Abstract
In the last few years, the flavored beer market has increased significantly. In particular, consumers showed a growing interest in citrus-flavored beers. Citrus fruits contain, among other class of compounds, terpenes and terpenoids and oxygenated heterocyclic compounds. The absence of a specific legislation concerning beer flavored production and ingredients reported on the labels makes these beers subject to possible adulterations. Solid phase micro extraction (SPME) followed by gas chromatographic–mass spectrometry (GC-MS) and gas chromatographic-flame ionization detector (GC-FID) analysis of the volatile profile together with the characterization of the oxygen heterocyclic compounds through high performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) demonstrated to be a powerful analytical strategy for quality control. In this study, we combined the volatile and non-volatile profiles of “citrus flavored mainstream beers”, in order to evaluate the authenticity and determine markers to prevent food frauds. The changes in the aroma composition of the unflavored types after the addition of peel, or citrus essential oil were also evaluated. The linear retention index (LRI) system was used for both techniques; in particular, its application in liquid chromatography is still limited and represents a novelty. The coupling of the high sensitivity of the HPLC MS/MS method with the LRI system, it has made possible for the first time a reliable identification and an accurate quantification of furocoumarins in citrus-flavored beers.
1. Introduction
Beer is one of the most produced alcoholic beverages in the world, its flavor and aroma resulting from a complex mixture of volatile and non-volatile components belonging to different chemical classes, most of which originated from malt, hops, and yeast used in brewing process.
Many of the volatile organic compounds present in beer aroma is directly inherent in the raw material or originated in process such as roasting malt, boiling wort, and flavor addition. These compounds, even if present in low concentration, can strongly influence the quality and character of the beer.
The flavor profile depends also on the storage conditions and changes during storage and aging of beer. Reaction initiated by light, oxygen, and temperature can result in the de novo formation of volatiles or can lead to increasing or decreasing concentrations of aroma compounds [1].
Therefore, the understanding and investigation of the volatiles generated in brewing process are important for beer characterization and for prediction of the aroma, in order to provide quality products and to promote the introduction on the market of new products that answer to the consumer demand.
Recently, fruit beers are getting very popular all over the world especially in the field of craft breweries. Particular widespread in Belgium are beers produced by adding cherry or raspberry to the production process. The addition of these fruits leads to an enhancement of flavors and might contribute to increase the content of bioactive compounds such as carotenoids and polyphenols that originate during beer refermentation and maturation.
In the last few year citrus-flavored beer are becoming increasingly popular and their request is more diffused on the marked.
Citrus metabolites are of vital importance to human health due to their bioactive properties. Citrus volatile fraction consists mostly of monoterpene and sesquiterpene hydrocarbons, their oxygenated derivatives, and aliphatic oxygenated compounds. Nonvolatile constituents instead, include flavonoids, alkaloids, limonoids, coumarins (Cs), furocoumarins (FCs), polymethoxyflavones (PMFs), carotenoids, and phenolic acids. Both volatile and nonvolatile metabolites have among the others anti-oxidative, anti-inflammatory, anti-cancerogen characteristic [2,3], as well as cardiovascular and neuroprotective effects. The addition of citrus peel, juice, pulp, or other citrus derivates can thus represent a way to increase the bioactive compounds present in beer, with potential health benefits.
Nevertheless, their production is not strictly regulated, and the ingredients on the label do not always clarify if peel, juice, synthetic aroma, or hydroalcoholic solution has been added to the wort during fermentation, and this can lead to possible adulterations.
The purpose of the present study was to characterize the volatile and non-volatile components of citrus-flavored beer to detect traceability and food authenticity marker for fraud prevention and consumer protection.
In fact, each species of Citrus is characterized by the presence of specific volatile and non-volatile components. The oxygen heterocyclic compound (OHC) profile, which is represented by Cs, FCs, and PMFs, is specific for the different types of Citrus; therefore, together with the volatile composition represents a powerful strategy for the quality control of Citrus products.
For this reason, we examined by High Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) analysis the oxygen heterocyclic compounds and the volatile compounds, respectively, of several citrus-flavored beers. A comparison was made with conventional no-fruit beers, and with the same no-fruit beers after the addition of lemon and orange peels or essential oils.
Moreover, the high sensitivity of the High Performance Liquid Chromatography-tandem Mass Spectrometry (HPLC MS/MS) method applied in combination with the Linear Retention Index (LRI) system leads to an accurate quantification of FCs. The determination of FCs in food and beverages is nowadays a topic of great relevance [4,5,6,7] by considering the latest scientific opinions [8,9] on this issue, which are still encouraging the development of analytical methods to determine FCs in food matrices, in order to estimate the dose-effects relationship and consequently update the current official regulation [10].
2. Materials and Methods
2.1. Samples
A total of 16 commercial beer’s samples among unflavored and flavored by different citrus species (3 control, 5 orange, 4 lemon, 2 bergamot, 2 mandarin) were purchased in local supermarkets and stored at 4 °C (Table 1). Three unflavored beers with different characteristic (American pale ale (APA), lager, and Italian grape ale (IGA); sample 1, 2, and 3, respectively) were used as negative control. Sample one was also used to create positive controls by the addition of 0.3% cold bergamot essential oil, orange peel 1.5% and lemon peel 1.8%, by maceration for 10 days. Prior to analysis the beer samples were brought to room temperature. The volatile fraction of the spiked beer was analyzed after 10 days and after 30 days to evaluate the stability of the products and the amount of possible marker compounds.

Table 1.
Samples analyzed. ABV, alcohol by volume.
In order to better understand the origin and the amount of the detected compounds, a comparison with the profile of citrus juices, nonalcoholic citrus flavored beverages, and essential oils has been also considered.
2.2. Standard Compounds (Reagents)
AC7-C40 Saturated Alkanes (1000 g/mL) standard mixture in hexane (49452-U) supplied by Merck Life Science (Darmstadt, Germany) was utilized for ALKANEs linear retention indices (LRIs) calculation.
The homologue series used for the LRI calculation in LC was composed by alkyl aryl ketones from acetofenone to heptanophenone, and each standard was furnished by Merck Life Science (Merck KGaA, Darmstadt, Germany).
Analytical standards (>95% purity) of Cs, FCs, and PMFs (angelicin, bergamottin, bergapten, byakangelicin, byakangelicol, cnidicin, cnidilin, epoxybergamottin, heraclenin, imperatorin, isobergapten, isoimperatorin, isopimpinellin, oxypeucedanin, oxypeucedanin hydrate, phellopterin, psoralen, 8-methoxypsoralen, 8-geranyloxypsoralen, aurapten, citropten, epoxyaurapten, herniarin, isomeranzin, meranzin, meranzin hydrate, 5-geranyloxy-7-methoxycoumarin, gardenin A, gardenin B, nobiletin, sinensetin, tangeretin, tetra-O-methylscutellarein, and 5-O-methylnobiletin) were furnished by Merck Life Science (Merck KGaA, Darmstadt, Germany). Tetrahydrofuran (THF), ethylacetate and ethanol HPLC grade, water and methanol both UHPLC-MS grade were provided by Merck Life Science. Stock solutions were prepared for all STDs at concentration 1000 mg/L. All standards and stock solutions were maintained at −18 °C before use.
2.3. Solid Phase Micro Extraction (SPME) and Liquid/Liquid Extraction Conditions
Solid phase micro extraction (SPME) extraction was carried out in the headspace mode (HS) using an automatic holder provided by Merck Life Science (Merck KGaA, Darmstadt, Germany) equipped with a fused silica fiber coated with a 50/30 μm layer of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) 1 cm long (Merck Life Science). The fiber was conditioned before the initial use according to the manufacturer’s instructions, and a cleaning step of 20 min at 10 °C below the fiber’s recommended maximum temperature was applied between consecutive analyses. Samples were conditioned in a thermostatic bath for 5 min at 37 °C with a stirring rate of 300 rpm before exposing the fiber for 30 min in the same conditions. After the extraction the analytes were thermally desorbed for 1 min at 260 °C in the GC injector port in splitless mode.
The liquid-liquid extraction of OHCs was carried out by the method previously optimized in our laboratory and recently applied for the analysis of cosmetics. Briefly, the samples were extracted by shaking in a separatory funnel 10 mL of beer, non-alcoholic drink or juice, with ethyl acetate in ratio 1:1 (v/v), for 10 min, three times. The supernatants were collected and dried through a parallel evaporator (GENEVAC, EZ-2). The residues were dissolved in ethanol and injected into the HPLC-MS/MS system, a triple quadrupole mass spectrometer LCMS-8060 (Shimadzu, 160 Duisburg, Germany). A proper dilution was made in case of compound out of the calibration curve linearity range.
For the same reason, sweet blood orange, green mandarin, yellow mandarin, and bergamot essential oils were diluted with ethanol 1:250 and 1:2500 (v/v), in order to quantify both most and less concentrated OHCs.
Before and after the sample analysis the homologous series was injected in order to apply the LRI system for the identification.
2.4. Gas Chromatographic-Mass Spectrometry (GC-MS) and Gas Chromatographic-Flame Ionization Detector (GC-FID) Analysis
The gas chromatographic-mass spectrometry (GC-MS) and gas chromatographic-flame ionization detector (GC-FID) analysis were carried out for quantitative and qualitative purposes, respectively.
GC-FID analyses were carried out on a GC2020 system (Shimadzu, Kyoto, Japan). For the separation an SLB-5ms fused-silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm df) provided by Merck Life Science (Merck KGaA, Darmstadt, Germany) was used. Helium was used as carrier gas, at a constant linear velocity of 30.0 cm/s which corresponding to an inlet pressure of 97.4 kPa. The injector temperature was set at 280 °C. The temperature program was the following: 40 °C held for 1 min to 350 °C at 3 °C/min, held for 5 min. The FID temperature was set at 280 °C (sampling rate 200 ms), hydrogen and air flows were 40 mL/min and 400 mL/min, respectively. Data were collected by LabSolution software ver. 5.92 (Shimadzu, Kyoto, Japan). Quantitative results were determined as peak area percentage without any correction. Samples were analyzed in triplicates.
GC-MS analyses were carried out on a GC-QP2020 system (Shimadzu, Kyoto, Japan). Column, oven temperature program and injection parameters were the same as for FID applications Helium was used as carrier gas, at a constant linear velocity of 30.0 cm/s which corresponding to an inlet pressure of 24.2 kPa. The interface and ion source temperatures were 250 °C and 200 °C, respectively. The acquisition was made in full scan mode in the mass range of 40–500 m/z, with a scanning rate interval of 0.2 s. Data handling was supported by GCMS solution ver. 4.30 software (Shimadzu, Kyoto, Japan). For the characterization, the following databases were used: W11N17 (Wiley11-Nist17, Wiley, Hoboken, USA; and FFNSC 4.0 (Shimadzu, Duisburg, German). The identification was performed applying two filters, namely spectral similarity match over 85% and linear retention index (LRI) match calculated using a C7–C40 saturated n-alkane homologue series with a filter window of ±10 LRI units.
2.5. LC–MS/MS Analysis
The analyses were carried out by using a Nexera X2 system coupled with a triple quadrupole mass spectrometer LCMS-8060 (Shimadzu, Duisburg, Germany). An APCI interface, set in positive ionization mode, was employed for the ionization process. The chromatographic system was equipped with two LC-30AD pumps, a SIL-30AC autosampler, a DGU-20A5R degassing unit and a CTO-20AC oven. The separation was achieved following the analytical conditions already optimized [11] and described as follows: The column was an Ascentis Express C18 column (50 × 4.6 mm, 2.7 μm) provided by Merck. The mobile phase was composed by solvent (A) water/methanol/THF (85:10:5, v:v:v) and solvent (B) methanol/THF (95:5, v:v) in gradient mode: 0–4.5 min, 15–28% B; 4.5–7.0 min, 28–60% B; 7.0–11.0 min, 60–85% B, hold for 3 min, at a flow rate of 2 mL min–1. The oven temperature was maintained at 40 °C. The injection volume was 2 μL.
The carry-over phenomenon was considered and evaluated in order to be avoided. MS parameters were as follows: Interface temperature was set at 450 °C; desolvation line (DL) and heat block temperatures were both 300 °C; nebulizing and drying gas flow were 3 and 15 L/min, respectively; the pressure of the CID gas was 270 kPa.
The homologous series was analyzed in Singol Ion Monitoring positive mode (SIM+). The m/z monitored for the alkyl aryl ketones were: m/z 121, m/z 135, m/z 149, m/z 163, m/z 177, m/z 191 for acetophenone, propiophenone, butyrophenone, valerophenone, hexanophenone, and heptanophenone, respectively.
Target analytes (Cs, FCs, and PMFs) were detected in both Full Scan and and Multiple Reaction Monitoring (MRM) acquisition mode.
The mass spectral range for the Full Scan analysis was 150–450 m/z, while the MRM acquisition was performed through a synchronized method, acquiring the specific transitions in selected time windows, according to the retention time of the analytes.
The MS and MS/MS libraries were created by collecting the spectra of the target in Full Scan and MRM mode, respectively.
The quantitative characterization was based on the external calibration, by creating calibration curves in MRM acquisition mode for each target. The weighting factors were applied in order to correct the data heteroscedasticity [12].
The method was validated according to the Eurachem guide [13], in terms of limit of detection (LODs), limit of quantification (LOQs), linearity range, reproducibility and repeatability.
Moreover, the method validation was implemented by the uncertainty determination, following the formula reported by Piotr Konieczka and Jacek Namiesnik [14]. According to their considerations, the uncertainty associated with the amount of sample used was considered negligible. The parameter related to the uncertainty of the calibration and linear regression method was also excluded, because all curves were corrected by proper weighting factors [12], then reducing the error due to heteroscedastic data.
A simplified formula, which considers the uncertainty associated with repeatability, with analyte concentration and with recovery (trueness) was adopted for the uncertainty determination.
The LRI were calculated applying the equation proposed by H. van den Dool and D. J. Kratz (Equation (1)) [15], developed for programmed-temperature retention index calculation.
3. Results and Discussion
3.1. Optimization of SPME and GC Conditions
The choice of the proper fiber coating is the most important step in the optimization of an SPME procedure, and depends on the physico-chemical properties of the solutes. Several coatings have been developed for a range of applications and various articles [16,17] report use of different kind of fibers for the extraction of volatile compounds in beer.
The same articles report the best sample volume to extract the volatile compounds obtaining a good number of peaks is 4 mL, with or without addition of an amount of NaCl between 0.5 g and 1.5 g.
For the selection of the best extraction conditions, an unflavored beer was used, and the total area of the peaks (both identified and unidentified fraction) was monitored during the phase of method optimization.
For the method optimization, considering the different nature of the compounds to investigate, Polydimethylsiloxane/Divinylbenzene (PDMS/DVB) 65 μm and DVB/CAR/PDMS 50/30 μm SPME fibers were tested (both provided by Merck Life Science, Merck KGaA, Darmstadt, Germany), and different analysis conditions were applied (time of sample conditioning; time of fiber exposition into the sample; temperature and stirring rate of sample conditioning and extraction, NaCl addition). Each parameter has been optimized performing analysis in triplicate. GC analyses were carried out using for each test a 20 mL vial with 2.5, 3, and 4 mL sample, respectively. The best results were obtained for a 2.5 mL sample volume and employing the triphasic fiber, that resulted to be the most useful in covering the wide range of beer’s volatile analytes. The addition of 0.5 mg NaCl was also tested and not substantial variation of the detected compounds amount has been observed.
Before solute adsorption onto the fiber coating, the beer samples underwent heating (conditioning) in order to saturate the vial headspace. Saturation times of 5, and 10 min were evaluated at the same temperature (37 °C, 45 °C, or 55 °C) employed for the extraction stage, and the analytical repeatability was excellent in both conditions.
A temperature variation in an HS-SPME system is quite complex because has a specific effect on the partitioning process of every volatile. In this investigation, an extraction temperature of 37 °C resulted the best compromise between equilibration time and method sensitivity.
Furthermore, three different fiber exposure times were tested: 20, 30, and 40 min. The highest volatile extraction yield was achieved after an exposure time of 30 min, and most of the heavier molecular weight volatiles remained substantially stable thereafter.
The GC results guaranteed high extraction yields for all beer components, confirming DVB/CAR/PDMS 50/30 μm fiber, conditioning time of 5 min at 37 °C, fiber exposure time of 30 min at the same temperature, and stirring rate of 300 rpm are the best choice for the extraction of volatiles components.
3.2. Volatile Fraction’s Analysis
The beer samples analyzed showed very different GC/MS profiles. About three-hundred compounds have been identified in total in the different samples, accounting for 89–97% of the total composition (Table 2 and Table S1).

Table 2.
Most abundant volatile compounds contained in the samples analysed, expressed in area% (± SD) as average of three measurements by GC–FID analysis.
The major compounds found in unflavored beer are alcohols and esters: Isopentyl alcohol, sec-butylcarbinol, isoamyl acetate, ethyl hexanoate, ethyl octanoate, phenethyl alcohol, 2-phenethyl acetate, ethyl decanoate, and 2-methylbutyl acetate.
Esters are one of the more volatile compounds in beer and hence influence greatly beer aroma. In moderate quantities, they can add a pleasant, full-bodied character to beer aroma. However, when present in excess they give beer aroma an overly fruity quality, which is considered undesirable by most consumers [18]. Concentrations of the esters strongly depend on the yeast strain, the density of the wort and the amount of oxygen available during fermentation [19].
One of the two main groups of volatile esters present in fermented beverages contains the acetate esters (in which the acid group is acetate and the alcohol group is ethanol or a complex alcohol derived from amino acid metabolism), such as ethyl acetate (solvent like aroma), isoamyl acetate (banana aroma) and phenyl ethyl acetate (roses, honey). The second group features the ethyl esters (in which the alcohol group is ethanol and the acid group is a medium-chain fatty acid) and includes ethyl hexanoate (anise seed, apple-like aroma), ethyl octanoate (sour apple aroma), and ethyl decanoate [20]. Between these esters, ethyl acetate is typically present in the highest concentration [21] and represents approximately one-third of all esters in beer.
Ethyl hexanoate, ethyl octanoate, and 2-phenethyl acetate are very common in beer [19] and are normally associated with fruity aroma impressions. 2-Methylbutyl acetate, and 2-phenethyl acetate are also important esters produced during fermentation, and their concentrations are normally above their flavor threshold value.
Beers produced in different countries do not differ greatly in their content of aliphatic fusel alcohol (higher alcohols formed during yeast fermentation of carbohydrates), although the amounts vary between the different beer types because their formation depends on the yeast used and, in particular, on fermentation conditions. Isopentyl alcohol is a product of the fermentation of starches, phenethyl alcohol instead an aromatic fusel alcohol with a relatively strong rose-like odor, it has been found to be present in beer in quantities from 4 to 102 mg/L. It does not occur in wort and is evidently synthesized from phenylalanine by yeast in manner analogous to the formation of fusel alcohols, as evidenced by Ehrlich’s early experiments [22].
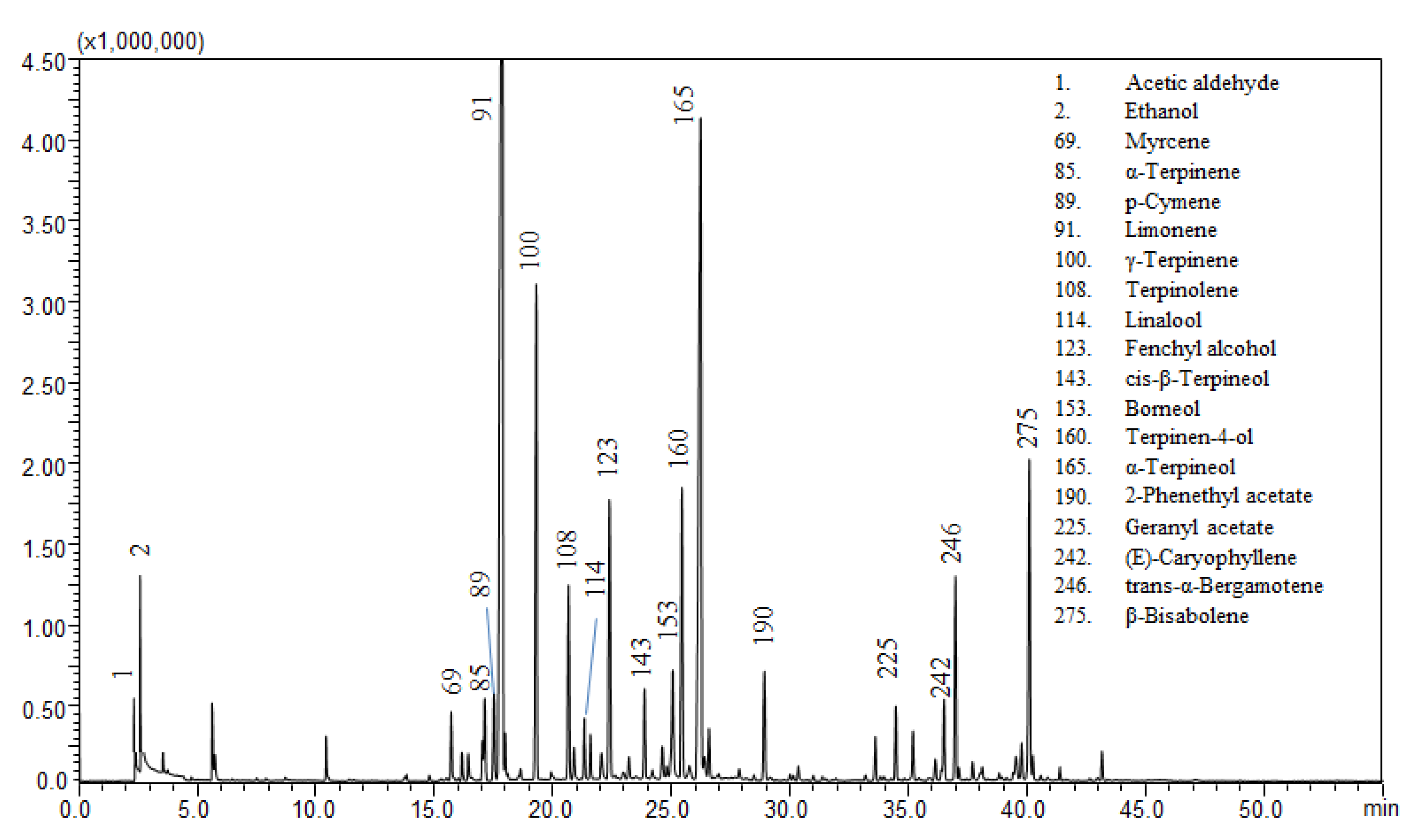
The most abundant compounds encountered in the lemon peel flavored beers were limonene, β-pinene, γ-terpinene, α-terpineol, neryl acetate, geranyl acetate, n-octanal, n-nonanal, n-decanal, linalool, and myrcene. Figure 1 shows the chromatogram of a lemon flavored beer in which lemon juice has been added as flavoring agent.
Figure 1.
GC-MS analysis of the volatile profile for sample 11.
Myrcene is the principal volatile of most hop varieties, and is a significant contributor to fresh hop aroma [23,24]. Its concentration decreases during the brewing process due to autoxidation, giving rise to multiple cyclic reaction products (e.g., α-pinene, β-pinene, camphene, p-cymene) and also forms terpenoids such as linalool, nerol, geraniol, citral, α-terpineol, or carvone [25].
Lemon essential oil and lemon juice contain a great amount of limonene, β-pinene, γ-terpinene, α-terpineol, neryl acetate, geranyl acetate, and myrcene as shown in Tables S2 and S3. The presence of those compounds in the lemon flavored beer analyzed is in agreement with the addition of natural aroma. n-nonanal, n-decanal, and linalool are also present in lemon essential oil, but they are not specific markers since they are common also in unflavored beer. The same consideration applies to fenchol, which is a hop-derived aroma compound commonly found in beer [26].
The analysis on orange flavored beer shown the most abundant compounds were ethyl octanoate, 2-phenethyl acetate, ethyl decanoate, phenethyl alcohol, isopentyl alcohol, isoamyl acetate, ethyl hexanoate, terpinen-4-ol, styrene, limonene, ethyl acetate, myrcene, ethyl dodecanoate. The presence of styrene, as reported by K. J. Schwarz et al. [27] is derived from the thermal decarboxylation of cinnamic acid during wort boiling or from enzymatic decarboxylation during fermentation. The production of styrene proceed in parallel but much faster than the decarboxylation of ferulic and p-cumaric acid to 4-vinylguaiacol and 4-vinylphenol by the same decarboxylase enzyme.
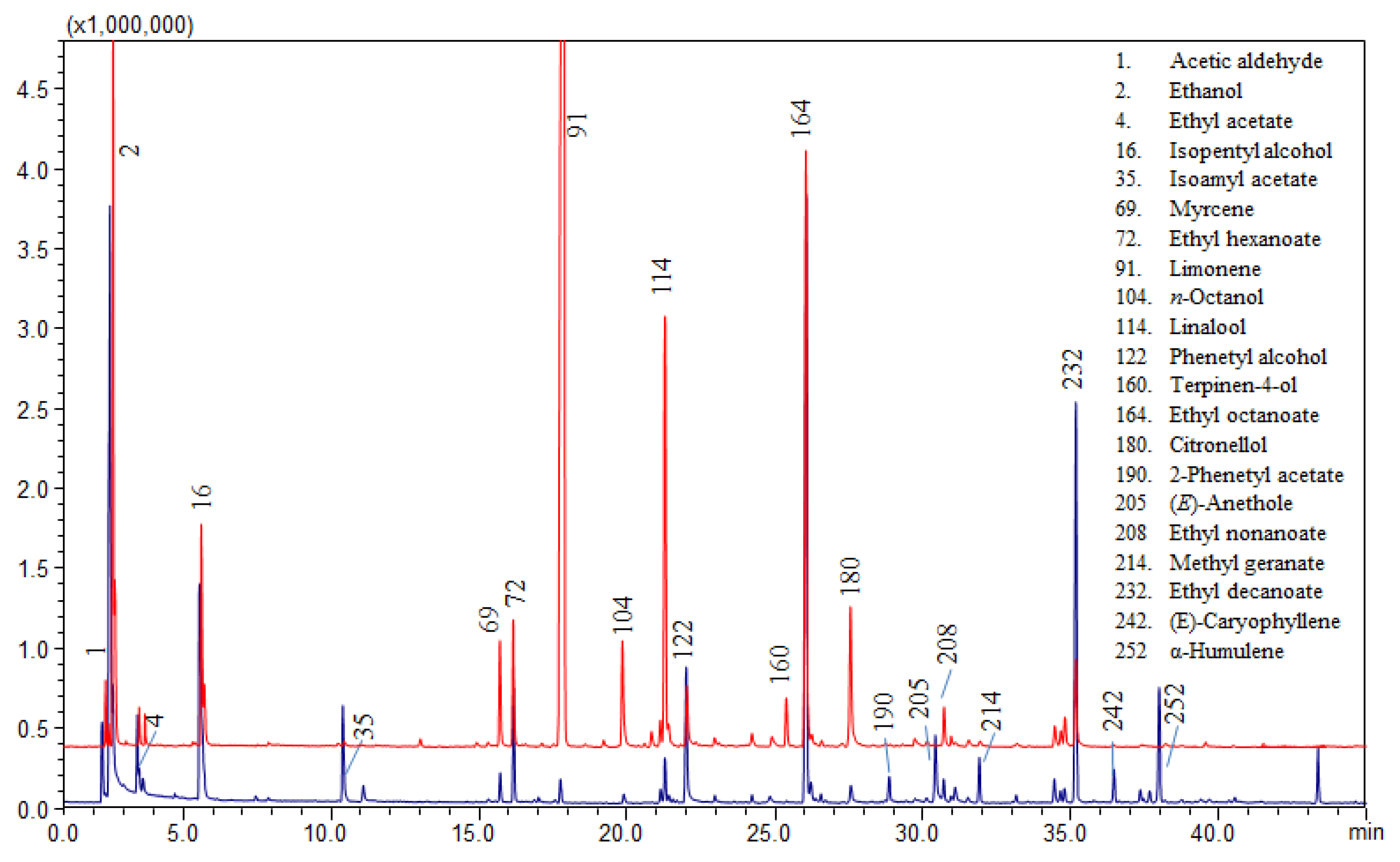
Limonene is the most abundant component in orange peel, followed by myrcene. In the analyzed beers, the signal of limonene is not as predominant as expected, since the height of the limonene signal is similar to that of other compounds. Figure 2 shows a comparison between the volatile profile of an orange flavored beer and an unflavored beer spiked with 1.5% of orange peel, in which limonene signal is considerably high. Furthermore the ratio between limonene and myrcene does not correspond to that typically found in orange essential oils (Table S2). The esters and alcohol encountered in high quantity are typical of beers and result from malt and yeast fermentation. The achieved results are not enough to confirm orange peel has been added during the brewing process, and the small amount of marker compounds found could depend on the addition of an exiguous quantity of orange peel as flavoring agent.
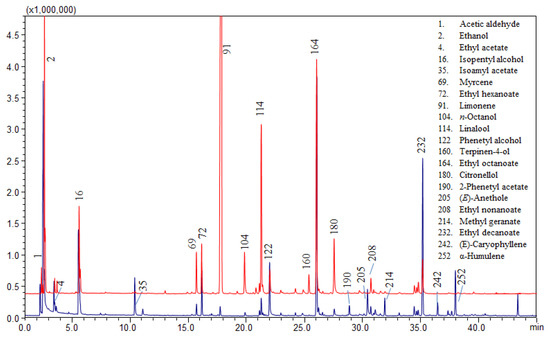
Figure 2.
Comparison between volatile profile of the orange flavored beer sample 4 (in blue) and sample 1 spiked with 1.5% orange peel (in red).
The data concerning the beers flavored with bergamot peel showed a great amounts of linalool, ethyl octanoate, isopentyl alcohol, isoamyl acetate, limonene, phenethyl alcohol, terpinen-4-ol, ethyl hexanoate, and 2-phenethyl acetate. This results are not consistent with bergamot essential oil (Table S2) and juice (Table S3) analysis, since linalyl acetate, a compound present in large quantity in both products, is totally absent in the beers under analysis. Based on the obtained data it is reasonable to think that an artificial aroma or another citrus aroma has been added during the brewing process.
The results of the investigations on mandarin beers showed the compounds in major quantity were ethyl octanoate, ethyl decanoate, isopentyl alcohol, limonene, ethyl dodecanoate, linalool, phenethyl alcohol, ethyl hexanoate, and γ-terpinene. The esters and alcohols detected have been found also in the other beer, and as previously discussed are typical of beer aroma. The presence of limonene and γ-terpinene, associated with the detection of methyl N-methylanthranilate, a typical compound of mandarin aroma can confirm the addition of mandarin.
In the beer 3, 4-ethyl guaiacol, and ethyl lactate have been also encountered, both considered as off-flavor marker in the beer, originated from Brettanomyces yeast activity [28].
The change in volatile aroma profile of unflavored beer after addition of orange peel was monitored after 10 and 30 days. As expected an increment of limonene and, linalool was observed. Furthermore, the presence of typical orange minority compounds as n-octanol, terpinen-4-ol and citronellol was noted (Figure S8). No meaningful variation in volatile composition and in the detected compound amount has been encountered leaving peels into the beer for more time.
In the same way, the addition of lemon peels led to an increment of limonene, γ-terpinene, and β-pinene (Figure S7), typical of the fruit volatile aroma profile. In addition, also in this case, no relevant variations have been observed after one month.
The addition of 0.3% of the bergamot E.O. to the beer led an increment of linalool, linalyl acetate, limonene, γ-terpinene, β-pinene, myrcene, sabinene, neryl acetate, geranyl acetate (Figure S9), typical of the bergamot aroma.
3.3. Non-Volatile Fraction’s Analysis
The characterization of the non-volatile fraction represents one of the tools applied to determine the authenticity of citrus essential oils. Cold pressed Citrus essential oils are, in fact, characterized by specific OHCs depending on the species [11,29,30]. When Citrus essential oils or peels are used as ingredients of a beer, the OHC qualitative profile of the final product must correspond to that of the citrus species declared on the label. Although so many factors influence the final aroma of the beer, the combination of both LC and GC analysis is a powerful strategy to notice potential discrepancy between the listed ingredient and the obtained results. In some case, differences with the expected OHC profile are due to the use of juice instead of peels, or due to the use of a different species respect to those declared, or in the wort cases due to the addition of single components of natural or synthetic origin. Sometimes altered qualitative profiles depend only on the use of mixed ingredients, such us peels and juice together, or on the use of different Citrus species in the same preparation.
Another aspect is the hydro-solubility of OHCs. Beer is mainly composed of water, and as a consequence, when peels are used as a flavor ingredient, the water solubility of OHCs influences the content of these molecules in the final preparation. Also, the preparation step when peels are added may change the extraction, for example if peels are boiled or used at the end of the preparation.
The optimized MRM transitions for all targets are reported in Table 3 together with the LRI values. The LRI system is here applied together with the MS/MS library (in MRM mode) as identification tool of OHCs, already successfully applied for the same purpose in essential oils and cosmetics. In particular, peaks with a high spectral similarity were excluded by the quantification when the LRI value was out of the set range, which is ± 4 units, according to the previous results. Concerning the quantitative point of view, a method based on the external calibration and the Eurachem validation procedure ensured the accuracy of the results reported in Table 4 and Table 5. Almost all calibration curves were in the ranges 0.001–1 mg/L, except for 8-geranyloxypsoralen, epoxyaurapten, meranzin hydrate, and nobiletin, with the lower point at 0.1 mg/L. LOQ resulted less than 10 g/L for all targets except for nobiletin, due to the high matrix effects, already demonstrated for this compound [31]. The accuracy was satisfactory for all the concentration tested, providing a correct quantitative determination of the analytes also if contained at trace level.

Table 3.
Oxygen heterocyclic compounds contained in the samples analyzed, with MRM transitions and linear retention index (LRI) value.

Table 4.
Oxygen heterocyclic compounds contained in the samples analysed, expressed in μg/L L (± expanded uncertainty) as average of three measurements.

Table 5.
Oxygen heterocyclic compounds contained in the samples analysed, expressed in g/L or mg/L (± expanded uncertainty) as average of three measurements.
Moreover, the method validation was implemented by the uncertainty determination, following the formula reported by Piotr Konieczka and Jacek Namiesnik [14] and reported in Table 4 and Table 5 for each compound.
Three unflavored beers (samples 1, 2, 3) were selected as negative control. They were chosen as representative of different types of beer (American Pale Ale, Lager and Italian Grape Ale) to test also the LC method and their influence as matrix on the targeted analysis.
The analysis of the negative control gave the idea of the method selectivity. No OHCs were detected and no false signals for both Q and q transitions were detected. This means that other compounds of the matrix (unflavored beer) do not affect the MRM signals of the targets.
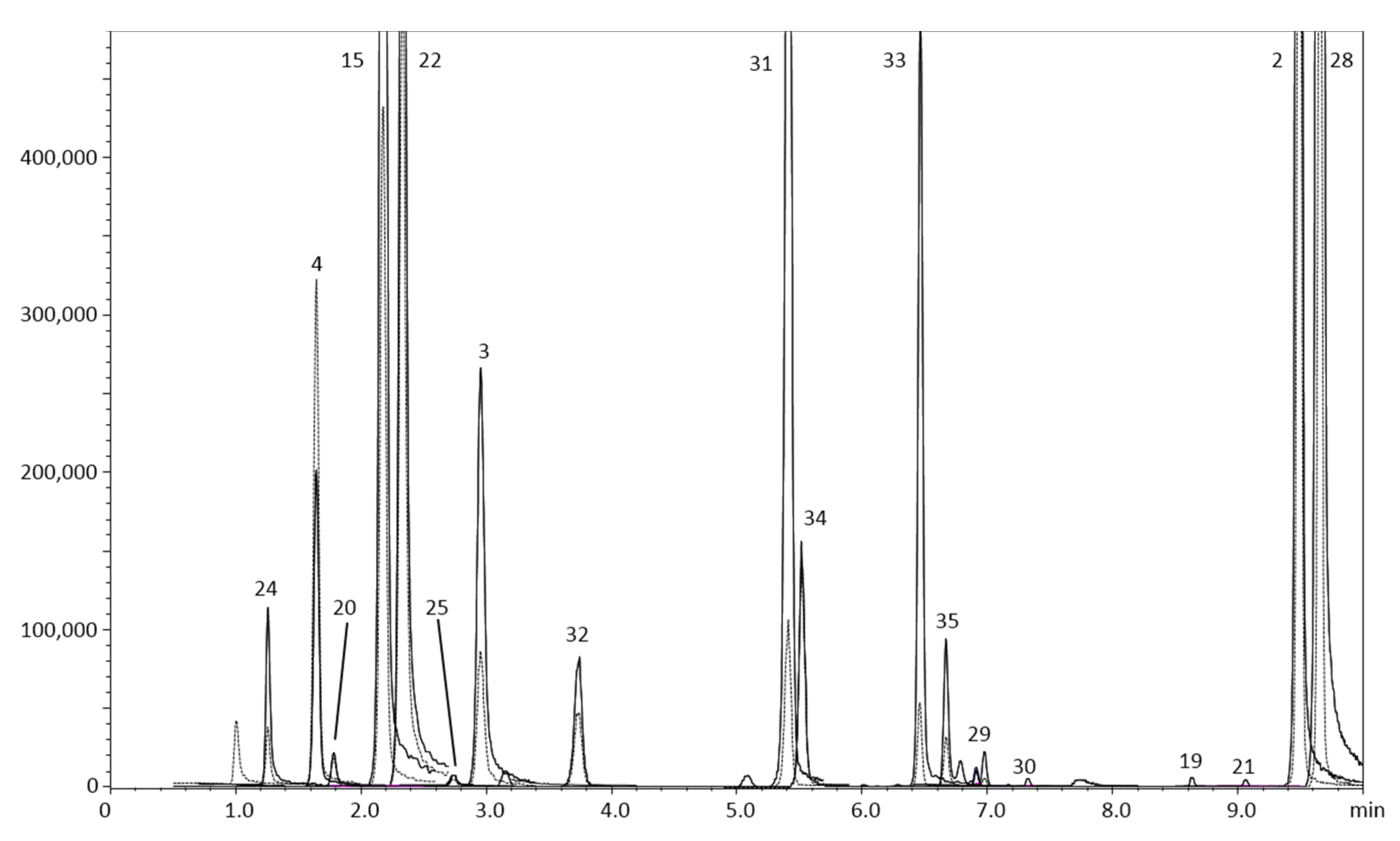
Figure 3 represents the qualitative profile of sample 10. The HPLC-MS/MS (in MRM mode) chromatogram reports the oxygen heterocyclic compounds detected in sample 10, which has been chosen as representative due to the fact that it resulted the richest one in terms of number of compounds identified.
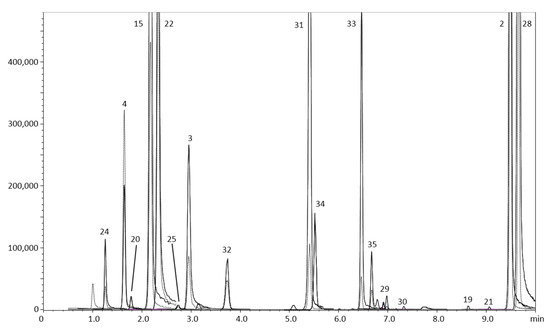
Figure 3.
HPLC-MS/MS (in MRM mode) of the oxygen heterocyclic compound profile for sample 10. Quantifier and qualifier ions in continuous and dotted line, respectively. Refer to Table 3 for peak numbers.
In this beer all the three classes of OHC are contained, FCs, Cs, and PMFs due to the addition of several types of Citrus in the preparation, as described in the label. The peak identification was carried out by using the developed MRM library, in combination with the LRI database previously realized.
The homologous series of the alkyl aryl ketones before the sample analysis ensured to apply the LRI system for the unambiguous identification. In some cases, false positive were avoided thanks to the difference in the LRI value.
Samples 4–8 are orange flavored, in all beers the citrus taste arises from the addition of peels, as declared on the label. The qualitative profiles are similar, apart from few Cs (herniarin, isomeranzin, and 5-geranyloxy-7-methoxycoumarin). Among FCs, in three samples out of five, bergamottin was detected, despite in concentration <LOQ. In this case, bergamottin shall be attributed on the addition of other ingredients that contain this furocoumarin, such as lemon or lime.
PMFs are the predominant class of compound. The maximum amount was detected in the sample 7, and in all samples nobiletin was the most abundant components, coherently with the composition of sweet orange essential oil.
All orange beers analyzed gave qualitative profiles corresponding to the addition of Citrus specie declared on the labels; their qualitative profiles are similar to those obtained for the commercial orange juice reported in Table 5.
Among lemon beers (9–12), sample 12 is the only one flavored exclusively with lemon peel, as reported on the label. However, this sample lacks some characteristic markers of lemon, such as bergamottin (FC) and some Cs, such as 5-geranyloxypsoralen and especially citropten. Is not possible to assume that the compounds cited above were not detected due to the low amount of peels used. In fact, regardless of the quantity of peels, the natural composition, means the ratio between the OHCs, should be maintained. One exception can be made for those compounds, which may not be soluble enough in the hydro-alcoholic solution, for instance bergamottin and 5-geranyloxy-7-methoxycoumarin; because they could be not easily extracted from the peels.
The discussion regarding samples 9–11 is complex due to the addition of different citrus ingredients in the preparation. Beers 9 and 11 were both flavored by lemon juice and natural aroma. Their qualitative profiles were identical, whereas the quantitative results were different due to the natural aroma employed. The attribution of the results was difficult, because the regulation does not impose to specify which kind of aroma were used.
Differently, the addition of other citrus juices in the sample 10 was made evident by the detection of compounds not contained in lemon, such as gardenin A and B, and more than 13 μg/L of 8-geranyloxypsoralen, highly contained in lime essential oil [11]. In particular, the presence of isopimpinellin and cnidilin, that are markers characteristic for lime essential oil [11,32], demonstrates the authenticity of sample 10 regarding the presence of lime juice, cited as an ingredient. Isopimpinellin was detected also in sample 9 and 11, despite at trace level and <LOQ, respectively, as a constituent of the citrus aroma reported on the label and not specified.
A positive control was created by adding 1.8% of lemon peel to sample 1, leaving macerate for 10 days.
Table 5 reports the results obtained, with a lack of PMFs and only 5-geranyloxy-7-methoxypsoralen as representative of the coumarin class.
The qualitative and quantitative OHC composition of both bergamot samples (13 and 14) would not seem to reflect what is declared on the label. For both beers, the most representative class of OHCs characterized was those of PMFs. However, it is well known that bergamot peels, and essential oils consequently, are rich in citropten, bergapten, 5-geranyloxy-7-methoxycoumarin, and bergamottin, the latter at concentration close to 20,000 mg/L [11].
Moreover, a commercial bergamot juice was analyzed giving more than 9 mg/L of bergamottin and almost 5 mg/L of bergapten, highlights that also using bergamot juice in the beer production, trace of them should be detected.
It can be assumed that bergamottin and 5-geranyloxy-7-methoxycumarin are not extracted as the least polar of the targets, but bergapten and citropten are polar enough to be extracted from the flavedo by the hydroalcoholic solution. In their scientific article Dugrand and co-workers [33] reported that in bergamot peel the amount of bergapten is even more than bergamottin, contrarily to the essential oils. As a consequence, the lack of these specific compounds could be justified.
An even more serious case would be if bergamot had not been used at all, and the citrus aroma was given by a completely different species, less valuable than bergamot.
In the sample 13 also lemon peel are listed as ingredient of the preparation, but neither citropten, the most abundant coumarin of Citrus limon, was detected.
The sample 14 was flavored by bergamot peel (0.3%) and coriander. The isocoumarins, coriandrones A and B (292 g/mol), together with coriandrin (230 g/mol) and dihydrocoriandrin (232 g/mol) are naturally occurring compounds in Coriander sativum [34]. These compounds had different molecular weights with respect to the targets object of this study, and did not interfere with the MRM transitions, the correct identification, and quantification of targeted OHCs.
Table 5 reports the quantitative profile of a bergamot non-alcoholic beverage in which consistent amounts of bergamottin, bergapten, citropten and 5-geranyloxy-7-methoxycoumarin (686, 471, 363, and 156; respectively) were found, as an example of authentic bergamot beverage.
Flavored mandarin beers were typically rich of PMFs. The species of mandarin used in the samples 15 and 1 were different, Citrus reticulata blanco and Citrus japonica, respectively.
To the best of the authors’ knowledge, there are not references about the OHC composition of kumquat fruit. Polymethoxyflavones were the dominant class in both samples, Cs and FCs were absent. Two mandarin cold pressed essential oils (green mandarin and yellow mandarin) were used as reference. Gardenin A and sinensetin were not detected in the latter one, meaning that samples 15 and 16, in which both targets are contained, were not flavored by yellow mandarin. Further confirmation was given by the results of green mandarin non-alcoholic beverage.
To summarize, the LC characterization revealed anomalies in different samples. Sample 12 due to the lack of coumarins, and both bergamot beers (13 and 14) due to the lack of specific compounds such us bergapten and bergamottin. The GC analyses confirmed the discrepancies between the volatile profile and the citrus ingredient declared on the label in the aforementioned samples.
This research highlights the importance to combine LC and GC techniques, for the analysis of OHCs and volatile in beers, such as in other kind of Citrus flavored beverages and food, to get an exhaustive characterization of the matrix. Even more, this approach was here applied to evaluate the authenticity of flavored beers, i.e., as the matching of the ingredients used and what is declared on the label.
The LRI system applied to LC library, in order to identify unambiguously the target could be consider a novelty in this field [31,35,36]; whereas its use in combination with Electron Impact libraries is a widely validated system [15].
In addition to the main purpose of the work, which is to determine any fraud in citrus-flavored beers, this research offers a starting point to evaluate the content of OHCs, especially focusing on FCs, in beverages.
Despite the adverse effects [37,38,39,40], FCs are not subject to any official regulation, which imposes the maximum amount in food and beverages. The only limitation imposed by the Regulation (EC) No 1334/2008 of the European Parliament on flavorings and certain food ingredients with flavoring properties for use in and on foods, recently consolidated (3 December 2020), refers to the unique compound coumarin, whose maximum quantity is set depending on the food product [10]. This decision reflects what has been suggested by the European Food Safety Authority (EFSA) in the same year [9] adopted on 8 July 2008. The discussion on this topic is currently extended to FCs and is still lively and inspired by numerous opinions issued by official bodies involved in this area of research, related to public health (e.g., Update of the toxicological assessment of furanocoumarins in foodstuffs [41].
According to this document, was still not possible to specify the dangerous dose related to the repeated dietary intake of furanocoumarins. In the same document the authors declared that “the typical consumption of foods containing furanocoumarins, including flavored soft drinks, leads to an exposure that remains well below the phototoxic dose range”. In sample 10 (see Table 4) a total of 256 μg/L of FCs was quantified in the richest sample.
The lowest furanocoumarin dose responsible for phototoxic effects in combination with UVA is approximately 14, 15 mg 8-methoxypsoralen (8-MOP) equivalents [4,42]. If we consider the assumption of 1L of beer, the maximum intake is still under the toxic doses reported in the same document [41]. Commercial bergamot juice resulted in a total furocoumarins amount equal to 14 mg/L which corresponds to the limit reported above.
Definitely, this study offers a detailed investigation of the OHCs contained in commonly consumed soft citrus drinks, from flavored beers to commercial juices.
4. Conclusions
In this paper, the beer volatile fingerprinting of citrus-flavored and unflavored beers was profiled using a HS-SPME extraction method followed by GC analysis. This approach allowed to establish the volatile pattern of beers in which the labels reported the addition of citrus peels as ingredient. The volatile fingerprint in fact constitutes a valuable approach providing useful and comprehensive insights to evaluate the impact of citrus flavor addition on the beer volatile composition. The OHCs characterization, carried out by the HPLC-MS/MS method in combination with the LRI system is complementary to GC data and represents a powerful tool for quality control and against food fraud.
Moreover, this study offers a detailed characterization of both volatile and non-volatile profile of several soft drinks; in particular the quantification of FCs results useful to add data about the content of these compounds in soft drinks that could be useful in the evaluation of potential toxic effects on human health.
Supplementary Materials
The following are available online at https://www.mdpi.com/2297-8739/8/2/18/s1, Table S1:Less prevalent volatile compounds contained in the samples analyzed, expressed in area% as average of three measurements; Table S2: Major volatile constituents of citrus essential oils, expressed in area% as average of three measurements; Table S3: Major volatile constituents of citrus juice and citrus non-alcoholic beverages, expressed in area% as average of three measurements; Figure S1: Influence of temperature on SPME method extraction optimization; Figure S2: Influence of time on SPME method extraction optimization; Figure S3: Influence of sample volume on SPME method extraction optimization; Figure S4: Influence of sample time conditioning on SPME method extraction optimization; Figure S5: Influence of stirring rate on SPME method extraction optimization; Figure S6: Influence of NaCl addition on SPME method extraction optimization; Figure S7: Comparison between volatile profile of sample 1 (in blue) and sample 1 spiked with 1.8% lemon peel (in red); Figure S8: Comparison between volatile profile of sample 1 (in blue) and sample 1 spiked with 1.5% orange peel (in red); Figure S9: Comparison between volatile profile of sample 1 (in blue) and sample 1 spiked with 0.3% bergamot E.O. (in red).
Author Contributions
Data curation, E.T., A.A., F.V., and G.M.; Supervision, P.D. and L.M.; Writing-original draft, E.T. and A.A.; Writing-review and editing, E.T. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by MIUR in the PNR 2015-2020 within the context of the project e-brewery-Rif ARS01_00582 “Virtualizzazione, sensing e IoT per l’innovazione del processo produttivo industriale delle bevande.
Acknowledgments
The authors gratefully acknowledge the Shimadzu Corporation and Merck Life Science (Merck KGaA, Darmstadt, Germany) for their continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dresel, M. Beer. In Handbook of Odor, 1st ed.; Buettner, A., Ed.; Springer: Cham, Switzerland, 2017; pp. 129–142. ISBN 978-3-319-26930-6. [Google Scholar]
- Shi, Y.-S.; Zhang, Y.; Li, H.-T.; Wu, C.-H.; El-Seedi, H.R.; Ye, W.-K.; Wang, Z.-W.; Li, C.-B.; Zhang, X.-F.; Kai, G. Limonoids from Citrus: Chemistry, anti-tumor potential, and other bioactivities. J. Funct. Foods 2020, 75, 104213. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, J.; Zimmerli, B.; Dick, R.; Panizzon, R.; Schlatter, C. Dietary intake and risk assessment of phototoxic furocoumarins in humans. Food Chem. Toxicol. 1991, 29, 523–530. [Google Scholar] [CrossRef]
- Eisenbrand, G. Toxicological Assessment of Furocoumarins in Foodstuffs. Mol. Nutr. Food Res. 2007, 51, 367–373. [Google Scholar] [CrossRef]
- Melough, M.M.; Gil Lee, S.; Cho, E.; Kim, K.; Provatas, A.A.; Perkins, C.; Park, M.K.; Qureshi, A.; Chun, O.K. Identification and Quantitation of Furocoumarins in Popularly Consumed Foods in the U.S. Using QuEChERS Extraction Coupled with UPLC-MS/MS Analysis. J. Agric. Food Chem. 2017, 65, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Prosen, H.; Kočar, D. Different sample preparation methods combined with LC–MS/MS and LC–UV for determination of some furocoumarin compounds in products containing citruses. Flavour Fragr. J. 2008, 23, 263–271. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to tertiary-Butylhydroquinone (TBHQ). EFSA J. 2004, 2, 84. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Coumarin in flavourings and other food ingredients with flavouring properties Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2008, 6, 1–15.
- European Commission Regulation (EC). No 1334/2008 on Flavourings and Certain Food Ingredients; Official Journal of the European Union: Brussels, Belgum, 2008; pp. 47–48. [Google Scholar]
- Russo, M.; Bonaccorsi, I.; Costa, R.; Trozzi, A.; Dugo, P.; Mondello, L. Reduced time HPLC analyses for fast quality control of citrus essential oils. J. Essent. Oil Res. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Gu, H.; Liu, G.; Wang, J.; Aubry, A.-F.; Arnold, M.E. Selecting the Correct Weighting Factors for Linear and Quadratic Calibration Curves with Least-Squares Regression Algorithm in Bioanalytical LC-MS/MS Assays and Impacts of Using Incorrect Weighting Factors on Curve Stability, Data Quality, and Assay Performance. Anal. Chem. 2014, 86, 8959–8966. [Google Scholar] [PubMed]
- Magnusson, B.; Örnemark, U. The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. In Method Performance Characteristics, 2nd ed.; Eurachem Guide; Eurachem: Uppsala, Sweden, 2014; pp. 19–40. ISBN 978-91-87461-59-0. [Google Scholar]
- Konieczka, P.; Namieśnik, J. Estimating uncertainty in analytical procedures based on chromatographic techniques. J. Chromatogr. 2010, 1217, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Torrelli, P.; Marini, F. Flavour component analysis by HS-SPME/GC–MS and chemometric modeling to characterize Pilsner-style Lager craft beers. Microchem. J. 2019, 149, 103991. [Google Scholar] [CrossRef]
- Zanella, D.; Anderson, H.E.; Selby, T.; Magnuson, R.H.; Liden, T.; Schug, K.A. Comparison of Headspace Solid-Phase Microextraction High Capacity Fiber Coatings Based on Dual Mass Spectrometric and Broadband Vacuum Ultraviolet Absorption Detection for Untargeted Analysis of Beer Volatiles using Gas Chromatography. Anal. Chim. Acta 2021, 1141, 91–99. [Google Scholar] [CrossRef]
- A Gee, D.; Ramirez, W.F. A FLAVOUR MODEL FOR BEER FERMENTATION. J. Inst. Brew. 1994, 100, 321–329. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2007, 74, 454–461. [Google Scholar] [CrossRef]
- Jespersen, L.; Jakobsen, M. Specific spoilage organisms in breweries and laboratory media for their detection. Int. J. Food Microbiol. 1996, 33, 139–155. [Google Scholar] [CrossRef]
- Äyräpää, T. Occurrence of phenethyl alcohol in beer. J. Inst. Brew. 1961, 67, 262–266. [Google Scholar] [CrossRef]
- Steinhaus, M.; Schieberle, P. Comparison of the Most Odor-Active Compounds in Fresh and Dried Hop Cones (Humulus lupulusL. Variety Spalter Select) Based on GC−Olfactometry and Odor Dilution Techniques. J. Agric. Food Chem. 2000, 48, 1776–1783. [Google Scholar] [CrossRef]
- Nance, M.R.; Setzer, W.N. Volatile components of aroma hops (Humulus lupulus L.) commonly used in beer brewing. J. Brew. Distill. 2011, 2, 16–22. [Google Scholar]
- Dieckmann, R.H.; Palamand, S.R. Autoxidation of some constituents of hops. I. Monoterpene hydrocarbon, myrcene. J. Agric. Food Chem. 1974, 22, 498–503. [Google Scholar] [CrossRef]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Stübner, R.; Methner, F.-J. Formation of styrene dependent on fermentation management during wheat beer production. Food Chem. 2012, 134, 2121–2125. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Dugo, L.; Stancanelli, R.; Dugo, G. LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J. Pharm. Biomed. Anal. 2000, 24, 147–154. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Q.; Simon, J.E.; Lou, S.-N.; Ho, C.-T. Authenticity analysis of citrus essential oils by HPLC-UV-MS on oxygenated heterocyclic components. J. Food Drug Anal. 2015, 23, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Arigò, A.; Rigano, F.; Micalizzi, G.; Dugo, P.; Mondello, L. Oxygen heterocyclic compound screening in Citrus essential oils by linear retention index approach applied to liquid chromatography coupled to photodiode array detector. Flavour Fragr. J. 2019, 34, 349–364. [Google Scholar] [CrossRef]
- Masson, J.; Liberto, E.; Beolor, J.-C.; Brevard, H.; Bicchi, C.; Rubiolo, P. Oxygenated heterocyclic compounds to differentiate Citrus spp. essential oils through metabolomic strategies. Food Chem. 2016, 206, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Dugrand, A.; Olry, A.; Duval, T.; Hehn, A.; Froelicher, Y.; Bourgaud, F. Coumarin and Furanocoumarin Quantitation in Citrus Peel via Ultraperformance Liquid Chromatography Coupled with Mass Spectrometry (UPLC-MS). J. Agric. Food Chem. 2013, 61, 10677–10684. [Google Scholar] [CrossRef]
- Önder, A. Coriander and Its Phytoconstituents for the Beneficial Effects. Potential Essent. Oils 2018, 165–185. [Google Scholar] [CrossRef]
- Rigano, F.; Oteri, M.; Russo, M.; Dugo, P.; Mondello, L. Proposal of a Linear Retention Index System for Improving Identification Reliability of Triacylglycerol Profiles in Lipid Samples by Liquid Chromatography Methods. Anal. Chem. 2018, 90, 3313–3320. [Google Scholar] [CrossRef]
- Rigano, F.; Russo, M.; Arigò, A.; Dugo, P.; Mondello, L. Combining linear retention index and electron ionization mass spectrometry for a reliable identification in nano liquid chromatography. J. Chromatogr. A 2020, 1610, 460581. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Han, J.; Feskanich, D.; Cho, E.; Stampfer, M.J.; Willett, W.C.; Qureshi, A.A. Citrus Consumption and Risk of Cutaneous Malignant Melanoma. J. Clin. Oncol. 2015, 33, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Cho, E.; Feskanich, D.; Li, W.-Q.; Sun, Q.; Han, J.; Qureshi, A.A. Citrus consumption and risk of basal cell carcinoma and squamous cell carcinoma of the skin. Carcinogenesis 2015, 36, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Melough, M.M.; Chun, O.K. Dietary furocoumarins and skin cancer: A review of current biological evidence. Food Chem. Toxicol. 2018, 122, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lake, B. Coumarin Metabolism, Toxicity and Carcinogenicity: Relevance for Human Risk Assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Guth, S.; Habermeyer, M.; Schrenk, D.; Eisenbrand, G. Update of the toxicological assessment of furanocoumarins in foodstuffs (Update of the SKLM statement of 23/24 September 2004)-Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 2011, 55, 807–810. [Google Scholar] [CrossRef]
- Brickl, R.; Schmid, J.; Koss, F.W. Pharmacokinetics and pharmacodynamics of psoralens after oral administration: Considerations and conclusions. Natl. Cancer Inst. Monogr. 1984, 66, 63–67. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).