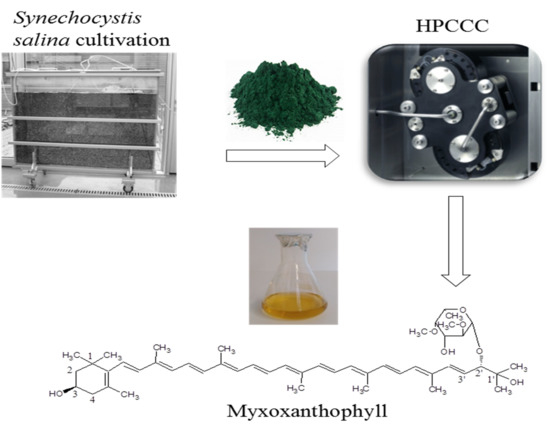
Separation of the Glycosylated Carotenoid Myxoxanthophyll from Synechocystis Salina by HPCCC and Evaluation of Its Antioxidant, Tyrosinase Inhibitory and Immune-Stimulating Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Production
2.2. Extract Preparation
2.3. Separation of Myxoxanthophyll Using HPCCC
2.3.1. HPCCC Equipment
2.3.2. Selection of the Suitable Biphasic Solvent System for HPCCC
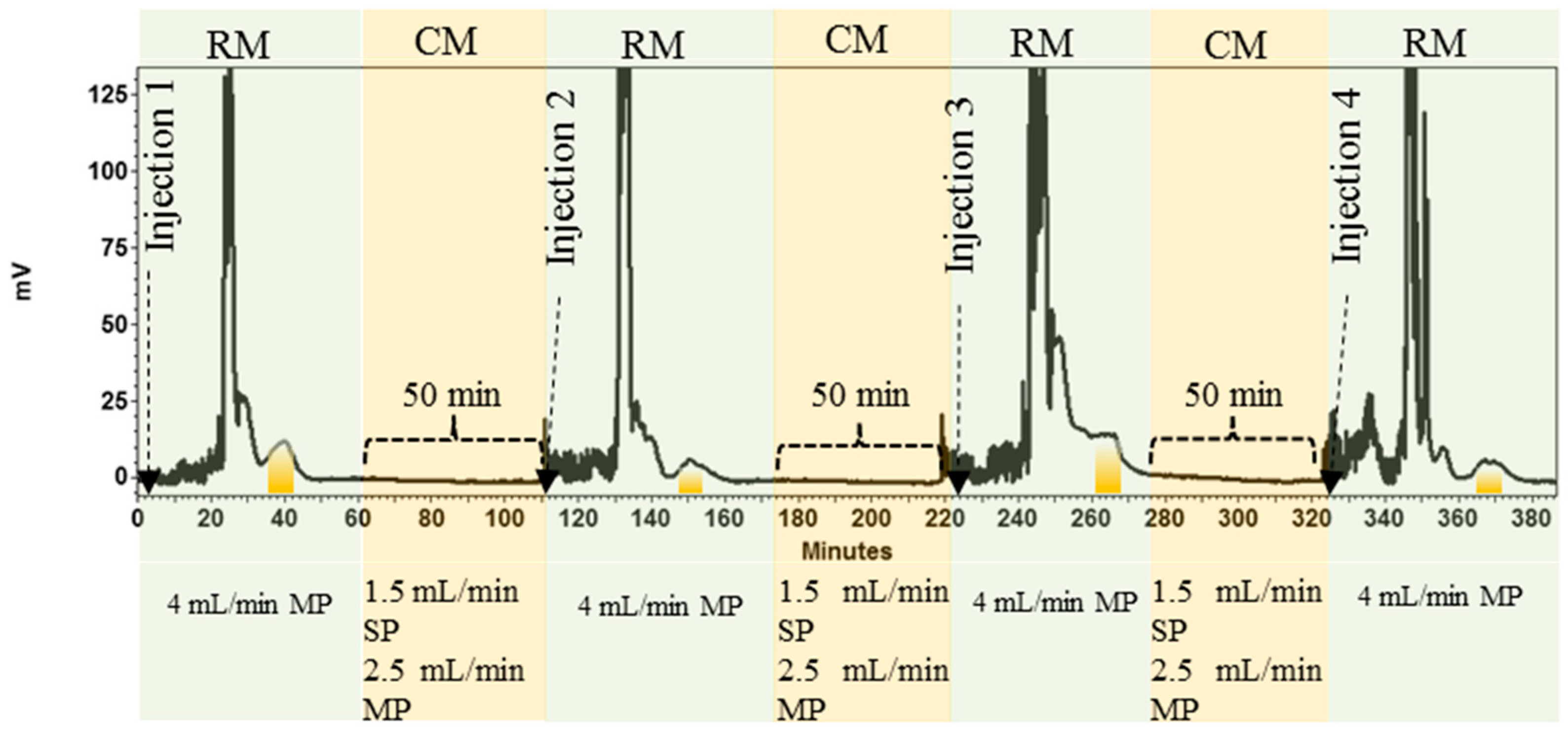
2.3.3. HPCCC Separation Process
2.4. Final Purification of the Myxoxanthophyll Fraction by Semi-Preparative HPLC
2.5. HPLC-DAD Analysis of Extract and Fractions
2.6. Confirmation of the Chemical Identity of the Purified Target Compound
2.7. Biological Evaluations
2.7.1. DPPH Free Radical Scavenging Activity
2.7.2. Inhibition of Tyrosinase Activity
2.7.3. Immune Cell Activation
2.8. Statistical Analysis
3. Results and Discussion
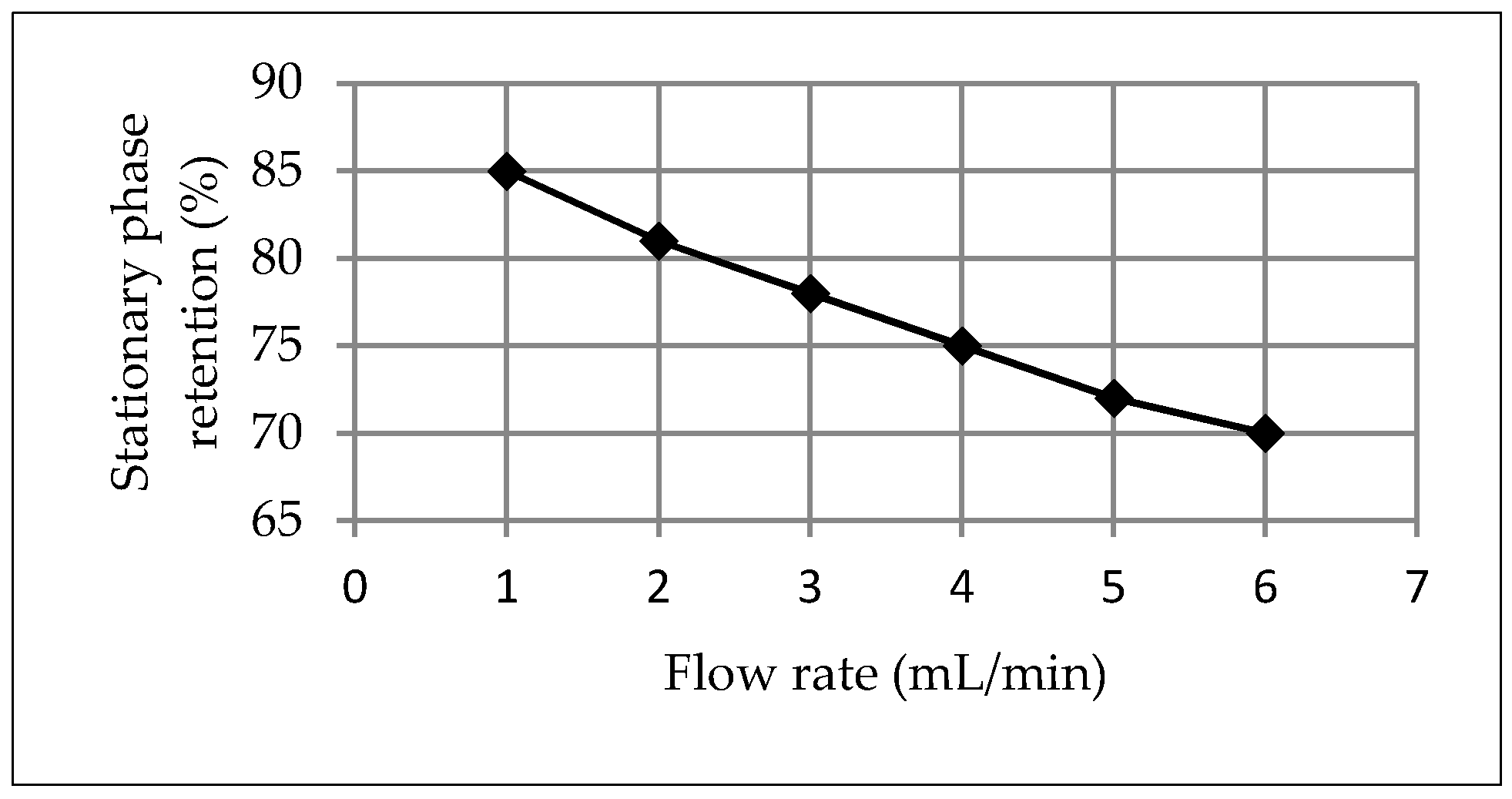
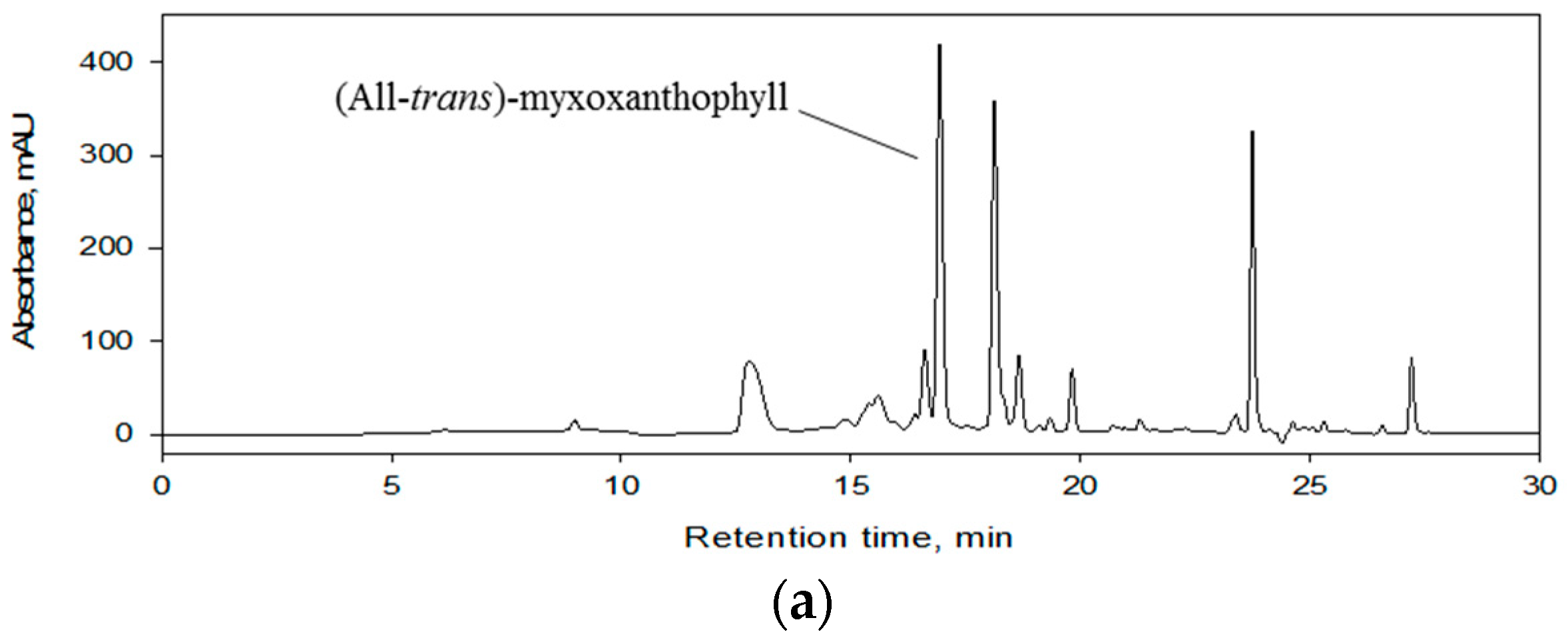
3.1. Development and Application of the HPCCC Method
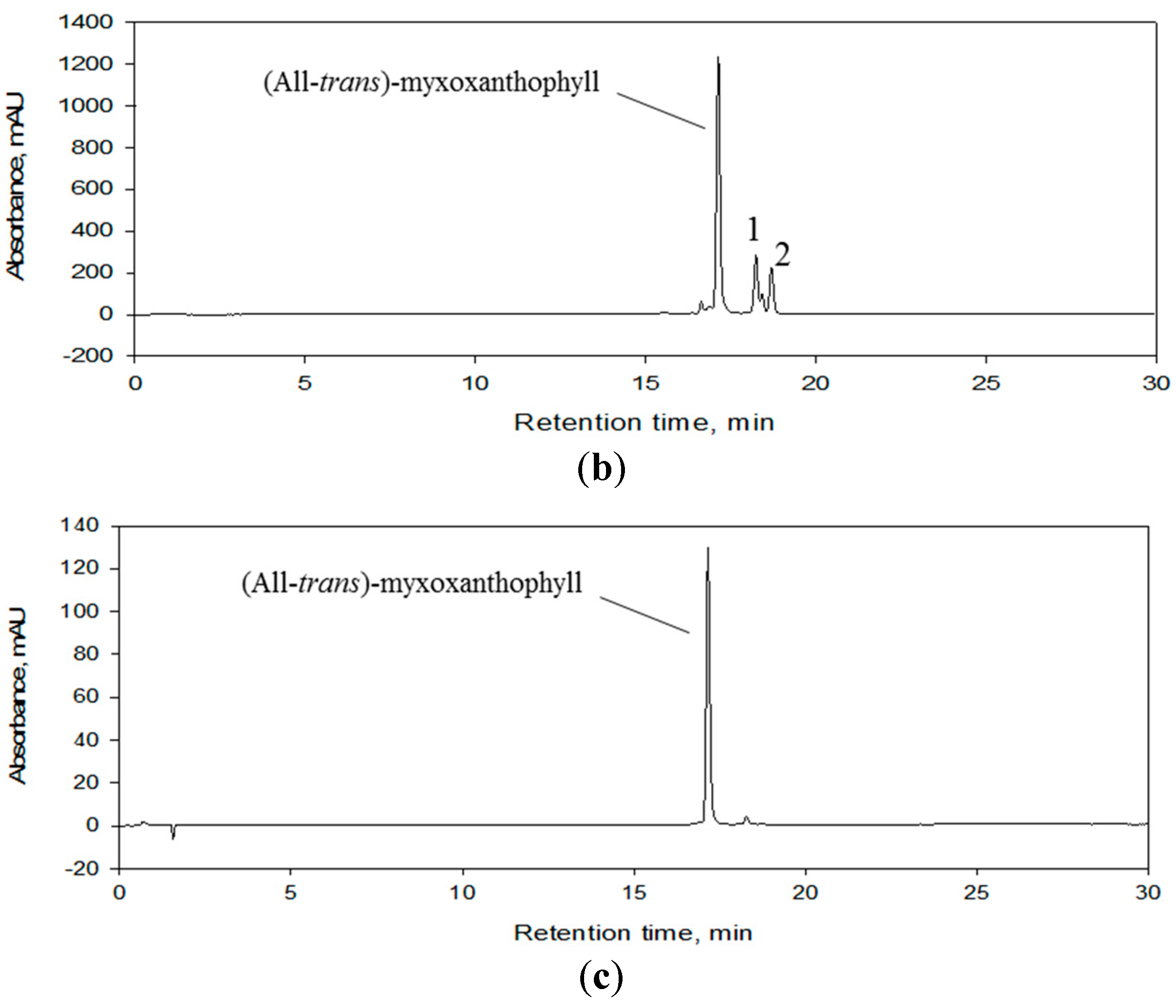
3.2. Final Purification of Myxoxanthophyll Fraction by Semi-Preparative HPLC
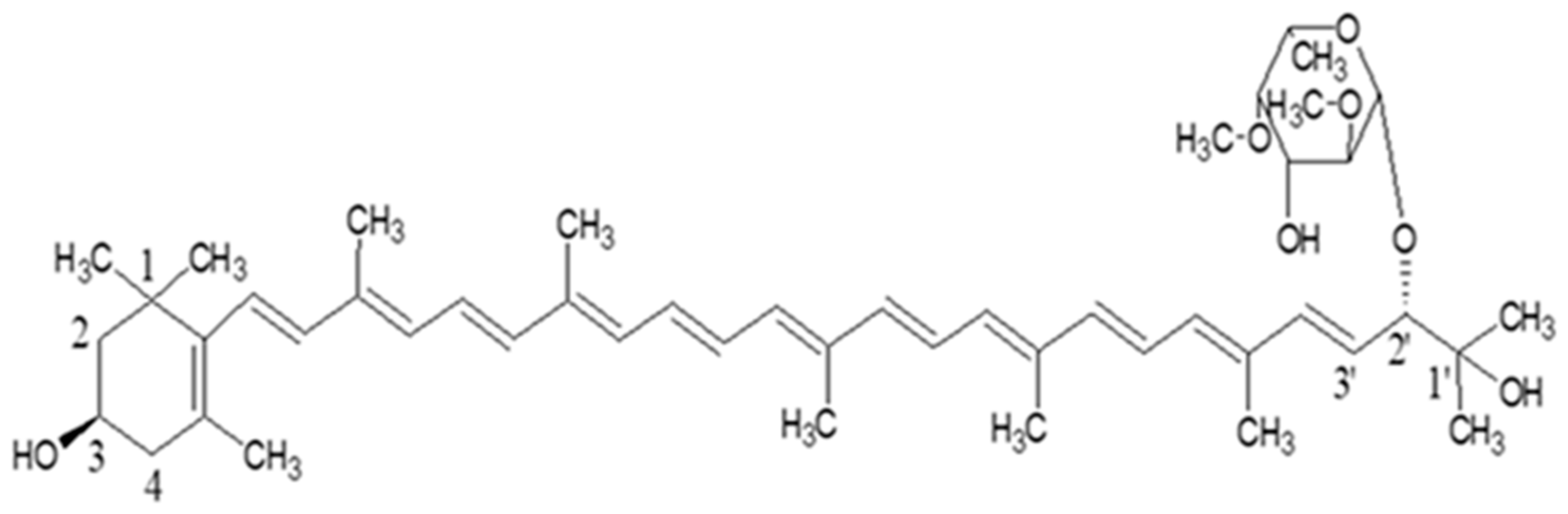
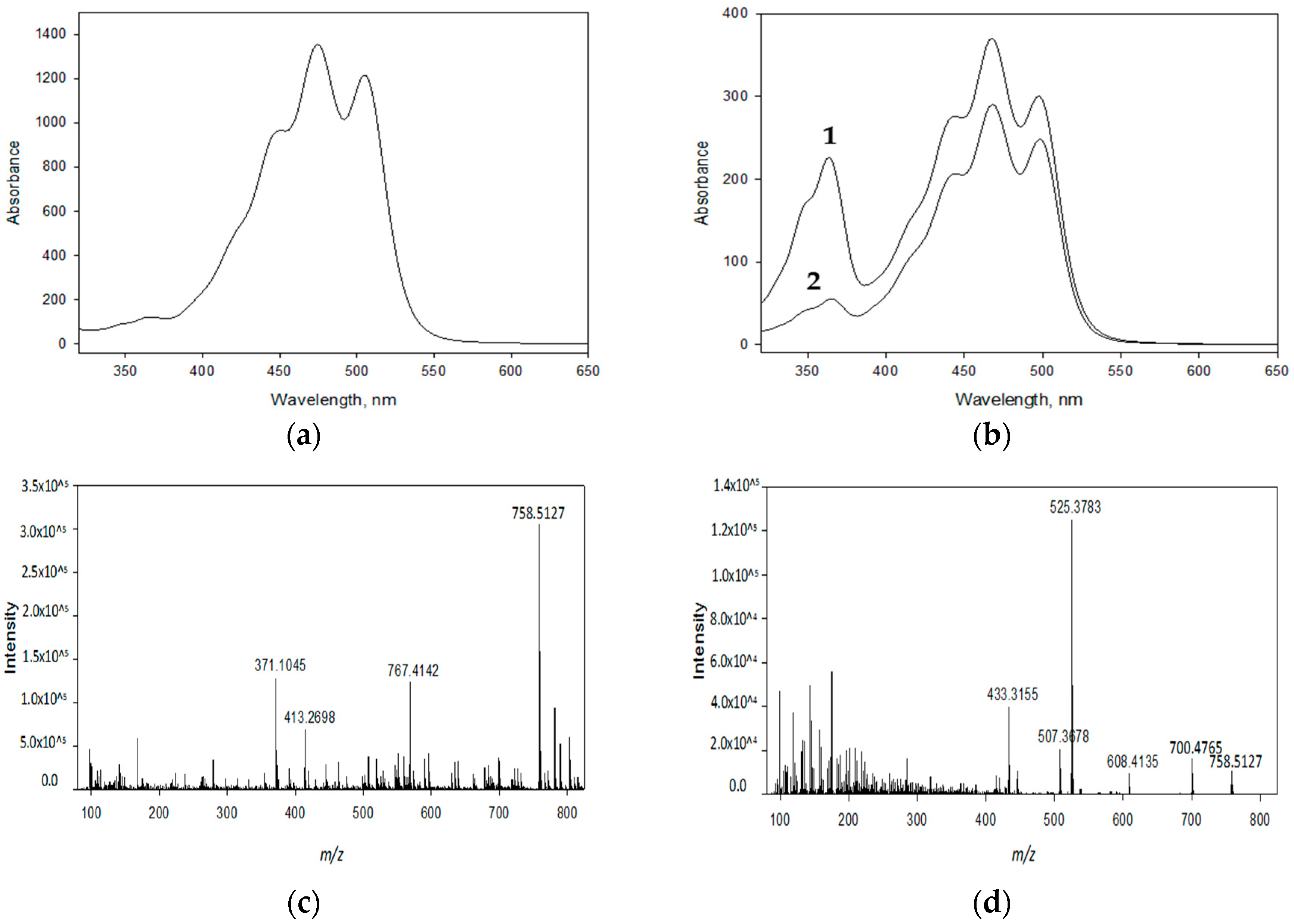
3.3. Identity Confirmation of the Isolated Target Compound
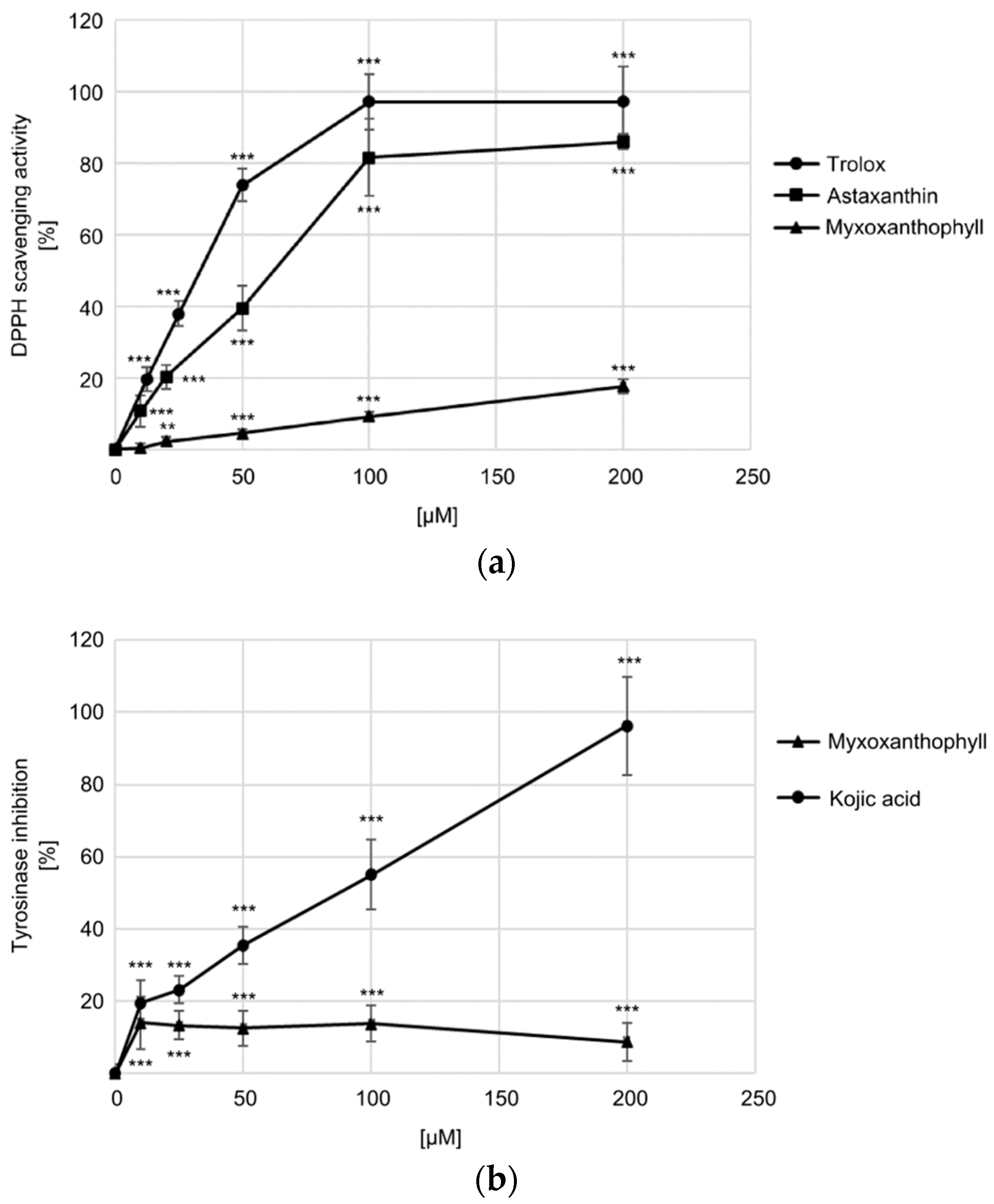
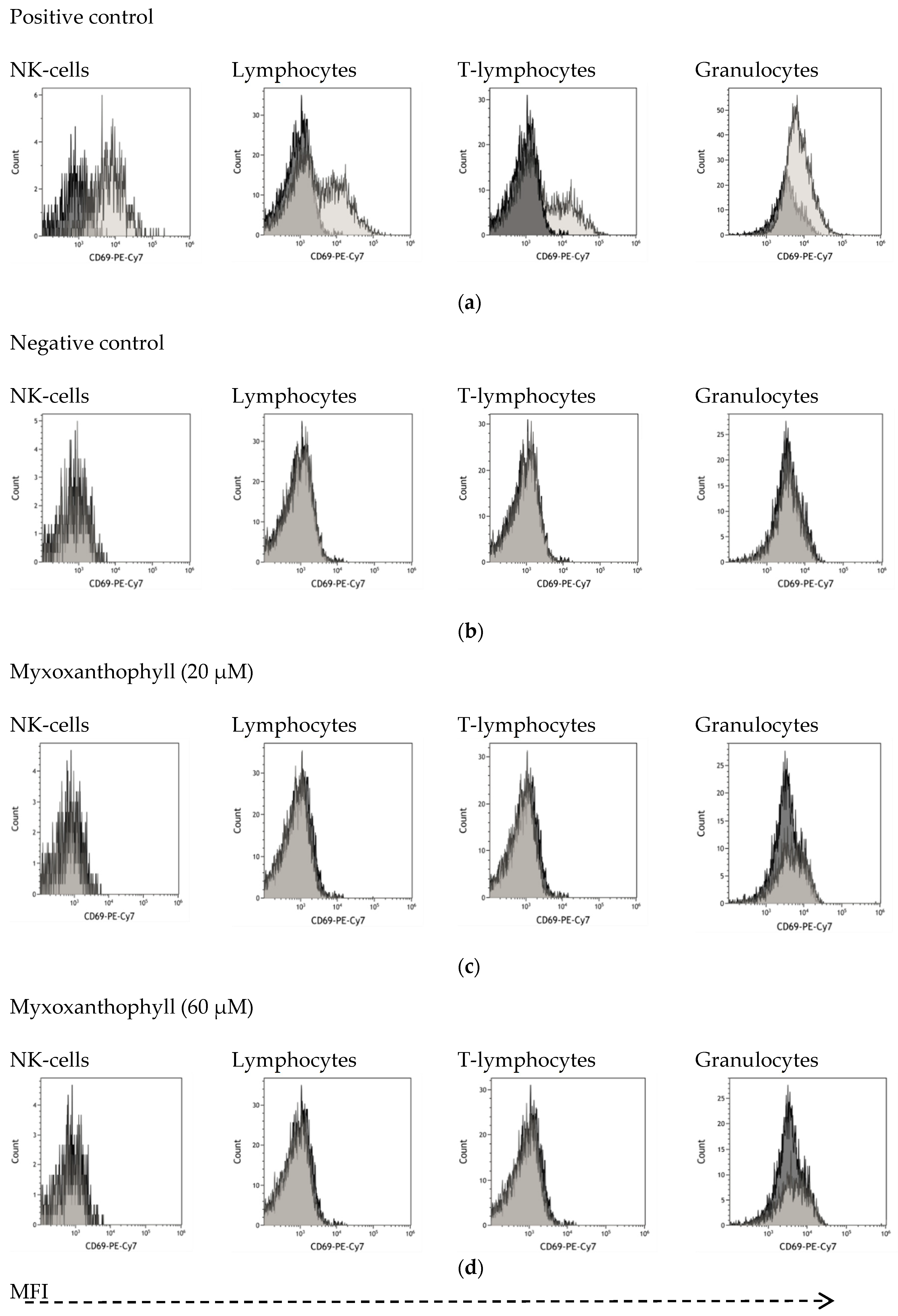
3.4. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, Y.; You, L.; Liu, D.; Hollinshead, W.; Tang, Y.J.; Zhang, F. Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar. Drugs 2013, 11, 2894–2916. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Pabbi, S.; Shukla, P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food Chem. Toxicol. 2018, 120, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.E.; van de Meene, A.M.L.; Roberson, R.W.; Vermaas, W.F.J. Myxoxanthophyll is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2005, 187, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef]
- Ghosh, T.; Bhayani, K.; Paliwal, C.; Maurya, R.; Chokshi, K.; Pancha, I.; Mishra, S. Cyanobacterial pigments as natural anti-hyperglycemic agents: An in vitro study. Front. Mar. Sci. 2016, 3, 146. [Google Scholar] [CrossRef]
- Steiger, S.; Schäfer, L.; Sandmann, G. High-light-dependent upregulation of carotenoids and their antioxidative properties in the cyanobacterium Synechocystis PCC 6803. J. Photochem. Photobiol. B Biol. 1999, 52, 14–18. [Google Scholar] [CrossRef]
- Jaeger, C.; Saettler, A.; Schroeder, K.R.; Roegner, M. Use of Myxoxanthophyll and/or Echinenon for the Prophylactic and/or Therapeutic Treatment of Undesirable Physical Conditions Caused or Promoted by Oxidative Processes. Patent DE10046838A1, Germany. 2000. Available online: https://patents.google.com/patent/DE10046838A1/en (accessed on 15 October 2020).
- Takaichi, S.; Maoka, T.; Masamoto, K. Myxoxanthophyll in Synechocystis sp. PCC 6803 is myxol 2′-dimethyl-fucoside, (3R,2′S)-myxol 2′-(2,4-di-O-methyl-α-L-fucoside), not rhamnoside. Plant Cell Physiol. 2001, 42, 756–762. [Google Scholar] [CrossRef]
- Sutherland, I.; Thickitt, C.; Douillet, N.; Freebairn, K.; Johns, D.; Mountain, C.; Wood, P.; Edwards, N.; Rooke, D.; Harris, G.; et al. Scalable technology for the extraction of pharmaceutics: Outcomes from a 3 year collaborative industry/academia research programme. J. Chromatogr. A 2013, 1282, 84–94. [Google Scholar] [CrossRef][Green Version]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Elfakir, C. New advances in countercurrent chromatography and centrifugal partition chromatography: Focus on coupling strategy. Anal. Bioanal. Chem. 2014, 406, 957–969. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.B.; Wong, R.; Ji, B.; Jiang, Y. Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1064, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Zou, D.; Chen, C.; You, J.; Zhou, G.; Sun, J.; Li, Y. Application of an efficient strategy based on liquid–liquid extraction, high-speed counter-current chromatography, and preparative HPLC for the rapid enrichment, separation, and purification of four anthraquinones from Rheum tanguticum. J. Sep. Sci. 2014, 37, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, H.L.; Zhao, X.H.; Li, J.; Shao, Y. Separation of four phenylpropanoid glycosides from a chinese herb by HSCCC. J. Chromatogr. Sci. 2015, 53, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Cheel, J.; Urajová, P.; Hájek, J.; Hrouzek, P.; Kuzma, M.; Bouju, E.; Faure, K.; Kopecký, J. Separation of cyclic lipopeptide puwainaphycins from cyanobacteria by countercurrent chromatography combined with polymeric resins and HPLC. Anal. Bioanal. Chem 2017, 409, 917–930. [Google Scholar] [CrossRef]
- Cheel, J.; Hájek, J.; Kuzma, M.; Saurav, K.; Smýkalová, I.; Ondráčková, E.; Urajová, P.; Vu, D.L.; Faure, K.; Kopecký, J.; et al. Application of HPCCC combined with polymeric resins and HPLC for the separation of cyclic lipopeptides muscotoxins A–C and their antimicrobial activity. Molecules 2018, 23, 2653. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Conway, W.D. Experimental observations of the hydrodynamic behavior of solvent systems in high-speed counter-current chromatography. III. Effects of physical properties of the solvent systems and operating temperature on the distribution of two-phase solvent systems. J. Chromatogr. A 1984, 301, 405–414. [Google Scholar] [CrossRef]
- Sutherland, I.A. Liquid stationary phase retention and resolution in hydrodynamic CCC. In Comprehensive Analytical Chemistry; Berthod, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 38, pp. 159–176. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Jiménez, M.; Chazarra, S.; Escribano, J.; Cabanes, J.; García-Carmona, F. Competitive inhibition of mushroom tyrosinase by 4-substituted benzaldehydes. J. Agric. Food Chem. 2001, 49, 4060–4063. [Google Scholar] [CrossRef]
- Tůmová, L.; Dučaiová, Z.; Cheel, J.; Vokřál, I.; Sepúlveda, B.; Vokurková, D. Azorella compacta infusion activates human immune cells and scavenges free radicals in vitro. Pharmacogn. Mag. 2017, 13, 260–264. [Google Scholar] [CrossRef]
- Berthod, A.; Faure, K. Separations with a liquid stationary phase: Countercurrent chromatography or centrifugal partition chromatography. In Analytical Separation Science, 1st ed.; Anderson, J.L., Berthod, A., Pino Estévez, V., Stalcup, A.M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1177–1206. [Google Scholar] [CrossRef]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- Fábryová, T.; Tůmová, L.; da Silva, D.C.; Pereira, D.M.; Andrade, P.B.; Valentão, P.; Hrouzek, P.; Kopecký, J.; Cheel, J. Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC). Algal Res. 2020, 49, 101947. [Google Scholar] [CrossRef]
- Lagarde, D.; Vermaas, W. The zeaxanthin biosynthesis enzyme IS-carotene hydroxylase is involved in myxoxanthophyll synthesis in Synechocystis sp. PCC 6803. FEBS Lett. 1999, 454, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Schieber, A.; Carle, R.J. Effects of heating and illumination on trans-cis isomerization and degradation of beta-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.S. A Review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. Int. J. Immunopharmacol. 1996, 18, 753–758. [Google Scholar] [CrossRef]
- Hughes, D.A. Effects of carotenoids on human immune function. Proc. Nutr. Soc. 1999, 58, 713–718. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Ramsdell, F.; Alderson, M.R. The activation antigen CD69. Stem Cells 1994, 12, 456–465. [Google Scholar] [CrossRef]
- Cheel, J.; Antwerpen, P.V.; Tůmová, L.; Onofre, G.; Vokurková, D.; Zouaoui-Boudjeltia, K.; Vanhaeverbeek, M.; Nève, J. Free radical-scavenging, antioxidant and immunostimulating effects of a licorice infusion (Glycyrrhiza glabra L.). Food Chem. 2010, 122, 508–517. [Google Scholar] [CrossRef]
- Cheel, J.; Onofre, G.; Vokurková, D.; Tůmová, L.; Neugebauerová, J. Licorice infusion: Chemical profile and effects on the activation and the cell cycle progression of human lymphocytes. Pharmacogn. Mag. 2010, 6, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Jurcic, K. Immunological studies of Revitonil®, a phytopharmaceutical containing Echinacea purpurea and Glycyrrhiza glabra root extract. Phytomedicine 2002, 9, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Zwickey, H.; Brush, J.; Iacullo, C.M.; Connelly, E.; Gregory, W.L.; Soumyanath, A.; Buresh, R. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD25 expression in humans: A pilot study. Phytother. Res. 2007, 21, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Brush, J.; Mendenhall, E.; Guggenheim, A.; Chan, T.; Connelly, E.; Soumyanath, A.; Buresh, R.; Barrett, R.; Zwickey, H. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD69 expression and immune cell activation in humans. Phytother. Res. 2006, 20, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal, B.; González, Á. Innate immune system. In Autoimmunity: From Bench to Bedside; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 2013; pp. 31–46. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459455/ (accessed on 15 October 2020).

| Solvent Systems | Composition | Relative Proportions of Solvents (v/v/v) | Phase Volume Ratio (UP/LP) | Settling Time (s) | Density Difference (LP−UP, g/mL) | Partition Coefficient (K) of MYX |
|---|---|---|---|---|---|---|
| 1 | n-Hep–EtOH–H2O | 5:4:0.5 | 0.93 | 8 | 0.11 | 0.019 |
| 2 | n-Hep–EtOH–H2O | 5:4:1 | 1.00 | 9 | 0.15 | 0.025 |
| 3 | n-Hep–EtOH–H2O | 5:4:1.5 | 0.94 | 8 | 0.17 | 0.045 |
| 4 | n-Hep–EtOH–H2O | 5:4:2 | 0.96 | 9 | 0.19 | 0.092 |
| 5 | n-Hep–EtOH–H2O | 4:4:2 | 0.75 | 16 | 0.19 | 0.200 |
| 6 | n-Hep–EtOH–H2O | 3:4:3 | 0.47 | 11 | 0.20 | 0.736 |
| 7 | n-Hep–EtOH–H2O | 2:4:4 | 0.30 | 12 | 0.22 | 1.517 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nováková, M.; Fábryová, T.; Vokurková, D.; Dolečková, I.; Kopecký, J.; Hrouzek, P.; Tůmová, L.; Cheel, J. Separation of the Glycosylated Carotenoid Myxoxanthophyll from Synechocystis Salina by HPCCC and Evaluation of Its Antioxidant, Tyrosinase Inhibitory and Immune-Stimulating Properties. Separations 2020, 7, 73. https://doi.org/10.3390/separations7040073
Nováková M, Fábryová T, Vokurková D, Dolečková I, Kopecký J, Hrouzek P, Tůmová L, Cheel J. Separation of the Glycosylated Carotenoid Myxoxanthophyll from Synechocystis Salina by HPCCC and Evaluation of Its Antioxidant, Tyrosinase Inhibitory and Immune-Stimulating Properties. Separations. 2020; 7(4):73. https://doi.org/10.3390/separations7040073
Chicago/Turabian StyleNováková, Michaela, Tereza Fábryová, Doris Vokurková, Iva Dolečková, Jiří Kopecký, Pavel Hrouzek, Lenka Tůmová, and José Cheel. 2020. "Separation of the Glycosylated Carotenoid Myxoxanthophyll from Synechocystis Salina by HPCCC and Evaluation of Its Antioxidant, Tyrosinase Inhibitory and Immune-Stimulating Properties" Separations 7, no. 4: 73. https://doi.org/10.3390/separations7040073
APA StyleNováková, M., Fábryová, T., Vokurková, D., Dolečková, I., Kopecký, J., Hrouzek, P., Tůmová, L., & Cheel, J. (2020). Separation of the Glycosylated Carotenoid Myxoxanthophyll from Synechocystis Salina by HPCCC and Evaluation of Its Antioxidant, Tyrosinase Inhibitory and Immune-Stimulating Properties. Separations, 7(4), 73. https://doi.org/10.3390/separations7040073