Plant Growth-Promoting Rhizobacteria Modulate the Concentration of Bioactive Compounds in Tomato Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Culturable Rhizospheric Bacteria
2.2. Plant Growth-Promoting Activities
2.3. Identification of Bacteria
2.4. Inoculation and Growth Conditions of Tomato Plants
2.5. Enzyme Extraction and Analysis
- (1)
- (A532 solution B − A600 solution B) − (A532 solution A − A600 solution A) = M1;
- (2)
- (A440 solution B − A600 solution B) 0.0571 = M2;
- (3)
- ((M1 − M2)/157000) 106 = nmol MDA mL−1 = M3;
- (4)
- M3/0.015 = nmol MDA gFW−1.
2.6. Gas Exchange Measurements
2.7. Brix Degrees Measurement
2.8. Lycopene Extraction and Measurement
2.9. Statistical Analysis
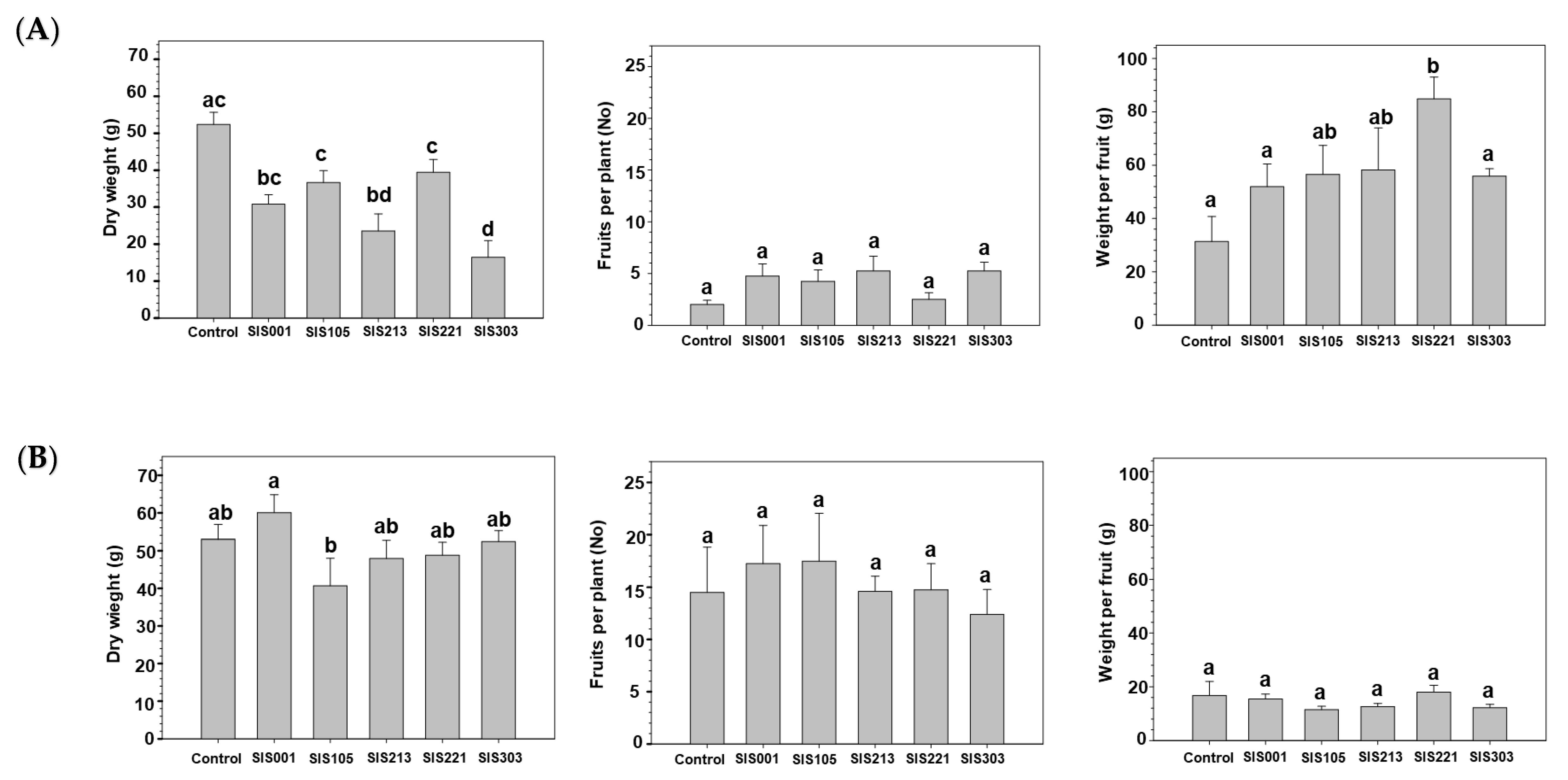
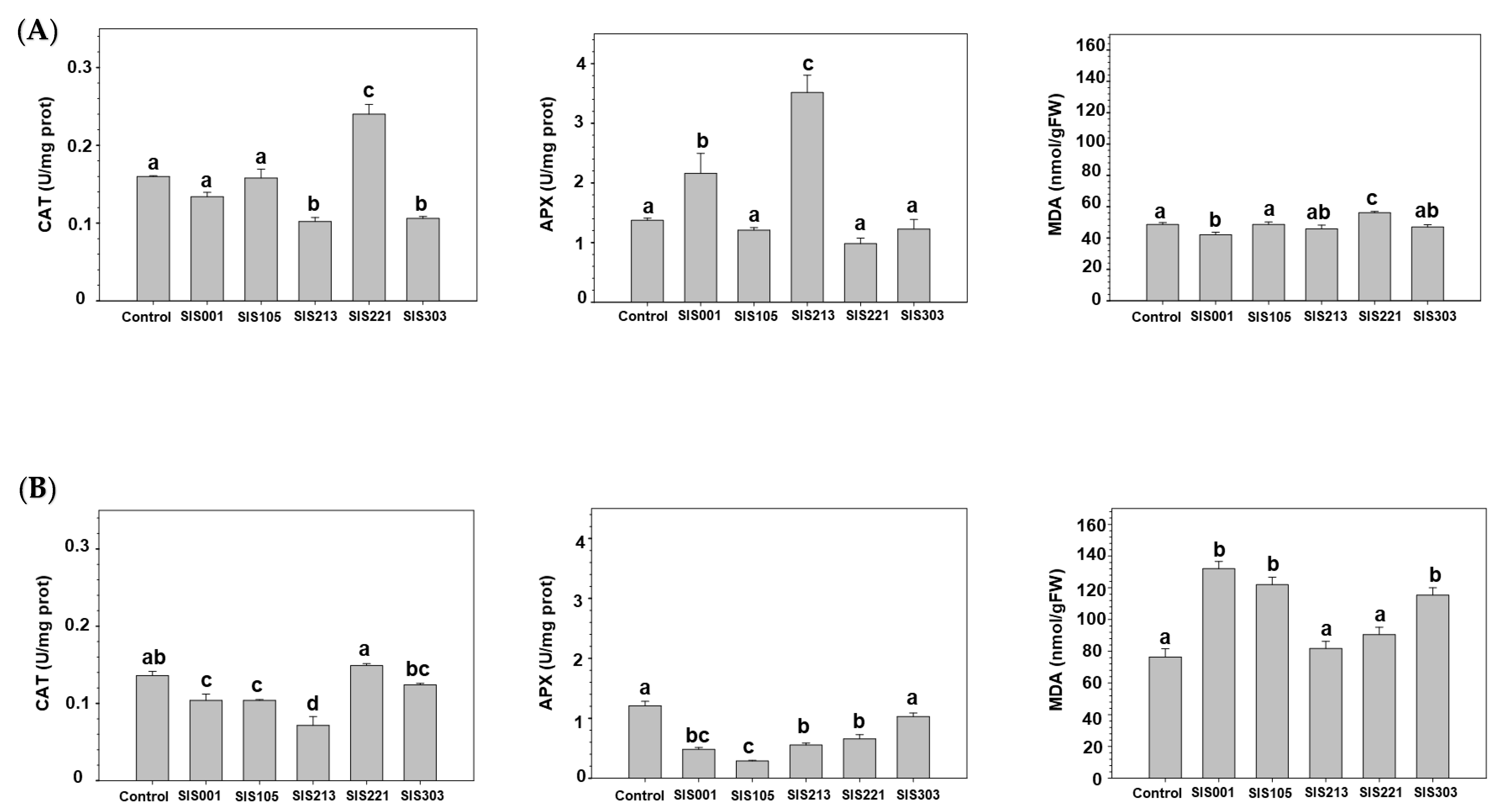
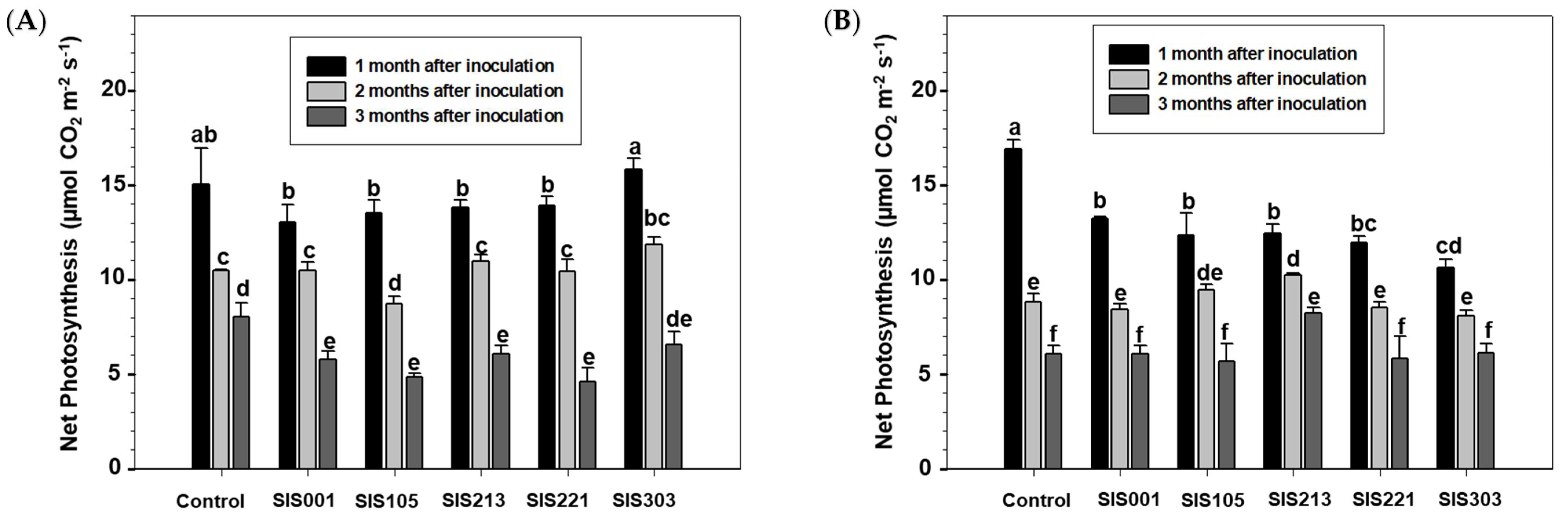
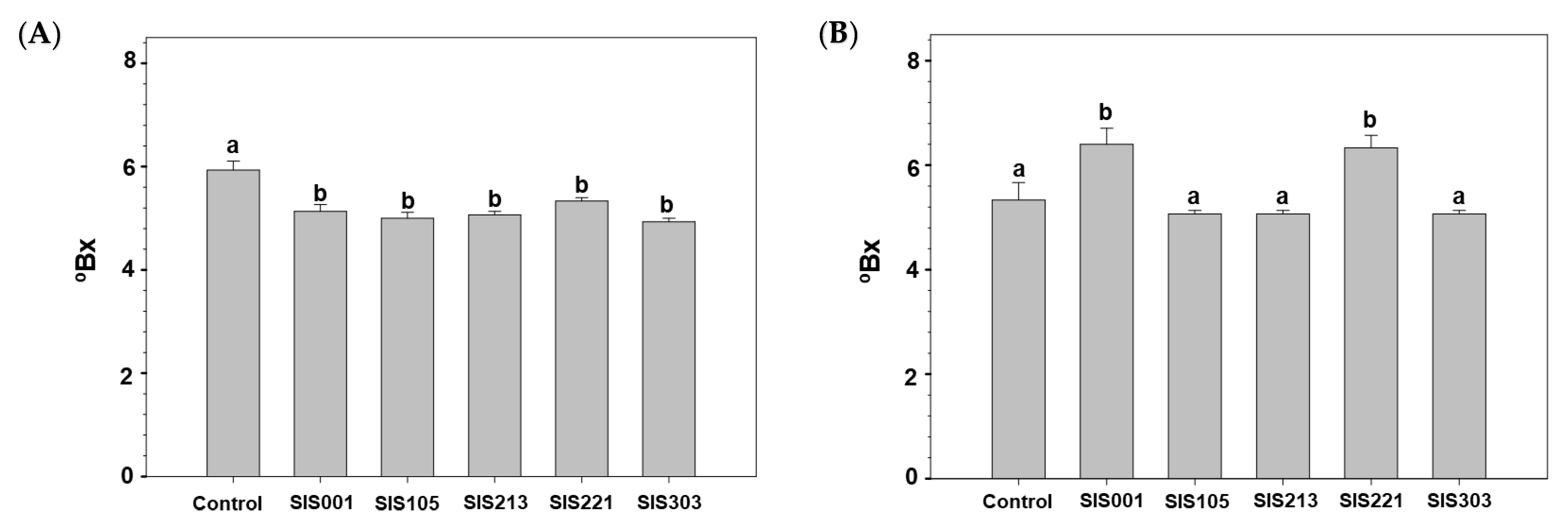
3. Results
3.1. Plant Growth-Promoting Rhizobacteria Isolation and Selection
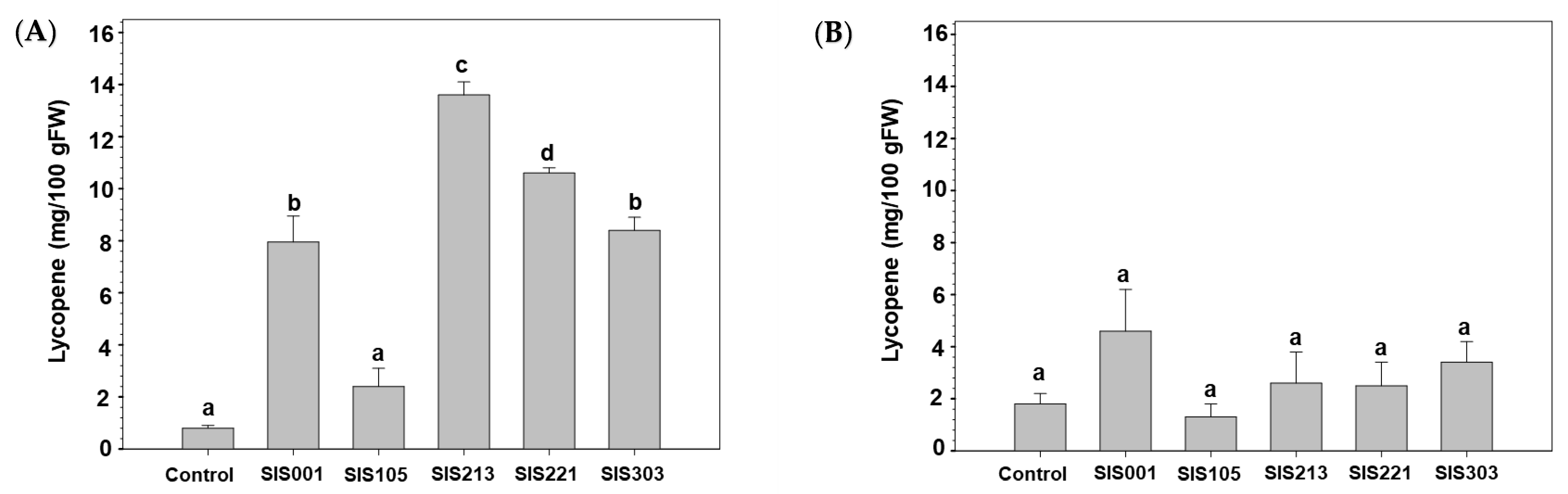
3.2. Tomato Responses to Inoculation with Selected PGPR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shine, H.-S.; Kumar Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; AL-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Gupta, A.; Gopal, M.; Thomas, G.V.; Manikandan, V.; Gajewski, J.; Thomas, G.; Seshagiri, S.; Schuster, S.C.; Rajesh, P.; Gupta, R. Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. PLoS ONE 2014, 9, e104259. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Khan, M.S. Evaluation of plant-growth promoting activities of rhizobacterium Pseudomonas putida under herbicide stress. Ann. Microbiol. 2012, 62, 1531–1540. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Singh, S.P.; Blázquez, M.A.; Sansinenea, E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549. [Google Scholar] [CrossRef]
- Jahanian, A.; Chaichi, M.R.; Rezaei, K.; Rezayazdi, K.; Khavazi, K. The effect of plant growth promoting rhizobacteria (PGPR) on germination and primary growth of artichoke (Cynaras colymus). Int. J. Agric. Crop Sci. 2012, 4, 923–929. [Google Scholar]
- Liu, J.; Tang, L.; Zhang, H.; Guo, C. Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. J. Sci. Food Agric. 2019, 99, 281–289. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Hou, J.; Tu, C.; Luo, Y.; Christie, P. Whole genome analysis of halotolerant and alkalotolerant plant growth-promoting rhizobacterium Klebsiella sp. D5A. Sci. Rep. 2016, 6, 26710. [Google Scholar] [CrossRef]
- Xie, J.; Shi, H.; Du, Z.; Wang, T.; Liu, X.; Chen, S. Comparative genomic and functional analysis reveals conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 2016, 6, 21329. [Google Scholar] [CrossRef]
- Guo, J.; Muhammad, H.; Lv, X.; Wei, T.; Ren, X.; Jia, H.; Atif, S.; Hua, L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: A review. Chemosphere 2020, 246, 125823. [Google Scholar] [CrossRef] [PubMed]
- Llangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Abd_Allah, E.F.; Hashem, A.; Ahmad, P. Plant growth promoting rhizobacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 2019, 217, 463–474. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. PGPR to alleviate salinity stress on plant growth. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 73–96. [Google Scholar]
- Fukami, J.; Ollero, F.J.; de la Osa, C.; Valderrama-Fernández, R.; Nogueira, M.A.; Megías, M.; Hungria, M. Antioxidant activity and induction of mechanisms of resistance to stresses related to the inoculation with Azospirillum brasilense. Arch. Microbiol. 2018, 200, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges-A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The tomato as a functional food. J. Nutr. 2005, 134, 1226–1230. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Carotenoids as antioxidants. Nutrition 2001, 17, 815–817. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; Bailey, M.J.; Ellis, R.J.; Purdy, K.J. Subgrouping of bacterial populations by cellular fatty acid composition. FEMS Microbiol. Lett. 1993, 102, 75–84. [Google Scholar] [CrossRef][Green Version]
- Lambrecht, M.; Okon, Y.; Vande Broek, A.; Vanderleyden, J. Indole-3-acetic: A reciprocal signaling molecule in bacteria-plant interactions. Trends Microbiol. 2000, 8, 298–300. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for insolating and characterizing ACC deaminase-containing plant growth- promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Carder, J.H. Detection and quantification of cellulase by congo red staining of substrates in a cup-plate diffusion assay. Anal. Biochem. 1986, 153, 75–79. [Google Scholar] [CrossRef]
- Vedder, E.B. Starch agar-A new culture medium for the gonococcus. J. Infect. Dis. 1915, 16, 385–388. [Google Scholar] [CrossRef]
- Bach, H.J.; Munch, J.C. Identification of bacterial sources of soil peptidases. Biol. Fertil. Soils 2001, 31, 219–224. [Google Scholar] [CrossRef]
- Roberge, M.R.; Knowles, R. Ureolysis, immobilization and nitrification in black spruce (Picea mariana Mill.) humus. R. Soil Sci. Soc. Am. Proc. 1996, 30, 201–204. [Google Scholar] [CrossRef]
- Peix, A.; Rivas-Boyero, A.; Mateos, P.F.; Rodríguez-Barrueco, C.; Martínez-Molina, E.; Velázquez, E. Growth promotion of chickpea and barley by phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biol. Biochem. 2001, 33, 103–110. [Google Scholar] [CrossRef]
- Rajawat, M.V.S.; Singh, S.; Tyagi, S.P.; Saxena, A.K. Plate assay for rapid screening of potassium solubilizing bacteria. Pedosphere 2016, 26, 768–773. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Meth. 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Hall, V.; O’Neill, G.L.; Magee, J.T.; Duerden, B.I. Development of amplified 16S ribosomal DNA restriction analysis for identification of Actinomyces species and comparison with Pyrolysis-Mass Spectrometry and conventional biochemical tests. J. Clin. Microbiol. 1999, 37, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Arias-Baldrich, C.; de la Osa, C.; Bosch, N.; Ruiz-Ballesta, I.; Monreal, J.A.; García-Mauriño, S. Enzymatic activity, gene expression and posttranslational modifications of photosynthetic and non-photosynthetic phosphoenolpyruvate carboxylase in ammonium-stressed sorghum plants. J. Plant Phys. 2017, 214, 39–47. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol. 1984, 25, 1285–1295. [Google Scholar]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Xu, Y.; Pan, S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.). Ultrason. Sonochem. 2013, 20, 1026. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Comp. Anal. 2002, 15, 309. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.C. Plant growth promotion under water: Decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Van de Poel, B.; Van Der Straeten, D. 1-aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 64. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases-potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [PubMed]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Marsic, N.K.; Vodnik, D.; Mikulic-Petkovsek, M.; Veberic, R.; Sircelj, H. Photosynthetic traits of plants and the biochemical profile of tomato fruits are influenced by grafting, salinity stress, and growing season. J. Agric. Food Chem. 2018, 66, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Dew, T.P.; Orfila, C.; Urwin, P.E. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2011, 59, 9673–9682. [Google Scholar] [CrossRef] [PubMed]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Stergiou, P.; Xanthou, M.-Z.; Kakabouki, I.; Vlachakis, D.; et al. Evaluation of plant growth promoting bacteria strains on growth, yield and quality of industrial tomato. Microorganisms 2021, 9, 2099. [Google Scholar] [CrossRef]
| Plant Growth-Promoting Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | RNA 16S | Chitinase Activity | Cellulase Activity | Amylolytic Activity | Protease Activity | Ureolytic Activity | ACC Deaminase | Phosphate Solubilisation | Potassium Solubilisation | Siderophore Production | IAA Production |
| SIS001 | Pantoea sp. | - | + | - | - | - | - | - | + | + | 6 mg/L |
| SIS105 | Pseudomonas sp. | - | - | + | - | - | + | + | + | + | 10 mg/L |
| SIS213 | Bacillus sp. | + | + | - | + | - | - | - | - | - | 10 mg/L |
| SIS221 | Pantoea sp. | - | + | - | - | - | - | + | + | - | 8 mg/L |
| SIS303 | Pseudomonas sp. | - | + | + | - | - | - | + | + | + | 13 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Osa, C.; Rodríguez-Carvajal, M.Á.; Gandullo, J.; Aranda, C.; Megías, M.; Ollero, F.J.; López-Baena, F.J.; Monreal, J.A. Plant Growth-Promoting Rhizobacteria Modulate the Concentration of Bioactive Compounds in Tomato Fruits. Separations 2021, 8, 223. https://doi.org/10.3390/separations8110223
de la Osa C, Rodríguez-Carvajal MÁ, Gandullo J, Aranda C, Megías M, Ollero FJ, López-Baena FJ, Monreal JA. Plant Growth-Promoting Rhizobacteria Modulate the Concentration of Bioactive Compounds in Tomato Fruits. Separations. 2021; 8(11):223. https://doi.org/10.3390/separations8110223
Chicago/Turabian Stylede la Osa, Clara, Miguel Ángel Rodríguez-Carvajal, Jacinto Gandullo, Clara Aranda, Manuel Megías, Francisco Javier Ollero, Francisco Javier López-Baena, and José Antonio Monreal. 2021. "Plant Growth-Promoting Rhizobacteria Modulate the Concentration of Bioactive Compounds in Tomato Fruits" Separations 8, no. 11: 223. https://doi.org/10.3390/separations8110223
APA Stylede la Osa, C., Rodríguez-Carvajal, M. Á., Gandullo, J., Aranda, C., Megías, M., Ollero, F. J., López-Baena, F. J., & Monreal, J. A. (2021). Plant Growth-Promoting Rhizobacteria Modulate the Concentration of Bioactive Compounds in Tomato Fruits. Separations, 8(11), 223. https://doi.org/10.3390/separations8110223







