Potentiality of Azolla pinnata R. Br. for Phytoremediation of Polluted Freshwater with Crude Petroleum Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Its Propagation
2.2. Assessment of Phytoremediation Potentiality
2.3. Determination of Petroleum Hydrocarbon Degradation
2.4. Fractionation of the Extracted TPH
2.5. Gas Chromatography for TSH Analysis
2.6. High-Performance Liquid Chromatography for TAH Analysis
2.7. Statistical Analysis
3. Results
3.1. Degradation of Total Petroleum Hydrocarbons
3.2. The Molecular Type Composition of PHs
3.3. The Analysis of Normal Paraffins of TSHs
3.4. The Analysis of Polycyclic Aromatic Hydrocarbons of TAHs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eshagberi, G.O. Toxic Effects of Water Soluble Fractions of Crude Oil, Diesel and Gasoline on Ceratophyllum Demersum. Int. J. Health Med. 2017, 2, 1–4. [Google Scholar]
- Kumari, A.; Kaur, R.; Kaur, R. A review on fate and remediation techniques of oil spills. Int. J. Res. Pharm. Sci. 2019, 10, 111. [Google Scholar]
- Bruckberger, M.C.; Morgan, M.J.; Walsh, T.; Bastow, T.P.; Prommer, H.; Mukhopadhyay, A.; Kaksonen, A.H.; Davis, G.; Puzon, G.J. Biodegradability of legacy crude oil contamination in Gulf War damaged groundwater wells in Northern Kuwait. Biodegradation 2019, 30, 71–85. [Google Scholar] [CrossRef]
- Zhang, B.; Matchinski, E.J.; Chen, B.; Ye, X.; Jing, L.; Lee, K. Chapter 21—Marine Oil Spills—Oil Pollution, Sources and Effects. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 391–406. [Google Scholar] [CrossRef]
- Chambers, L.E.; Patterson, T.; Hobday, A.J.; Arnould, J.P.Y.; Tuck, G.N.; Wilcox, C.; Dann, P. Determining trends and environmental drivers from long-term marine mammal and seabird data: Examples from Southern Australia. Reg. Environ. Chang. 2015, 15, 197–209. [Google Scholar] [CrossRef]
- Henkel, L.A.; Nevins, H.; Martin, M.; Sugarman, S.; Harvey, J.T.; Ziccardi, M.H. Chronic oiling of marine birds in California by natural petroleum seeps, shipwrecks, and other sources. Mar. Pollut. Bull. 2014, 79, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Qu, C.; Li, B.; Wu, H.; Wang, S.; Giesy, J.P. Multi-pathway assessment of human health risk posed by polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2015, 37, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Sarria-Villa, R.; Ocampo-Duque, W.; Páez, M.; Schuhmacher, M. Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Sci. Total Environ. 2016, 540, 455–465. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Schädle, T.; Pejcic, B.; Myers, M.; Mizaikoff, B. Fingerprinting Oils in Water via Their Dissolved VOC Pattern Using Mid-Infrared Sensors. Anal. Chem. 2014, 86, 9512–9517. [Google Scholar] [CrossRef]
- Liu, K.; Han, W.; Pan, W.-P.; Riley, J.T. Polycyclic aromatic hydrocarbon (PAH) emissions from a coal-fired pilot FBC system. J. Hazard. Mater. 2001, 84, 175–188. [Google Scholar] [CrossRef]
- Tewari, S.; Sirvaiya, A. Oil spill remediation and its regulation. Int. J. Eng. Res. Gen. Sci. 2015, 1, 2394–8299. [Google Scholar]
- Dave, D.; Ghaly, A.E. Remediation technologies for marine oil spills: A critical review and comparative analysis. Am. J. Environ. Sci. 2011, 7, 423. [Google Scholar] [CrossRef]
- Lessard, R.; Demarco, G. The Significance of Oil Spill Dispersants. Spill Sci. Technol. Bull. 2000, 6, 59–68. [Google Scholar] [CrossRef]
- Nomack, M.; Cleveland, C. “Oil Spill Control Technologies”. Encyclopedia of Earth. Available online: http://editors.eol.org/eoearth/wiki/spill_response_(Oil_spill_control_technologies) (accessed on 22 February 2021).
- Vergetis, E. Oil Pollution in Greek Seas and Spill Confrontation Means-Methods; National Technical University: Athens, Greece, 2002. [Google Scholar]
- Fingas, M.; Fieldhouse, B. Review of solidifiers. In Oil Spill Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 713–733. [Google Scholar]
- Board, M.; Council, N.R. Using Oil Spill Dispersants on the Sea; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Buist, I.; McCourt, J.; Potter, S.; Ross, S.; Trudel, K. In Situ Burning. Pure Appl. Chem. 1999, 71, 43–65. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Atlas, R.M. Bioremediation of petroleum pollutants. Int. Biodeterior. Biodegrad. 1995, 35, 317–327. [Google Scholar] [CrossRef]
- Duran, R.; Cravo-Laureau, C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J. Remediation processes for petroleum oil polluted soil. Indian J Biotechnol 2017, 16, 157–163. [Google Scholar]
- Atta, A.M.; Mohamed, N.H.; Hegazy, A.K.; Moustafa, Y.M.; Mohamed, R.R.; Safwat, G.; Diab, A.A. Green technology for remediation of water polluted with petroleum crude oil: Using of Eichhorniacrassipes (Mart.) Solms combined with magnetic nanoparticles capped with myrrh resources of Saudi Arabia. Nanomaterials 2020, 10, 262. [Google Scholar] [CrossRef]
- Azab, E.; Kebeish, R.; Hegazy, A.K. Expression of the human gene CYP1A2 enhances tolerance and detoxification of the phenylurea herbicide linuron in Arabidopsis thaliana plants and Escherichia coli. Environ. Pollut. 2018, 238, 281–290. [Google Scholar] [CrossRef]
- Azab, E.; Soror, A.-f.S. Physiological Behavior of the Aquatic Plant Azolla sp. in Response to Organic and Inorganic Fertilizers. Plants 2020, 9, 924. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Emam, M.H.; Lovett-Doust, L.; Azab, E.; El-Khatib, A.A. Response of duckweed to lead exposure: Phytomining, bioindicators and bioremediation. Desalin Water Treat. 2017, 70, 227–234. [Google Scholar] [CrossRef]
- Jan, S.; Parray, J.A. Metal Tolerance Strategy in Plants. In Approaches to Heavy Metal Tolerance in Plants; Springer: Singapore, 2016; pp. 19–32. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A.K. Monitoring the Efficiency of Rhazyastricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants 2020, 9, 1057. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A.K.; Gobouri, A.A.; Elkelish, A. Impact of Transgenic Arabidopsis thaliana Plants on Herbicide Isoproturon Phytoremediation through Expressing Human Cytochrome P450-1A2. Biology 2020, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K. Plant succession and its optimization on tar-polluted coasts in the Arabian Gulf region. Environ. Conserv. 1997, 149–158. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Afifi, S.Y.; Alatar, A.A.; Kabiel, H.E.; Emam, M.H. Response of crop plant growth and radionuclides uptake to organic and chemical fertilization of black sand soil. Phyton 2013, 53, 87–111. [Google Scholar]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of soils polluted with crude petroleum oil using Bassiascoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. A Review on Phytoremediation of Crude Oil Spills. Water AirSoil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, Q.; Peng, S.; Li, K. Promoted biodegradation and microbiological effects of petroleum hydrocarbons by Impatiens balsamina L. with strong endurance. J. Hazard. Mater. 2010, 183, 731–737. [Google Scholar] [CrossRef]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Choppala, G.; Kirkham, M.B.; Bolan, N.S. Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. J. Hazard. Mater. 2021, 401, 123282. [Google Scholar] [CrossRef]
- Sun, T.R.; Cang, L.; Wang, Q.Y.; Zhou, D.M.; Cheng, J.M.; Xu, H. Roles of abiotic losses, microbes, plant roots, and root exudates on phytoremediation of PAHs in a barren soil. J. Hazard. Mater. 2010, 176, 919–925. [Google Scholar] [CrossRef]
- Akhundova, E.; Atakishiyeva, Y. Interaction Between Plants and Biosurfactant Producing Microorganisms in Petroleum Contaminated Absheron Soils. In Phytoremediation for Green Energy; Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M.S.A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 115–122. [Google Scholar] [CrossRef]
- Farraji, H.; Robinson, B.; Mohajeri, P.; Abedi, T. Phytoremediation: Green technology for improving aquatic and terrestrial environments. Nippon J. Environ. Sci. 2020, 1. [Google Scholar] [CrossRef]
- Dhir, B. Phytoremediation: Role of Aquatic Plants in Environmental Clean-up; Springer: New Delhi, India, 2013; Volume 14, pp. 1–111. [Google Scholar] [CrossRef]
- Sood, A.; Uniyal, P.L.; Prasanna, R.; Ahluwalia, A.S. Phytoremediation Potential of Aquatic Macrophyte, Azolla. AMBIO 2012, 41, 122–137. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Hafez, R.M.; Hegazy, A.K.; Fattah, A.M.A.-E.; Mohamed, N.H. Variations of structural and functional traits of Azollapinnata R. Br. in response to crude oil pollution in arid regions. Sustainability 2021, 13, 2142. [Google Scholar] [CrossRef]
- Eribo, O.; Kadiri, M. Growth performance and phytoremediation ability of Azolla pinnata in produced water. J. Appl. Sci. Environ. Manag. 2017, 20, 1053. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1971; p. 61. [Google Scholar]
- Das, B.; Deka, S. A cost-effective and environmentally sustainable process for phycoremediation of oil field formation water for its safe disposal and reuse. Sci. Rep. 2019, 9, 15232. [Google Scholar] [CrossRef] [PubMed]
- Patowary, K.; Saikia, R.R.; Kalita, M.C.; Deka, S. Degradation of polyaromatic hydrocarbons employing biosurfactant-producing Bacillus pumilus KS2. Ann. Microbiol. 2015, 65, 225–234. [Google Scholar] [CrossRef]
- Mishra, S.; Jyot, J.; Kuhad, R.C.; Lal, B. In situ bioremediation potential of an oily sludge-degrading bacterial consortium. Curr. Microbiol. 2001, 43, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lal, B.; Khanna, S. Degradation of crude oil by Acinetobacter calcoaceticus and Alcaligenesodorans. J. Appl. Bacteriol. 1996, 81, 355–362. [Google Scholar]
- Azeez, N.M.; Sabbar, A.A. Efficiency of duckweed (Lemna minor L.) in phytotreatment of wastewater pollutants from Basrah oil refinery. J. Appl. Phytotechnology Environ. Sanit. 2012, 1, 163–172. [Google Scholar]
- Ekperusi, A.O.; Nwachukwu, E.O.; Sikoki, F.D. Assessing and Modelling the Efficacy of Lemnapaucicostata for the Phytoremediation of Petroleum Hydrocarbons in Crude Oil-Contaminated Wetlands. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Atyah, B.S.; Al-Mayaly, I. Biodegradation of Crude Oil by Anabaena variabilis Isolated from Al-Dora Refinery. IOSR J. Pharm. Biol. Sci. 2018, 13, 51. [Google Scholar]
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Suja, F.; Anuar, N.; Idris, M. Preliminary test of hydrocarbon exposure on Azollapinnata in phytoremediation process. In Proceedings of the International Conference on Environment, Energy and Biotechnology, Singapore, 5–6 May 2012; pp. 244–247. [Google Scholar]
- Nikolaeva, O.; Karpukhin, M.; Streletskii, R.; Rozanova, M.; Chistova, O.; Panina, N. Linking pollution of roadside soils and ecotoxicological responses of five higher plants. Ecotoxicol. Environ. Saf. 2021, 208, 111586. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Maslennikov, P.; Novikova, A.; Kozhikin, M. Effect of Crude Oil on Growth, Oxidative Stress and Response of Antioxidative System of Two Rye (Secalecereale L.) Varieties. Plants 2021, 10, 157. [Google Scholar] [CrossRef]
- Ali, L.N.; Mantoura, R.F.C.; Rowland, S.J. The dissolution and photodegradation of Kuwaiti crude oil in sea water. Part 1: Quantitative dissolution and analysis of the seawater-soluble fraction. Mar. Environ. Res. 1995, 40, 1–17. [Google Scholar] [CrossRef]
- Balasubramaniyam, A. The Influence of Plants in the Remediation of Petroleum Hydrocarbon-Contaminated Sites. Pharm. Anal. Chem. Open Access 2015, 1. [Google Scholar] [CrossRef]
- Adlan, N.A.; Sabri, S.; Masomian, M.; Ali, M.S.M.; Rahman, R.N.Z.R.A. Microbial Biodegradation of Paraffin Wax in Malaysian Crude Oil Mediated by Degradative Enzymes. Front. Microbiol. 2020, 11, 2145. [Google Scholar] [CrossRef] [PubMed]
- Wasoh, H.; Veeraswamy, K.; Gunasekaran, B.; Shukor, M.Y. Biodegradation of hydrocarbon sludge by Pseudomonas sp. strain UPM-KV. J. Environ. Microbiol. Toxicol. 2019, 7, 10–15. [Google Scholar]
- Borriss, R. Phytostimulation and biocontrol by the plant-associated Bacillus amyloliquefaciens FZB42: An Update. In Phyto-Microbiome in Stress Regulation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–20. [Google Scholar]
- Zhang, Z.Z.; Su, S.M.; Luo, Y.J.; Lu, M. Improvement of natural microbial remediation of petroleum-polluted soil using graminaceous plants. Water Sci. Technol. 2009, 59, 1025–1035. [Google Scholar] [CrossRef]
- Vieira, P.A.; Vieira, R.B. Biodegradation of diesel oil and gasoline contaminated effluent employing intermittent aeration. J. Hazard. Mater. 2009, 168, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Chi, Z.; Ma, Z.C.; Zhou, H.X.; Liu, G.L.; Lee, C.F.; Chi, Z.M. Hydrocarbons, the advanced biofuels produced by different organisms, the evidence that alkanes in petroleum can be renewable. Appl. Microbiol. Biotechnol. 2015, 99, 7481–7494. [Google Scholar] [CrossRef] [PubMed]
- Bagaeva, T.V.; Zinurova, E.E. Comparative characterization of extracellular and intracellular hydrocarbons of Clostridium pasteurianum. Biochemistry 2004, 69, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, Y.A.; Panikov, N.S.; Lukin, S.M.; Osipov, G.A. Saturated C21–C33Hydrocarbons Are Involved in the Self-Regulation of Pseudomonas fluorescensAdhesion to a Glass Surface. Microbiology 2001, 70, 138–144. [Google Scholar] [CrossRef]
- Martirani-Von Abercron, S.-M.; Pacheco, D.; Benito-Santano, P.; Marín, P.; Marqués, S. Polycyclic Aromatic Hydrocarbon-Induced Changes in Bacterial Community Structure under Anoxic Nitrate Reducing Conditions. Front. Microbiol. 2016, 7, 1775. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Naidu, R. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by novel bacterial consortia tolerant to diverse physical settings–assessments in liquid-and slurry-phase systems. Int. Biodeterior. Biodegrad. 2016, 108, 149–157. [Google Scholar] [CrossRef]
- Li, P.-H.; Wang, Y.; Li, Y.-H.; Wai, K.-M.; Li, H.-L.; Tong, L. Gas-particle partitioning and precipitation scavenging of polycyclic aromatic hydrocarbons (PAHs) in the free troposphere in southern China. Atmos. Environ. 2016, 128, 165–174. [Google Scholar] [CrossRef]
- Dean-Ross, D.; Moody, J.; Cerniglia, C.E. Utilization of mixtures of polycyclic aromatic hydrocarbons by bacteria isolated from contaminated sediment. FEMS Microbiol. Ecol. 2002, 41, 1–7. [Google Scholar] [CrossRef]
- Seo, J.-S.; Keum, Y.-S.; Li, Q.X. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Heath 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Lee, S.-E.; Seo, J.-S.; Keum, Y.-S.; Lee, K.-J.; Li, Q.X. Fluoranthene metabolism and associated proteins inMycobacterium sp. JS14. Proteomics 2007, 7, 2059–2069. [Google Scholar] [CrossRef]
- Yu, H. Environmental carcinogenic polycyclic aromatic hydrocarbons: Photochemistry and phototoxicity. J. Environ. Sci. Heal. Part C 2002, 20, 149–183. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Habe, H.; Yamane, H.; Nojiri, H. Characterization of Novel Carbazole Catabolism Genes from Gram-Positive Carbazole Degrader Nocardioides aromaticivorans IC177. Appl. Environ. Microbiol. 2006, 72, 3321–3329. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Karimi, B.; Habibi, M.; Esvand, M. Biodegradation of naphthalene using Pseudomonas aeruginosa by up flow anoxic-aerobic continuous flow combined bioreactor. J. Environ. Health Sci. Eng. 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Weitkamp, E.A.; Sage, A.M.; Pierce, J.R.; Donahue, N.M.; Robinson, A.L. Organic aerosol formation from photochemical oxidation of diesel exhaust in a smog chamber. Environ. Sci. Technol. 2007, 41, 6969–6975. [Google Scholar] [CrossRef] [PubMed]
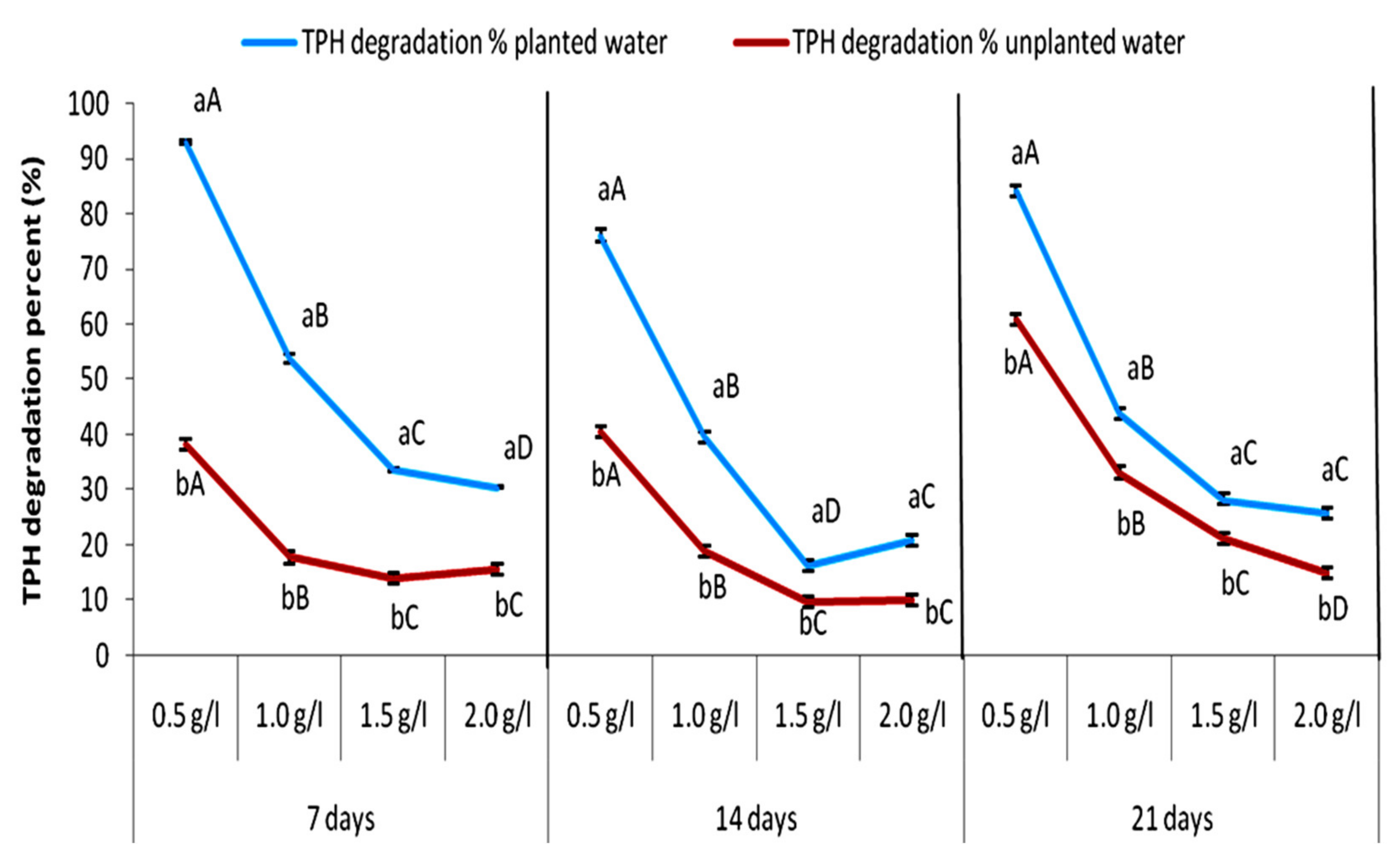
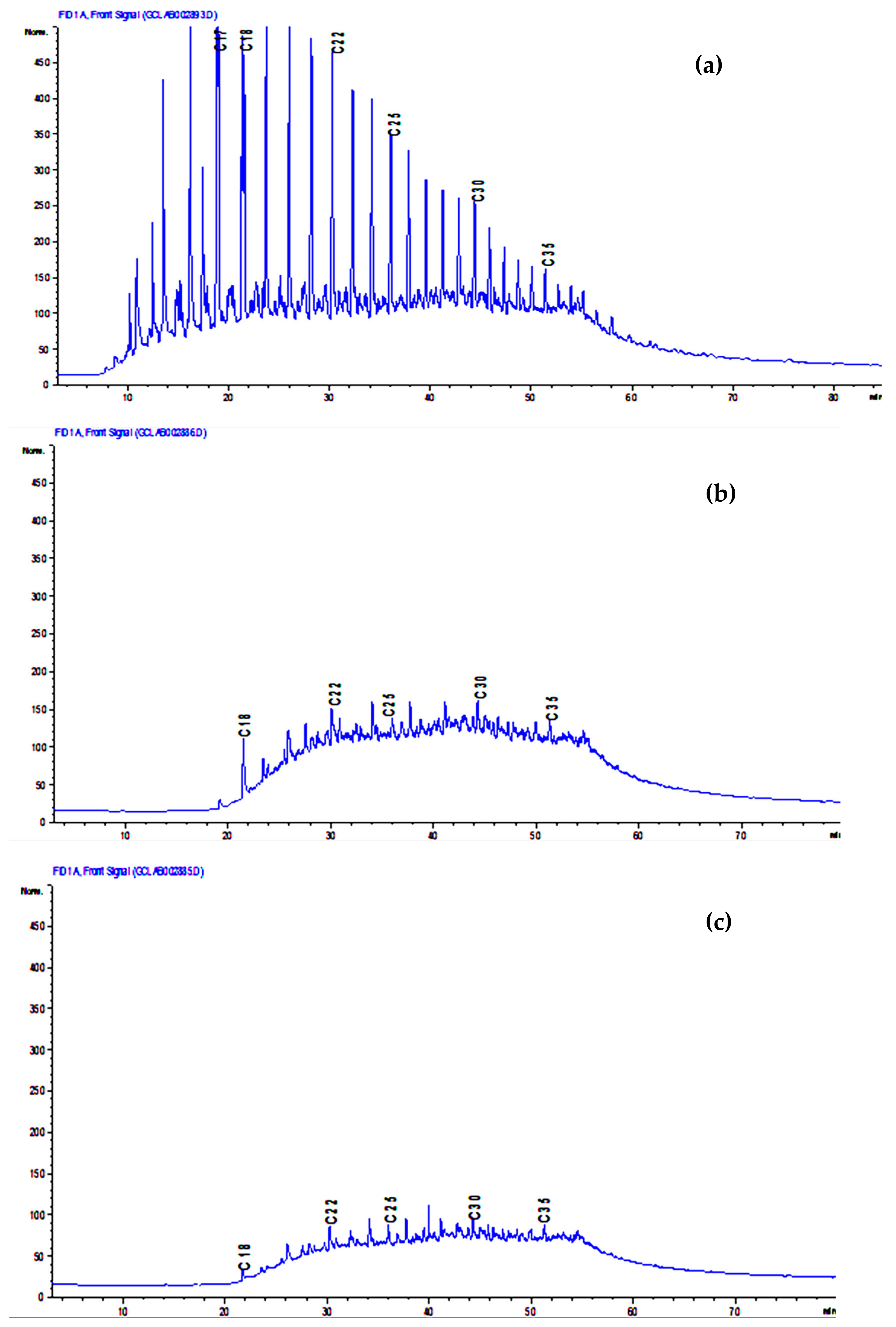
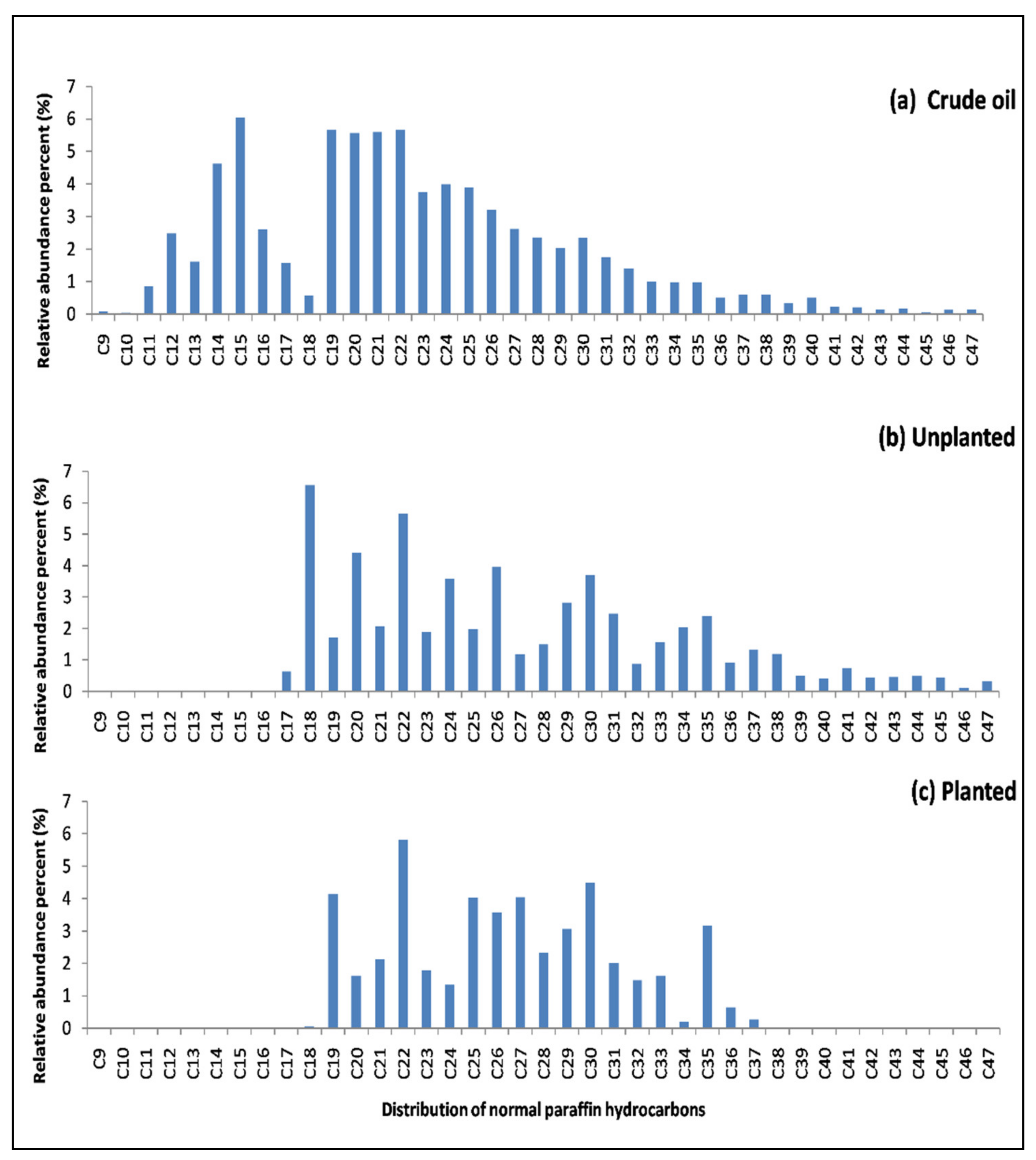
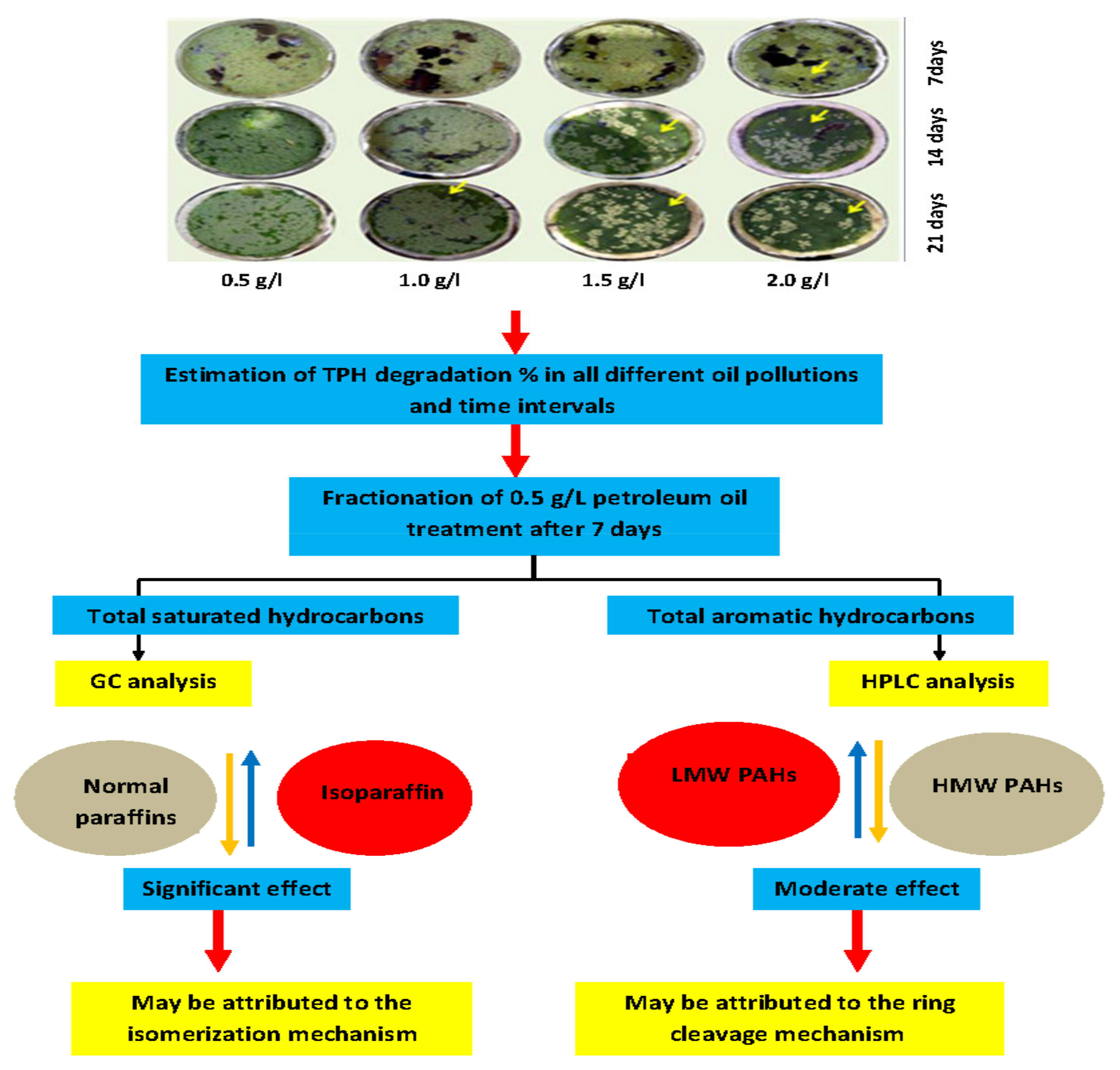
| Molecular Type Composition (wt.%) | 0.5 g Crude Oil | Unplanted Polluted Water | Planted Polluted Water |
|---|---|---|---|
| Total saturated hydrocarbons | 77.92 ± 0.04 c | 79.80 ± 0.12 b | 81.67 ± 0.18 a |
| Normal paraffins | 77.03 | 58.39 | 47.88 |
| Isoparaffins | 0.89 | 21.41 | 33.79 |
| Total aromatic hydrocarbons | 22.10 ± 0.03 a | 20.27 ± 0.18 b | 18.67 ± 0.18 c |
| Category | Carbon Number | 0.5 g Crude Oil | 0.5 g/L Planted Water | 0.5 g/L Unplanted Water |
|---|---|---|---|---|
| First Class | C9 | 0.096 | 0 | 0 |
| C10 | 0.048 | 0 | 0 | |
| C11 | 0.856 | 0 | 0 | |
| C12 | 2.48 | 0 | 0 | |
| C13 | 1.619 | 0 | 0 | |
| C14 | 4.622 | 0 | 0 | |
| C15 | 6.036 | 0 | 0 | |
| C16 | 2.6 | 0 | 0 | |
| Second Class | C18 | 0.568 | 0.069 | 6.553 |
| C34 | 0.992 | 0.201 | 2.042 | |
| C37 | 0.602 | 0.274 | 1.325 | |
| C38 | 0.608 | 0 | 1.193 | |
| C39 | 0.346 | 0 | 0.505 | |
| C41 | 0.233 | 0 | 0.753 | |
| C42 | 0.223 | 0 | 0.452 | |
| C43 | 0.15 | 0 | 0.468 | |
| C44 | 0.172 | 0 | 0.49 | |
| C45 | 0.058 | 0 | 0.441 | |
| C47 | 0.158 | 0 | 0.331 | |
| Third Class | C17 | 1.578 | 0 | 0.636 |
| C20 | 5.562 | 1.626 | 4.395 | |
| C23 | 3.753 | 1.799 | 1.89 | |
| C24 | 3.987 | 1.344 | 3.585 | |
| C40 | 0.502 | 0 | 0.411 | |
| C46 | 0.141 | 0 | 0.105 | |
| Fourth Class | C19 | 5.652 | 4.133 | 1.722 |
| C21 | 5.599 | 2.139 | 2.078 | |
| C22 | 5.654 | 5.808 | 5.642 | |
| C25 | 3.906 | 4.028 | 1.98 | |
| C27 | 2.614 | 4.046 | 1.173 | |
| C28 | 2.364 | 2.342 | 1.503 | |
| C32 | 1.4 | 1.485 | 0.882 | |
| Fifth Class | C26 | 3.21 | 3.574 | 3.962 |
| C29 | 2.041 | 3.062 | 2.819 | |
| C30 | 2.345 | 4.491 | 3.693 | |
| C31 | 1.764 | 2.018 | 2.469 | |
| C33 | 1.006 | 1.634 | 1.568 | |
| C35 | 0.981 | 3.155 | 2.399 | |
| C36 | 0.508 | 0.655 | 0.927 |
| Number of Rings | PAHs | 0.5 g/L Oil before Use | 0.5 g/L Oil After | |
|---|---|---|---|---|
| Planted Water | Unplanted Water | |||
| 2 rings | Nap | 0 | 0 | 0 |
| % 2 rings/total PAHs | 0 | 0 | 0 | |
| 3 rings | A | 0.24 | 0 | 0 |
| Ace | 0 | 0 | 8.5 | |
| F | 0.32 | 0.36 | 0.41 | |
| Phe | 0 | 0 | 0 | |
| Ant | 0.12 | 0.34 | 0.21 | |
| % 3 rings/total PAHs | 3.012 | 3.655 | 43.804 | |
| 4 rings | Flu | 0 | 0 | 0.012 |
| Pyr | 0 | 0 | 0.093 | |
| BaA | 0.86 | 3.53 | 0 | |
| Chr | 0.14 | 0 | 0.52 | |
| % 4 ring/total PAHs | 4.429 | 18.433 | 3.002 | |
| 5 rings | BbF | 0.21 | 12.86 | 9.74 |
| BkF | 0.26 | 0.25 | 0.23 | |
| BaP | 0.71 | 0.31 | 0.09 | |
| DahA | 10.49 | 0 | 0.17 | |
| % 5 ring/total PAHs | 51.683 | 70.078 | 49.135 | |
| 6 rings | Bp | 7.58 | 1.47 | 0.84 |
| IP | 1.64 | 0.01 | 0 | |
| % 6 ring/total PAHs | 40.833 | 7.728 | 4.035 | |
| Total PAHs | 22.58 | 19.15 | 20.82 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.A.; Hegazy, A.K.; Mohamed, N.H.; Hafez, R.M.; Azab, E.; Gobouri, A.A.; Saad, H.A.; Fattah, A.M.A.-E.; Mustafa, Y.M. Potentiality of Azolla pinnata R. Br. for Phytoremediation of Polluted Freshwater with Crude Petroleum Oil. Separations 2021, 8, 39. https://doi.org/10.3390/separations8040039
Mostafa AA, Hegazy AK, Mohamed NH, Hafez RM, Azab E, Gobouri AA, Saad HA, Fattah AMA-E, Mustafa YM. Potentiality of Azolla pinnata R. Br. for Phytoremediation of Polluted Freshwater with Crude Petroleum Oil. Separations. 2021; 8(4):39. https://doi.org/10.3390/separations8040039
Chicago/Turabian StyleMostafa, Aya A., Ahmad K. Hegazy, Nermen H. Mohamed, Rehab M. Hafez, Ehab Azab, Adil A. Gobouri, Hosam A. Saad, Azza M. Abd-El Fattah, and Yasser M. Mustafa. 2021. "Potentiality of Azolla pinnata R. Br. for Phytoremediation of Polluted Freshwater with Crude Petroleum Oil" Separations 8, no. 4: 39. https://doi.org/10.3390/separations8040039
APA StyleMostafa, A. A., Hegazy, A. K., Mohamed, N. H., Hafez, R. M., Azab, E., Gobouri, A. A., Saad, H. A., Fattah, A. M. A.-E., & Mustafa, Y. M. (2021). Potentiality of Azolla pinnata R. Br. for Phytoremediation of Polluted Freshwater with Crude Petroleum Oil. Separations, 8(4), 39. https://doi.org/10.3390/separations8040039








