II) and Rh(III) precipitation (less than 5%) occurred, regardless of the HCl concentration. This result clearly indicates that 2EHA acts as a selective precipitant for Pt(IV) under a wide range of HCl concentrations.
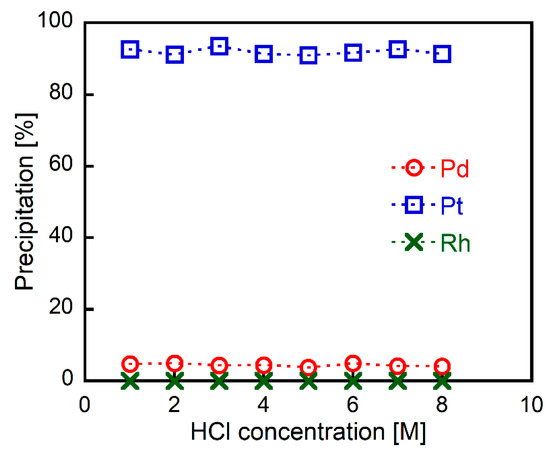
Figure 1.
The effect of HCl concentration on metal precipitation (2EHA/Pt = 50 mol/mol, 30 min of shaking) from HCl solutions containing Pd(II), Pt(IV), and Rh(III) (1.0 mmol/L each).
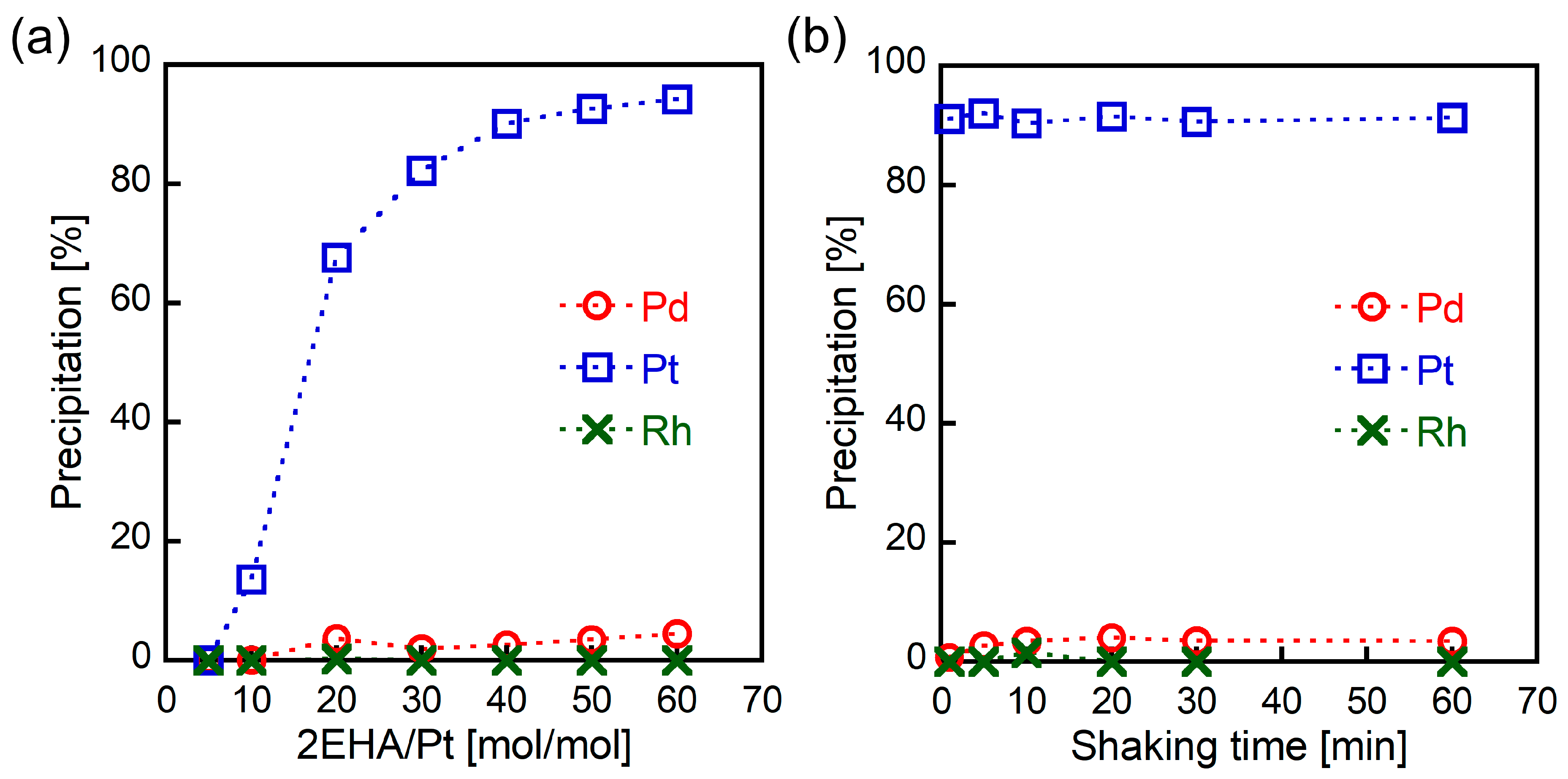
The effect of 2EHA loading on metal precipitation was examined to optimize selective Pt(IV) precipitation. The Pt(IV) precipitation rate increased with higher 2EHA loading (Figure 2a) and exceeded 90% at a 2EHA/Pt ratio of 50 mol/mol. In all cases, almost no Pd(II) and Rh(III) was observed. The dependence of metal precipitation on shaking time was also investigated. As shown in Figure 2b, the Pt(IV) precipitation rate reached 90% in just 1 min, whereas Pd(II) and Pt(IV) did not precipitate even after prolonged shaking. Thus, the results indicate that selective Pt(IV) precipitation can be achieved using high dosages of 2EHA with a short shaking time.
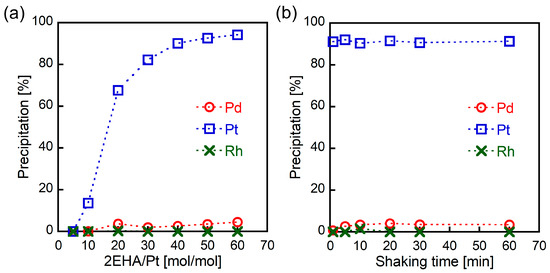
Figure 2.
The effect of (a) 2EHA loading (6 M HCl, 30 min of shaking) and (b) shaking time (6 M HCl, 2EHA/Pt = 50 mol/mol) on metal precipitation from HCl solutions containing Pd(II), Pt(IV), and Rh(III) (1.0 mmol/L each).
3.2. Separation of Pt(IV) from Base Metals
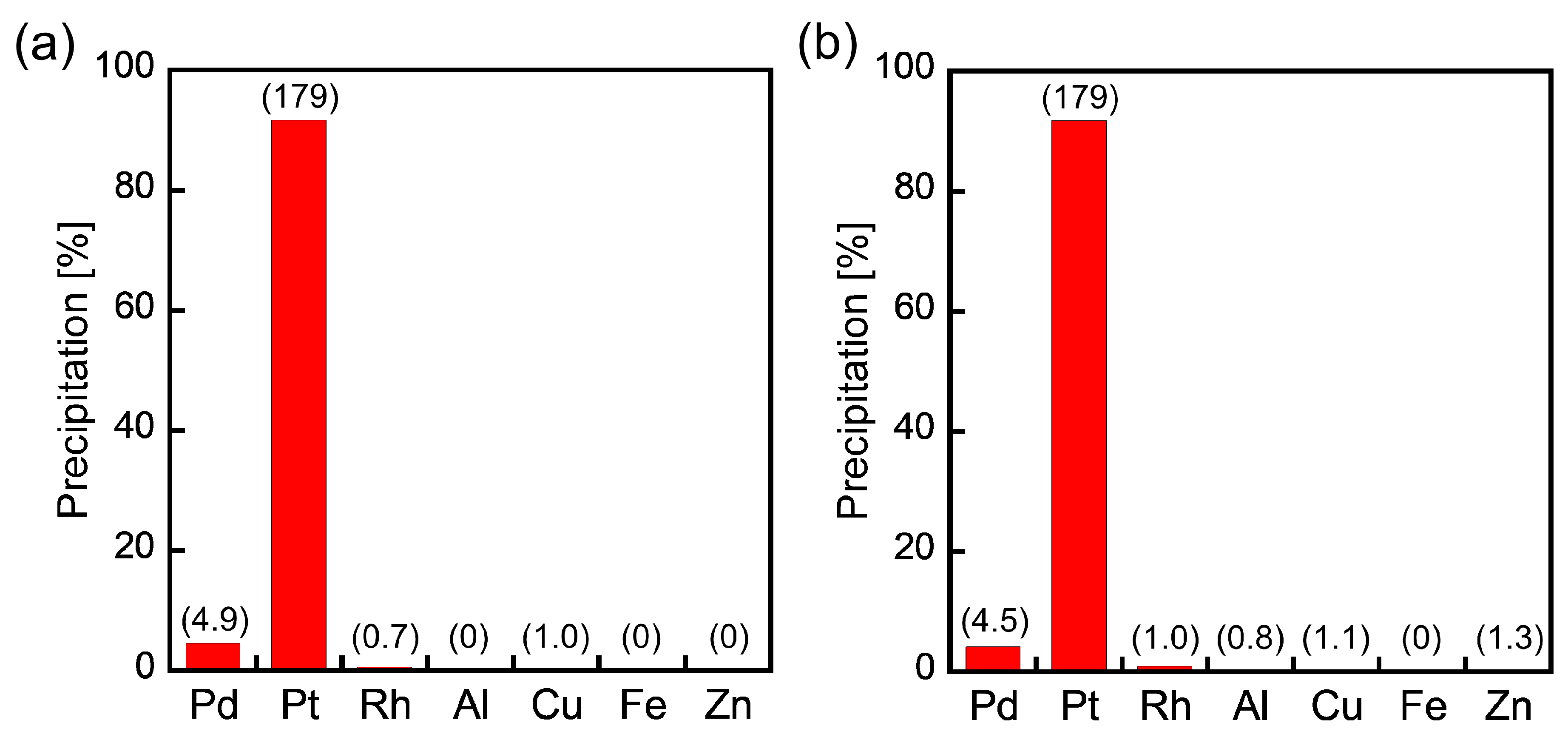
In actual PGM separation procedures, metal-containing solutions (metal-leaching solutions) typically include relatively high concentrations of base metals, such as Al, Cu, Fe, and Zn. Therefore, we studied the selective Pt(IV) precipitation from HCl solutions containing base metals as well as Pd(II) and Rh(III). The precipitated rates of the metals were evaluated by applying ICP-AES to water solutions in which the resulting precipitates were dissolved. As shown in Figure 3a, 2EHA could selectively separate Pt(IV) from 1000 ppm base metals, and higher concentrations of base metals (10,000 ppm each) had no impact on selective Pt(IV) precipitation (Figure 3b). Further, the presence of base metals did not induce co-precipitation of Pd(II) and Rh(III). The high selectivity for Pt(IV) over base metals demonstrates the practicability of metal precipitation using 2EHA.
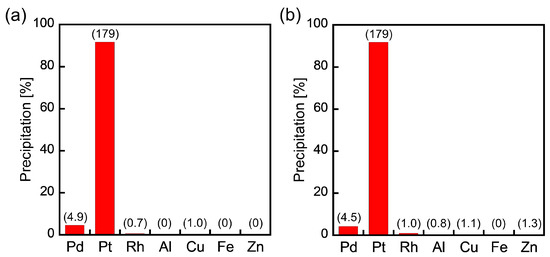
Figure 3.
Metal precipitation from HCl solutions containing PGMs (Pd(II), Pt(IV), Rh(III): 1.0 mmol/L each) and base metals (a) at 1000 ppm each and (b) 10,000 ppm each. The numbers in parentheses are metal concentrations (ppm) of water solutions determined by ICP-AES. The maximum metal concentrations (the cases of 100% precipitation) are 106 ppm for Pd(II), 195 ppm for Pt(IV), 103 ppm for Rh(III), and 1000 or 10,000 ppm for Al, Cu, Fe, and Zn.
3.3. Recovery of 2EHA from HCl Solutions
The recovery of 2EHA from HCl solutions after Pt(IV) precipitation was examined because a large amount of 2EHA remained in the solutions. Since 2EHA forms an amphiphilic structure in HCl solutions (hydrophilic ammonium group and hydrophobic 2-ethylhexyl group), 2EHA adsorption was performed using hydrophobic porous resins (styrene-divinylbenzene resin: Sepabeads SP850). Here, 2EHA was effectively adsorbed on the resins and stripped using methanol, allowing approximately 94% of the 2EHA to be recovered as hydrochloride salt.
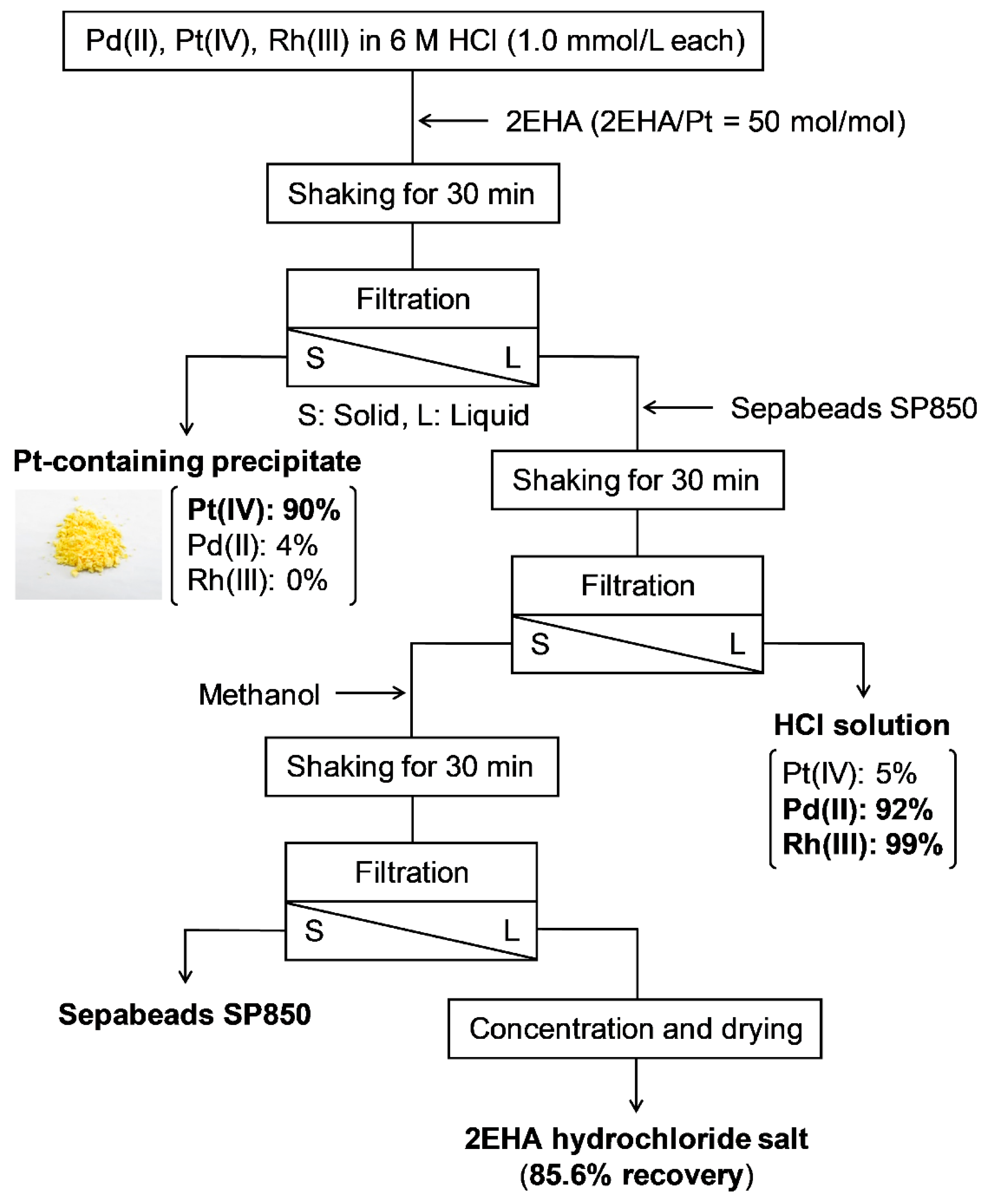
We also investigated 2EHA recovery from the metal-containing solution after Pt(IV) recovery (Figure 4). Although some of the used 2EHA was precipitated and filtered out with Pt(IV), as much as 85.6% of the total 2EHA feed was recovered. Ninety percent of the Pt(IV) was recovered as a precipitate, whereas 5% of Pt(IV), 92% of Pd(II), and 99% of Rh(III) remained in the solution after 2EHA recovery. Notably, no chemical structural change arose from decomposition in the recovered 2EHA hydrochloride salt (Figure 5). Furthermore, as the employed porous resin (SP850) did not possess polar units and charged functionalities to adsorb metals, Pd(II) and Pt(IV) remained in the solution.
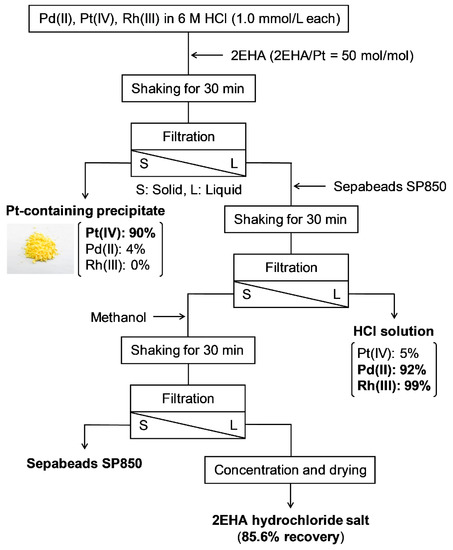
Figure 4.
Scheme of metal precipitation and 2EHA recovery from an HCl solution containing Pd(II), Pt(IV), and Rh(III).
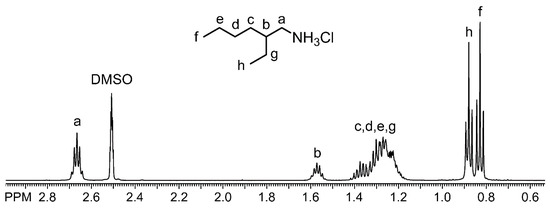
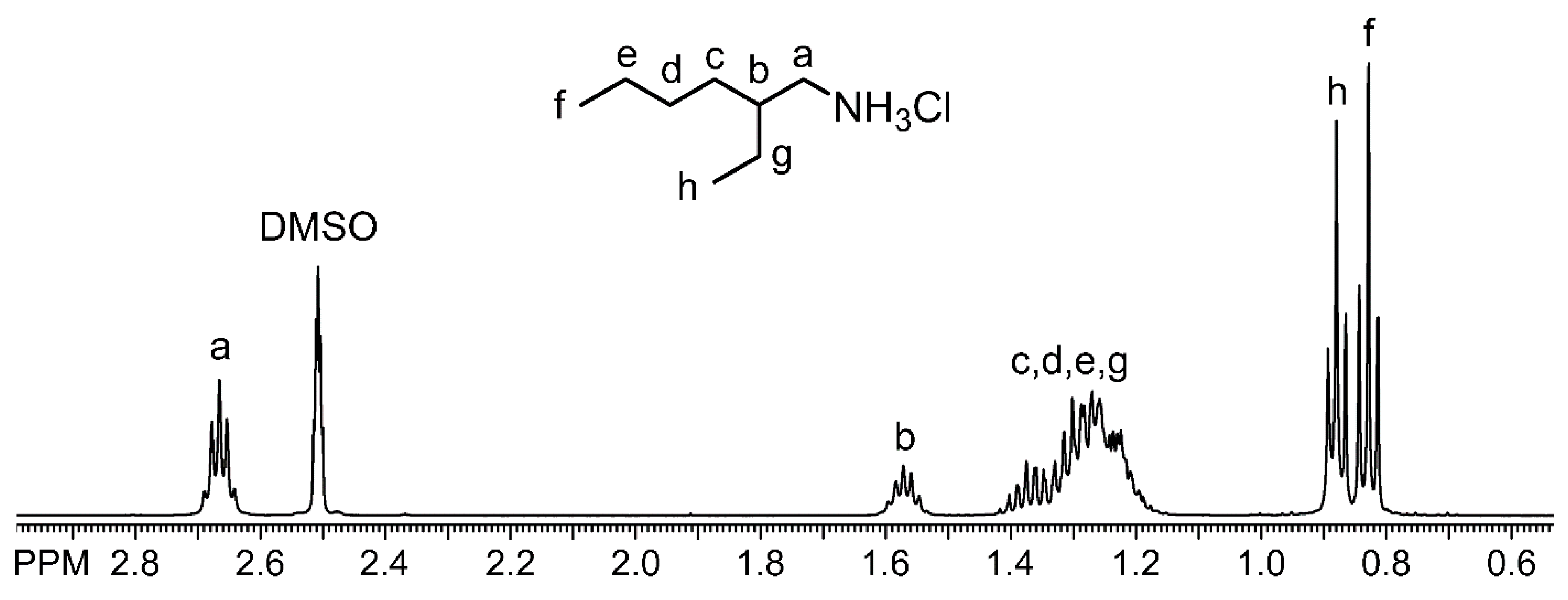
Figure 5.
1H NMR spectrum of the recovered 2EHA hydrochloride salt in DMSO-d6.
3.4. Analysis of Pt(IV)-Containing Precipitate
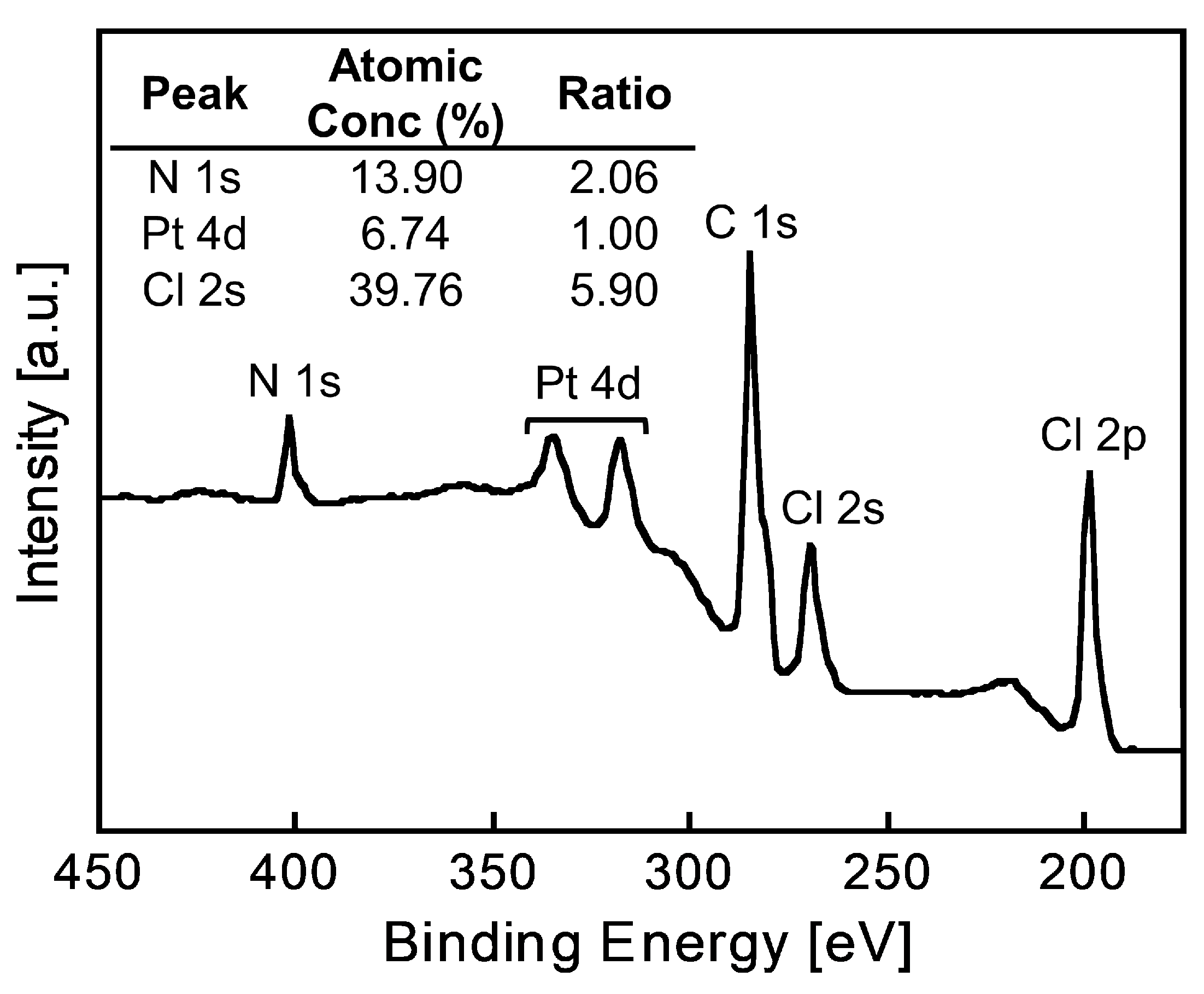
The elemental composition of the Pt(IV)-containing precipitate was evaluated using XPS. The precipitate used for the measurements was prepared from a 6 M HCl solution containing Pt(IV) using 2EHA, which was washed with a small amount of 6 M HCl to remove excess 2EHA prior to the measurements. The obtained spectrum showed characteristic XPS peaks assigned to Pt 4d, C 1s, N 1s, Cl 2s, and Cl 2p (Figure 6). The atomic ratios were calculated from the photoelectron peak areas based on atomic sensitivity factors. The Pt:Cl ratio was calculated as 1.0:5.9, indicating the presence of [PtCl6]2− in the precipitate. This result was consistent with the fact that Pt(IV) exists as a chloro-complex anion, [PtCl6]2−, in HCl solutions [13]. Given the N:Pt ratio of 2.1:1.0, the precipitate was composed of two 2EHA molecules and one chloro-complex of Pt(IV). Since 2EHA forms an ammonium cation, the expected structure of the precipitate is an ion-pair comprising the [PtCl6]2−/ammonium cation of 2EHA in a 1:2 ratio (Figure 7).
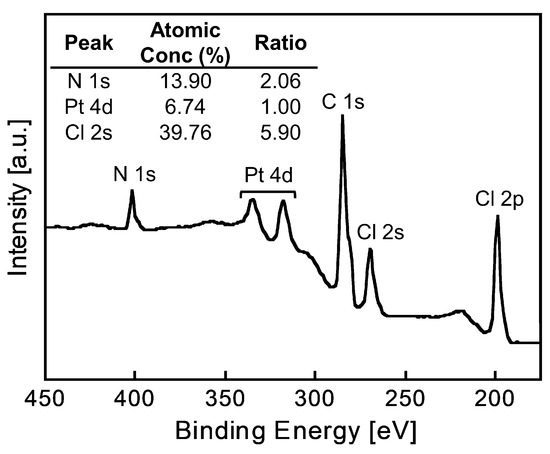
Figure 6.
XPS spectrum of the Pt(IV)-containing precipitate.
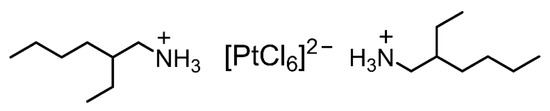
Figure 7.
Expected structure of the ion-pair comprising one [PtCl6]2− and two ammonium cations of 2EHA.
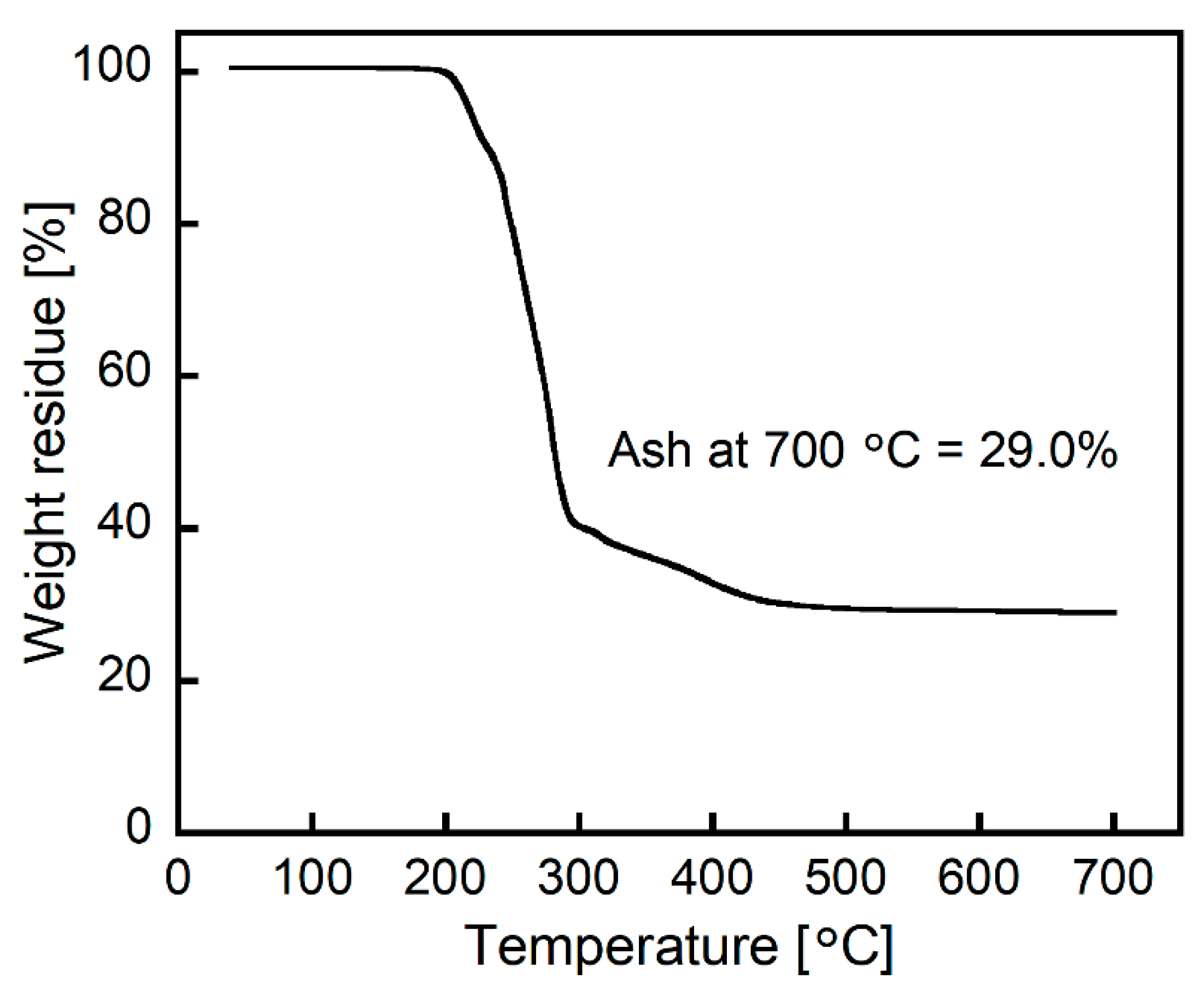
The Pt(IV)-containing precipitate was examined via TG analysis. The obtained TG curve showed the weight loss stemming from the decomposition and volatilization of 2EHA and chlorine over a temperature range of 200–450 °C (Figure 8). After heating at 700 °C in air, the resultant residue was 29.0% of its original weight. The combustion of PtCl4 is known to yield zerovalent Pt (Pt(0)), indicating that the resultant residue after TG analysis was Pt(0) [22]. Furthermore, the combustion of the ion-pair shown in Figure 7 yields 29.1% of Pt(0). The good match in the weight percentages of Pt(0) between the Pt(IV)-containing precipitate and the expected ion-pair strongly supports the formation of the ion-pair comprising one [PtCl6]2− and two ammonium cations.
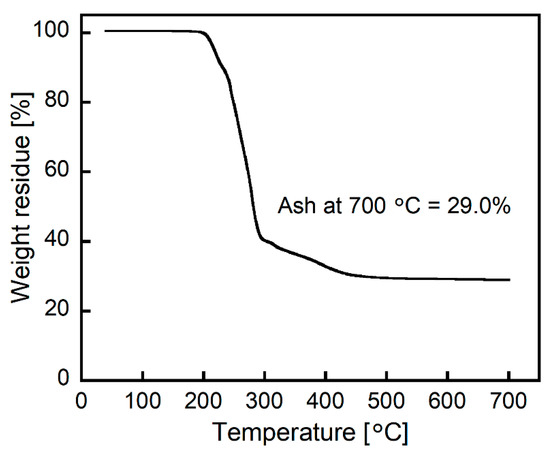
Figure 8.
TG curve of the Pt(IV)-containing precipitate at a heating rate of 10 °C/min under airflow (200 mL/min).
3.5. Mechanism of Selective Pt(IV) Precipitation
Aliphatic primary amines with linear alkyl chains can intrinsically precipitate Pd(II), Pt(IV), and Rh(III) from HCl solutions [21]. However, no precipitation of Pd(II) was observed using primary amines with short alkyl chains (carbon numbers < 9) [21], as the high hydrophilicity of their ammonium results in highly water-soluble ion-pairs between [PdCl4]2− and the ammoniums. The lack of Pd(II) precipitation using 2EHA, which has eight carbon chains, is also due to the high solubility of the Pd(II)-containing ion-pair in HCl solutions. In contrast, Pt(IV) can be precipitated using 2EHA by forming a Pt(IV)-containing ion-pair, as shown in Figure 1. This result agrees well with the fact that alkyl amines longer than hexyl can precipitate Pt(IV) [21]. Unlike in the case of Pd(II) and Pt(IV), Rh(III) precipitation behavior differs dramatically in the presence of 2EHA and normal alkyl amines; 2EHA cannot precipitate Rh(III) regardless of the HCl concentration. It has been reported that normal alkyl amines and aromatic primary monoamines can precipitate Rh(III), especially at high HCl concentrations, by forming unique ion-pairs comprising [RhCl6]3−/ammonium cations/chloride anions in a 1:6:3 ratio (Rh(III) hexa-ammonium) [18,21]. Because the ammoniums in Rh(III) hexa-ammonium surround [RhCl6]3− and are closely packed, its formation strongly depends on the steric effect of the ammonium. The steric hindrance of 2EHA, which has a branched alkyl chain, effectively inhibits the formation of Rh(III) hexa-ammonium, resulting in no Rh(III) precipitation. Therefore, the selective Pt(IV) precipitation achieved in this study was attributable to the high hydrophilicity and steric hindrance of 2EHA.
4. Conclusions
Pt(IV)-selective precipitation from HCl solutions containing Pd(II) and Rh(III) was achieved under a wide range of HCl concentrations using 2EHA as a precipitant. Furthermore, the selective precipitation of Pt(IV) was achieved from HCl solutions with high levels of base metals, such as Al, Cu, Fe, and Zn. The 2EHA remaining in the HCl solution after Pt(IV) precipitation was recovered at high rates without degradation using hydrophobic porous resins. XPS and TG measurements revealed that the Pt(IV)-containing precipitate was an ion-pair comprised of [PtCl6]2− and 2EHA ammonium cations in a 1:2 ratio. The steric hindrance and high hydrophilicity of 2EHA suppressed the formation of Rh(III) hexa-ammonium and Pd(II)-containing precipitates, respectively, resulting in the selective precipitation of Pt(IV). Therefore, Pt(IV) precipitation using 2EHA is a promising candidate for Pt recycling and purification.
Author Contributions
Conceptualization, K.M.; Funding acquisition, K.M.; Investigation, Y.S. and Y.H.; Supervision, K.M.; Writing–original draft, K.M. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant-in-Aid for Scientific Research (C) 20K05578), the Environment Research and Technology Development Fund (JPMEERF20193R02) of the Environmental Restoration and Conservation Agency of Japan, and Akita University Support for Fostering Research Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank Hiroshi Katagiri (Yamagata University) for valuable discussions regarding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alonso, E.; Field, F.R.; Kirchain, R.E. Platinum availability for future automotive technologies. Environ. Sci. Technol. 2012, 46, 12986–12993. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Rhamdhani, M.A.; Brooks, G.; Masood, S. Metal extraction processes for electronic waste and existing industrial routes: A review and Australian perspective. Resources 2014, 3, 152–179. [Google Scholar] [CrossRef]
- Froehlich, P.; Lorenz, T.; Martin, G.; Brett, B.; Bertau, M. Valuable metals—Recovery processes, current trends, and recycling strategies. Angew. Chem. Int. Ed. 2017, 56, 2544–2580. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling the platinum group metals: A European perspective. Platin. Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Separation of Pt (IV), Pd (II), Ru (III) and Rh (III) from model chloride solutions by liquid-liquid extraction with phosphonium ionic liquids. Sep. Purif. Technol. 2019, 212, 791–801. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K. Palladium extraction with N,N,N′,N′-tetra-n-octyl-thiodiglycolamide. Miner. Eng. 2008, 21, 483–488. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Development of tertiary thioamide derivatives to recover palladium (II) from simulated complex chloride solutions. Hydrometallurgy 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, J.Y.; Lee, M.S. Separation of Pt (IV) and Rh (III) from chloride solution by solvent extraction with amine and neutral extractants. Mater. Trans. 2011, 52, 2071–2076. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wieczorek, D. Extraction and separation of palladium (II), platinum (IV), gold (III) and rhodium (III) using piperidine-based extractants. Hydrometallurgy 2018, 175, 359–366. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery form real samples. Hydrometallurgy 2013, 134–135, 11–18. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S.; Senanayake, G. Separation of Pt (IV), Rh (III) and Fe (III) in acid chloride leach solutions of glass scraps by solvent extraction with various extractants. Hydrometallurgy 2018, 175, 232–239. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Rydberg, J.; Cox, M.; Musikas, C.; Choppin, G.R. Solvent Extraction Principles and Practice, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Nikoloski, A.N.; Ang, K.L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extract. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Matsumoto, K.; Yamakawa, S.; Jikei, M. Selective recovery of platinum (IV) from palladium (II)-containing solution using 4-(hexyloxy)aniline. Chem. Lett. 2017, 46, 22–24. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamakawa, S.; Sezaki, Y.; Katagiri, H.; Jikei, M. Preferential precipitation and selective separation of Rh(III) from Pd(II) and Pt(IV) using 4-alkylanilines as precipitants. ACS Omega 2019, 4, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Hata, Y.; Sezaki, Y.; Katagiri, H.; Jikei, M. Highly selective Rh(III) recovery from HCl solutions using aromatic primary diamines via formation of three-dimensional ionic crystals. ACS Omega 2019, 4, 14613–14620. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yamakawa, S.; Haga, K.; Ishibashi, K.; Jikei, M.; Shibayama, A. Selective and preferential separation of rhodium (III) from palladium (II) and platinum (IV) using a m-phenylene diamine-containing precipitant. Sci. Rep. 2019, 9, 12414. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sezaki, Y.; Yamakawa, S.; Hata, Y.; Jikei, M. Selective and mutual separation of palladium (II), platinum (IV), and rhodium (III) using aliphatic primary amines. Metals 2020, 10, 324. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Maniecki, T.P. Influence of atmosphere kind on temperature programmed decomposition of noble metal chlorides. Thermochim. Acta 2005, 435, 151–161. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).