Abstract
The presence of free silanols on alkyl-bonded reversed-phase stationary phases is responsible for broad and asymmetrical peaks when basic drugs are chromatographed with conventional octadecylsilane (C18) columns due to ionic interactions. In the last few years, ionic liquids (ILs) have attracted attention to reduce this undesirable silanol activity. ILs should be considered as dual modifiers (with a cationic and anionic character), which means that both cations and anions are able to adsorb on the stationary phase, creating a positively or negatively charged layer, depending on the relative adsorption. The accessibility of basic compounds to the silanols is prevented by both the IL cation and anion, improving the peak profiles. A comparative study of the performance of six imidazolium-based ILs, differing in their cation/anions, as modifiers of the chromatographic behavior of a group of ten β-adrenoceptor antagonists, is addressed. Mobile phases containing cationic amines (triethylamine and dimethyloctylamine) were used as a reference for the interpretation of the results. Using a mathematical model based on two chemical equilibria, the association constants between the solutes and modified stationary phase as well as those between solutes and the additive in the mobile phase were estimated. These values, together with the changes in retention and peak shape, were used to obtain conclusions about the retention mechanism, changes in the nature of the chromatographic system, and silanol suppression effect.
1. Introduction
Alkyl-bonded stationary phases (commonly octadecylsilane or C18) with silica as support are extensively used in reversed-phase liquid chromatography (RPLC) due to their capability to separate a wide variety of compounds [1,2]. Solutes are mainly retained according to their hydrophobicity. However, in the silica derivatization process, free silanol groups remain on the stationary phase in a significant amount due to steric problems which vary with the brand and manufacturer. Silanols’ average protonation constants range from ca. log K = 4.5 to ca. 7 depending on the type of silica, which means that they are weakly acidic [3]. In typical RPLC columns, the working pH range varies between 2 and 8. This means that silanols are mainly negatively charged and are able to establish additional ion-exchange interactions with cationic compounds, thus increasing their retention. The kinetics of this process are slow, and, consequently, broad and asymmetrical peaks are obtained, which also affects peak resolution [4,5,6,7]. Many basic compounds have a great interest in the pharmaceutical industry, but they are bases exposed to silanol interaction. Consequently, the reduction of the “silanol effect” is of special importance in the analysis of this kind of compounds [8,9].
In order to minimize or suppress the negative influence of residual silanols on the chromatographic separation of basic drugs, different types of additives in the hydro-organic mobile phases have been used [5,10,11], since this approach has the advantage of using conventional alkyl-bonded silica phase columns (octyl or octadecyl). Among the common reagents traditionally used with this purpose (i.e., amines, surfactants, etc.), ionic liquids (ILs) have become a serious alternative [12,13,14,15,16,17].
ILs are molten salts with low melting points, often arbitrarily fixed at or below 100 °C [18,19,20]. Though they also have a non-molecular nature, ILs are made by the essence of cations and anions—the same number of positive cations associated with negative anions—so that the whole liquid is electrically neutral. One of the most important features of ILs is their extremely low vapor pressure [18,19,20], which has gained them the label of “green” solvents. However, several reports in the last few years have specified a certain reticence to the term “green” [21,22], since some ILs are not as safe and non-toxic as it was thought, especially during their synthesis. It should be noted that the properties of ILs can be tuned, including their toxicity.
When ILs are used as mobile phase additives (e.g., imidazolium ILs), they become just salts with hydrophobic cations and anions. Since both the cation and the anion are capable of interacting with the stationary phase, they have a dual character [17]. In previous work, it was observed that the adsorption effect on the alkyl-bonded stationary phase was larger for bulky imidazolium ILs [23,24]. The extension of the interactions of the IL cation and anion with the stationary phase and ion-pairing, ion-exchange, and hydrophobic partitioning present in the chromatographic process complicates the interpretation of the mechanism of retention. On the other hand, the wide variety of cation–anion combinations complicates the selection of the most convenient IL for a particular application (more than two thousand ILs were known in 2014) [19].
In order to gain more insight in the behavior of ILs as silanol suppressors, the goal of this work was to estimate the association constants between solutes and the modified stationary phase as well as between solutes and the additive in the mobile phase in order to find out whether the effect of the cation and anion can also be analyzed using mathematical analysis. As probe ILs, different imidazolium-based ones associated to chloride ([Cl]−) or tetrafluoroborate ([BF4]−) anions were added to acetonitrile–water mobile phases. With comparative purposes, two amines, triethylamine and dimethyloctylamine, usually added to improve the peak shape of basic compounds, were also studied as mobile phase additives. The results were also commented in terms of retention and peak tailing through the chromatograms of separation of mixtures of a group of probe compounds. With this purpose, a group of basic β-adrenoceptor antagonists was selected. β-adrenoceptor antagonists are drugs commercialized and used for the treatment of diverse cardiac diseases [25].
2. Materials and Methods
2.1. Chemicals
The following β-adrenoceptor antagonists were used as probe compounds: Acebutolol, atenolol, carteolol, celiprolol, esmolol, metoprolol, nadolol, oxprenolol, pindolol, and timolol (all from Sigma, St. Louis, MO, USA). The drugs were solubilized in a small amount of acetonitrile and diluted with water. The concentration of the working solutions was 40 μg/mL.
The hydro-organic RPLC mobile phases contained acetonitrile (Scharlab, Barcelona, Spain) and one of the following ionic liquids, whose structures and some relevant properties are shown in Table 1: 1-ethyl-3-methylimidazolium chloride ([C2MIM][Cl]), 1-buyl-3-methylimidazolium chloride ([C4MIM][Cl]), 1-hexyl-3-methylimidazolium chloride ([C6MIM][Cl]), 1-ethyl-3-methylimidazolium tetrafluoroborate ([C2MIM][BF4]), 1-butyl-3-methylimidazolium tetrafluoroborate ([C4MIM][BF4]), and 1-hexyl-3-methylimidazolium tetrafluoroborate ([C6MIM][BF4]) (all from Sigma). For comparison purposes, mobile phases containing triethylamine (TEA) (Sigma) or dimethyloctylamine (DMOA) (Sigma) and acetonitrile were prepared. Occasionally, acetonitrile–water mixtures in the absence of an additive were used. In all cases, mobile phases were buffered at pH 3 with 0.01 M citric acid monohydrate and sodium hydroxide (Panreac, Barcelona). Uracil (Across Organics, Geel, Belgium) was used as dead time marker.

Table 1.
Structure and properties of the ionic liquids.
Nanopure water (Barnstead, Sybron, Boston, MA, USA) was used throughout. The drug solutions and mobile phases were filtered through 0.45 μm Nylon membranes (Micron Separations, Westboro, MA, USA).
2.2. Instrumentation
An Agilent chromatograph (Waldbronn, Germany), equipped with an isocratic pump (Series 1200), an autosampler, a UV-visible detector (Series 1100) set at 254 nm (except for timolol, which was detected at 300 nm), and an HPChemStation (Agilent, B.02.01)—for data acquisition—was used.
The analytical column (a Kromasil C18 column, Análisis Vínicos, Ciudad Real, Spain) had the following characteristics: 150 × 4.6 mm i.d., 5 μm particle size, 19% carbon load, 320 m2/g surface area, and 110 Å pore diameter. The column was connected to a similar 30 mm guard column. The flow-rate was 1 mL/min. Duplicate injections were made using an injection volume of 20 μL.
2.3. Experimental Designs
Based on previous experience [23,24], a fixed concentration of 15% acetonitrile was selected for the mobile phases. An ionic liquid (Table 1) or TEA was then added at concentrations 10, 20, 30 and 40 mM. ([C2MIM][BF4]) was added at 5, 10, 20 and 30 mM, and DMOA at 1.25, 2.5, 5 and 10 mM. The selected mobile phase compositions guaranteed the elution of the assayed compounds in practical analysis times.
3. Results and Discussion
3.1. Solute-Stationary Phase and Solute-Mobile Phase Interactions
Micellar liquid chromatography is a reversed-phase chromatographic mode that uses mobile phases containing a surfactant above its critical micelle concentration [26]. Under these conditions, surfactant monomers aggregate to form micelles in the mobile phase and are also able to adsorb on the stationary phase modifying its properties.
From the early steps of micellar liquid chromatography, there was an interest in suggesting possible retention mechanisms. One of the first proposals was the three phase model approach (stationary phase, water/organic solvent, and micelle) associated to equations that describe solute retention [27,28]. Arunyanart and Cline-Love addressed two chemical equilibria in a micellar RPLC system [28]:
A + S ⇆ AS
A + M ⇆ AM
These equations involve the association of the solute with the stationary phase binding sites (S) and in bulk water (A) or with a monomer of surfactant in the micelle dissolved in the mobile phase (M). The corresponding constants KWS and KAM for Equations (1) and (2), respectively, express the displacement of these equilibria. Considering this, the retention factor (k) is given by:
with being the phase ratio (the ratio between the volume of active surface of the stationary phase and the column dead volume) and [M] being the molar concentration of surfactant monomers forming micelles. Including [S] (which is constant or practically constant), together with in the partition constant KWS, Equation (3) can be rewritten as:
where KAS () and KAM are the solute-stationary phase and solute-micelle association constants, respectively.
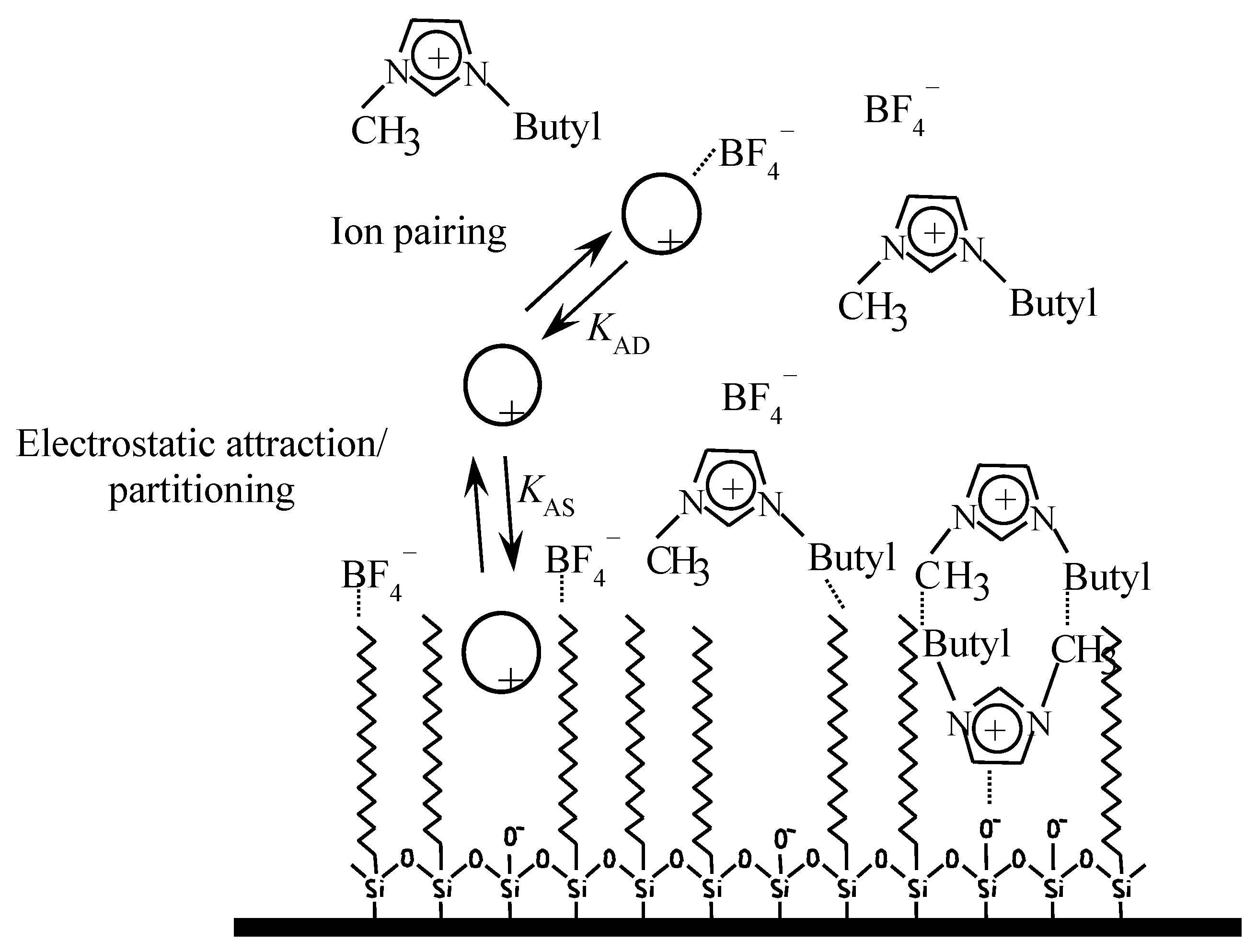
The linear plots of 1/k versus the surfactant concentration are an evidence of the saturation of the stationary phase by the surfactant monomers. The extrapolation of the linear segments allows for the measurement of the strength of the interaction between the solute and stationary phase, expressed as 1/KAS. The slope of the fitted equation (Equation 4) estimates KAM. The studied ILs and amines experience similar equilibria to those expressed by Equations (1) and (2) (Figure 1).
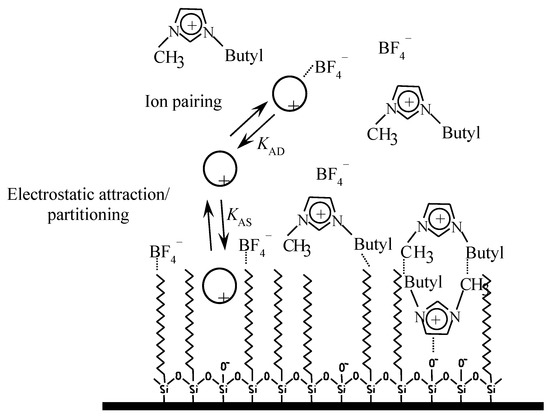
Figure 1.
Solute environment in an octadecylsilane (C18) stationary phase in the presence of [C4MIM][BF4].
The six selected ILs in this work ([C2MIM][Cl], [C4MIM][Cl], [C6MIM][Cl], [C2MIM][BF4], [C4MIM][BF4], and [C6MIM][BF4]) possess cations and anions with different adsorption capabilities for the stationary phase [29]. In previous work [23,24], it was checked that the cation can establish electrostatic and hydrophobic interactions with the anionic silanols and the alkyl bonded phase, respectively, whereas the anion interacts hydrophobically with the stationary phase. This gives rise to an asymmetric bilayer, positively or negatively charged depending on the relative adsorption of the IL. Consequently, cationic drugs are retained as a consequence of a mixed mechanism that involves ion-pairing, ion exchange, and hydrophobic partitioning. In the mobile phase, ion-pair interactions can be also established with the IL anion. The importance and extension of these interactions complicates the interpretation of the solutes’ behavior in the chromatographic system.
The chromatographic behavior of the β-adrenoceptor antagonists with mobile phases containing the six assayed ILs was previously studied [24]. In general, it was observed that the retention factors of the β-adrenoceptor antagonists decreased with the first addition of IL, this change being more intense for the [C6MIM] cation. Successive additions of the ILs decreased the retention factors. For ILs associated to [BF4]−, the retention times of the β-adrenoceptor antagonists were longer with respect to the presence of chlorides, a fact which can be explained by the stronger adsorption of [BF4]− on the C18 column [29].
Therefore, Equation (4) was fitted to these data obtained in the presence of the indicated ILs and amines to measure the strength of the interaction of solutes with stationary phases modified by adsorption of the indicated additives. The calculated association constants are given in Table 2, Table 3 and Table 4. KAD is the solute–additive association constant analogous to KAM in the presence of surfactant (See Equation (4) and Figure 1).

Table 2.
Association constants between the solute and stationary phase (KAS) and between the solute and additive in the mobile phase (KAD) in the presence of chloride ionic liquids (ILs).

Table 3.
Association constants between the solute and stationary phase (KAS) and between the solute and additive in the mobile phase (KAD) in the presence of tetrafluoroborate ILs.

Table 4.
Association constants between the solute and stationary phase (KAS) and between the solute and additive in the mobile phase (KAD) in the presence of amines.
As observed, the lowest values for KAS were obtained for the [C6MIM] cation, whereas the highest values corresponded to [C2MIM]+, thus suggesting a higher affinity of the β-adrenoceptor antagonists to the stationary phase modified with this IL. These observations indicate that the adsorption for [BF4]− is low or moderate with respect to [Cl]−. Additionally, [C6MIM]+ interacts stronger with the alkyl-bonded stationary phase (with respect to [C4MIM]+ and [C2MIM]+), which repels β-adrenoceptor antagonists and decreases the retention to a higher extent. As for the amines (Table 4), the KAS values were larger in the presence of TEA and lower in the presence of DMOA. This again suggests the higher affinity of the β-adrenoceptor antagonists to the stationary phase modified by TEA, which shows a low or moderate adsorption, whereas DMOA interacts to a greater extent with the alkyl-bonded stationary phase which repels the β-adrenoceptor antagonists.
On the other hand, the KAD values were significantly lower in the presence of [C2MIM]+, obtaining negative values in some cases, especially when this cation was associated to [BF4]−, which seemed nonsensical. However, in the micellar RPLC literature, this behavior was observed and described as “anti-binding” [30]. The anti-binding behavior would have been produced by electrostatic repulsion between the solutes and the additive in the mobile phase, both bearing a charge of the same sign. It should be also considered that the repulsion between charged solutes and the stationary phase (without other kinds of interaction) would make the solutes elute within the void volume region. In the presence of TEA, the KAD values were also slightly negative (see Table 4). However, the high and positive values obtained for DMOA suggest an important affinity of the solutes for this amine in the mobile phase.
3.2. Separation of Mixtures of β-Adrenoceptor Antagonists
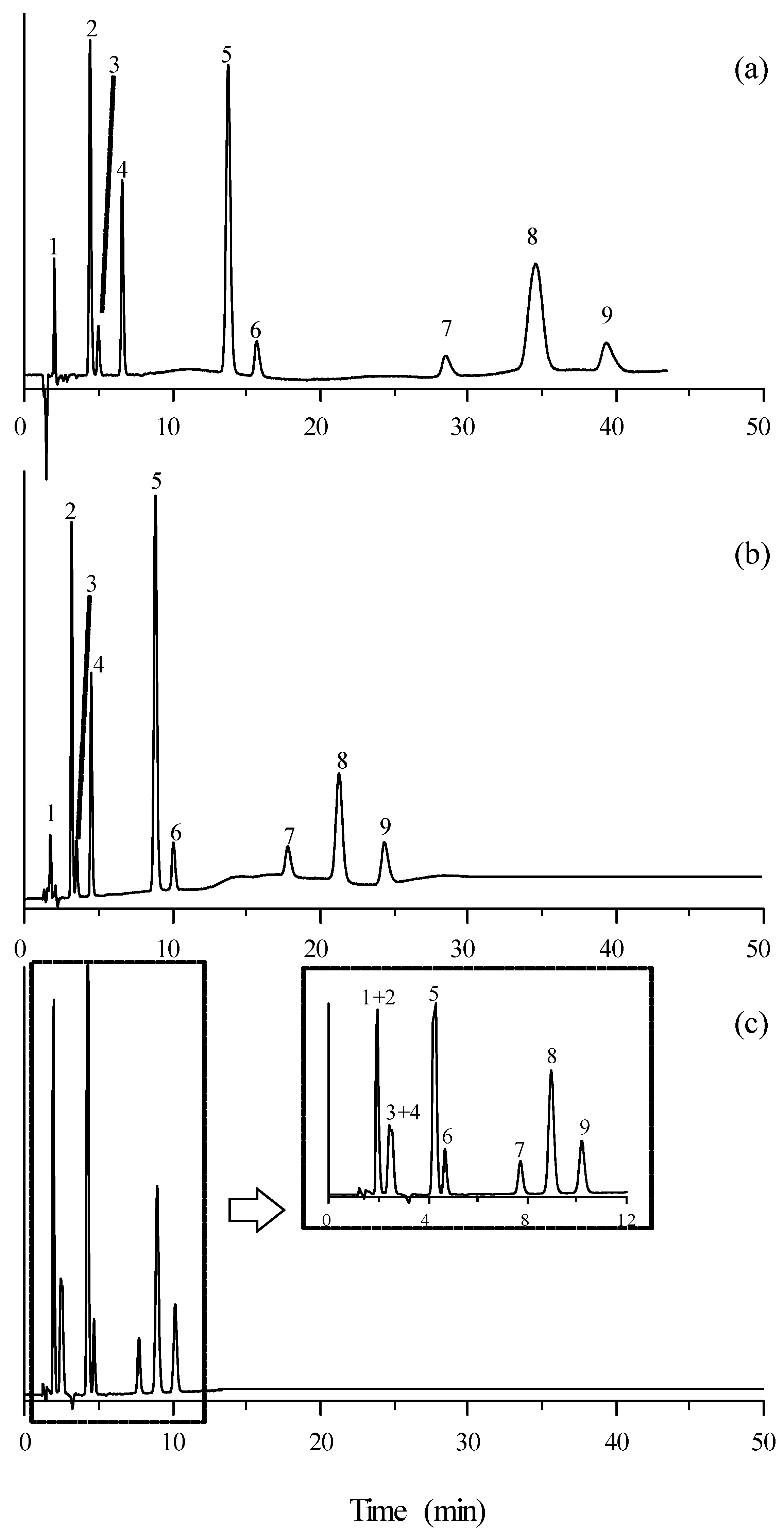
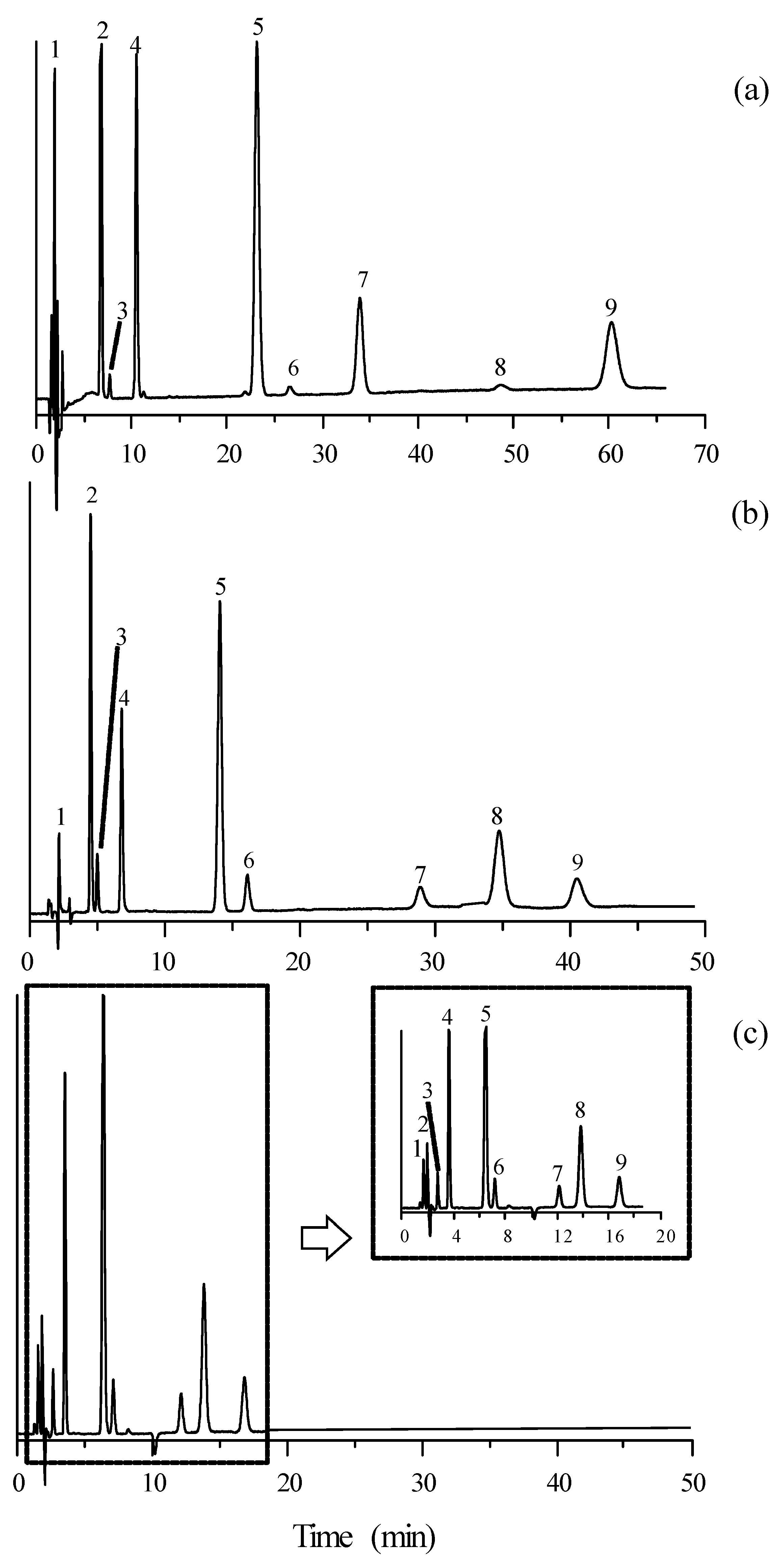
The chromatograms obtained for mixtures of the selected compounds eluted with a mobile phase containing 15% acetonitrile and 10 mM of the studied ILs (except timolol, which absorbed at 300 nm) are depicted in Figure 2 and Figure 3. It should be first observed that the elution order of the mixture of compounds did not change when varying the mobile phase additive. As commented, the retention times were larger in the presence of [C2MIM]+ and for those ILs associated to [BF4]−. The resolution was satisfactory in all cases, although it was poorer in the presence of [C6MIM]+. However, the retention times were more practical (<20 min) with this cation.
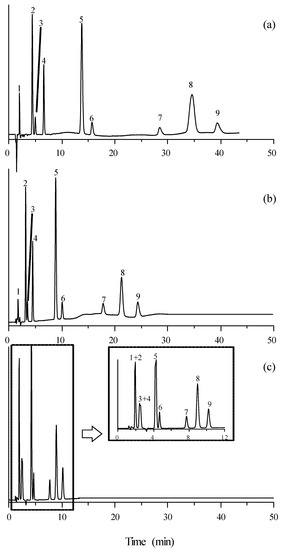
Figure 2.
Chromatograms of the set of β-adrenoceptor antagonists detected at 254 nm, eluted with mobile phases containing 15% acetonitrile and 10 mM ionic liquid: (a) [C2MIM][Cl], (b) [C4MIM][Cl], and (c) [C6MIM][Cl]. Solute identity: (1) Atenolol, (2) carteolol, (3) nadolol, (4) pindolol, (5) acebutolol, (6) metoprolol, (7) esmolol, (8) celiprolol, and (9) oxprenolol.
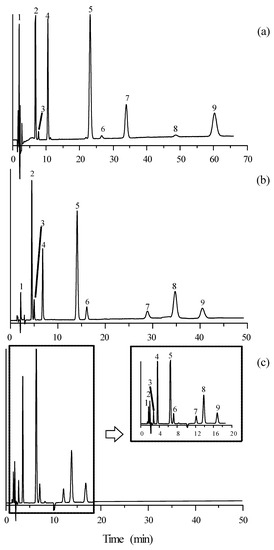
Figure 3.
Chromatograms of the set of β-adrenoceptor antagonists detected at 254 nm, eluted with mobile phases containing 15% acetonitrile and 10 mM ionic liquid: (a) [C2MIM][BF4], (b) [C4MIM][BF4], and (c) [C6MIM][BF4]. See Figure 2 for solute identity.
On the other hand, it should be noted that the peaks are highly symmetrical, especially in the presence of [C6MIM]+. This suggests that all these additives efficiently hinder the access of the β-adrenoceptor antagonists to the residual free silanols on the stationary phase (even for [C2MIM]⋅[Cl], [C2MIM][BF4] and [C4MIM][BF4], for which the retention of the probe compounds did not change at increasing concentrations of the additives—at least not significantly).
4. Conclusions
In previous work, it was checked that the chromatographic behavior of basic drugs in terms of retention and peak shape provided information about the solute interactions in the mobile phase and the stationary phase [23]. This research was here extended through a mathematical treatment to estimate the association constants between solutes and the modified stationary phase as well as between solutes and the additive in the mobile phase. The results were compared with those obtained in the presence of the amines TEA and DMOA, which also showed a diverse affinity for the stationary phase, to assist in the interpretation of the results.
The estimation of the solute-stationary phase and solute-mobile phase association constants (KAS and KAD from Equation (4)) indicate that the influence of both the cation and the anion should be considered, and, by extension, the interactions taking place simultaneously inside the column.
The low KAS values obtained for the bulky cations indicate a strong association of these reagents to the stationary phase, as they coat the surface of the stationary phase and form a double layer that hinders interaction with the residual silanols. In previous work [24], it was established that additives of small size, such as [C2MIM][Cl], [C2MIM][BF4] and TEA interact directly with the silanols and are less effective, whereas those of larger size associate with the alkyl-bonded chains and significantly improve the chromatographic peak shape. This was checked among the ILs studied in this work. Those of larger size ([C6MIM]+) significantly improved the chromatographic peak shape when compared with ILs of smaller size ([C2MIM]+ and [C4MIM]+). [C6MIM][Cl] and [C6MIM][BF4] showed the most interesting characteristics for the chromatographic performance of basic compounds—they gave rise to symmetrical peaks that elute at short retention times.
Author Contributions
M.J.R.-Á.; methodology; investigation; writing-original draft preparation; writing-review and editing.
Funding
This research was funded by the Ministry of Economy and Competitiveness (MINECO, Spain), grant number CTQ2016–75644-P, by FEDER funds, and DIRECCIÓ GENERAL D’UNIVERSITAT, INVESTIGACIÓ I CIÈNCIA (Generalitat Valenciana, Spain), grant number PROMETEO/2016/128.
Acknowledgments
This work was supported by Project CTQ2016–75644-P funded by the Ministry of Economy and Competitiveness (MINECO, Spain) and FEDER funds, and Project PROMETEO/2016/128 funded by the Direcció General d’Universitat, Investigació i Ciència (Generalitat Valenciana, Spain).
Conflicts of Interest
The author declares no conflict of interest.
References
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography, 3rd ed.; Wiley: New York, NY, USA, 2010. [Google Scholar]
- García-Alvarez-Coque, M.C.; Ramis-Ramos, G.; Baeza-Baeza, J.J. Reversed phase liquid chromatography. In Analytical Separation Science Series; Anderson, J., Berthod, A., Pino, V., Stalcup, A.M., Eds.; Wiley: New York, NY, USA, 2015; Volume 1, pp. 159–198. [Google Scholar]
- Méndez, A.; Bosch, E.; Rosés, M.; Neue, U.D. Comparison of the acidity of residual silanol groups in several liquid chromatography columns. J. Chromatogr. A 2003, 986, 33–44. [Google Scholar] [CrossRef]
- Nawrocki, J. The silanol group and its role in liquid chromatography. J. Chromatogr. A 1997, 779, 29–71. [Google Scholar] [CrossRef]
- Reta, M.; Carr, P.W. Comparative study of divalent metals and amines as silanol-blocking agents in reversed-phase liquid chromatography. J. Chromatogr. A 1999, 855, 121–127. [Google Scholar] [CrossRef]
- Neue, U.D.; Tran, K.; Méndez, A.; Carr, P.W. The combined effect of silanols and the reversed-phase ligand on the retention of positively charged analytes. J. Chromatogr. A 2005, 1063, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, H.; Blay, Ch.; Saar, J. Reversed phase chromatography: The mystery of surface silanols. Chromatographia 2005, 62, S19–S29. [Google Scholar] [CrossRef]
- Ruiz-Angel, M.J.; Carda-Broch, S.; Torres-Lapasió, J.R.; García-Álvarez-Coque, M.C. Retention mechanisms in micellar liquid chromatography, J. Chromatrogr. A 2009, 1216, 1798–1814. [Google Scholar] [CrossRef]
- McCalley, D.V. The challenges of the analysis of basic compounds by high performance liquid chromatography: Some possible approaches for improved separations. J. Chromatogr. A 2010, 1217, 858–880. [Google Scholar] [CrossRef]
- Calabuig-Hernández, S.; García-Alvarez-Coque, M.C.; Ruiz-Angel, M.J. Performance of amines as silanol suppressors in reversed-phase liquid chromatography. J. Chromatogr. 2016, 1465, 98–106. [Google Scholar] [CrossRef]
- Ruiz-Ángel, M.J.; Torres-Lapasió, J.R.; García-Álvarez-Coque, M.C.; Carda-Broch, S. Retention Mechanisms for Basic Drugs in the Submicellar and Micellar Reversed-Phase Liquid Chromatographic Modes. Anal. Chem. 2008, 80, 9705–9713. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Hernández-Borges, J.; Rodríguez-Delgado, M.A. Ionic liquids as mobile phase additives for the high-performance liquid chromatographic analysis of fluoroquinolone antibiotics in water samples. Anal. Bioanal. Chem. 2008, 392, 1439–1446. [Google Scholar] [CrossRef]
- Martín-Calero, A.; Pino, V.; Ayala, J.H.; González, V.; Afonso, A.M. Ionic liquids as mobile phase additives in high-performance liquid chromatography with electrochemical detection: Application to the determination of heterocyclic aromatic amines in meat-based infant foods. Talanta 2009, 79, 590–597. [Google Scholar] [CrossRef]
- Martín-Calero, A.; Tejral, G.; Ayala, J.H.; González, V.; Afonso, A.M. Suitability of ionic liquids as mobile-phase additives in HPLC with fluorescence and UV detection for the determination of heterocyclic aromatic amines. J. Sep. Sci. 2010, 33, 182–190. [Google Scholar] [CrossRef]
- Petruczynik, A. Effect of ionic liquid additives to mobile phase on separation and system efficiency for HPLC of selected alkaloids on different stationary phases. J. Chromatogr. Sci. 2012, 50, 287–293. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, A.; Liu, R.; Zhang, Y. Simultaneous determination of fangchinoline and tetrandrine in Stephania tetrandra S. Moore by using 1-alkyl-3-methylimidazolium- based ionic liquids as the RP-HPLC mobile phase additives. Anal. Chim. Acta 2013, 767, 148–154. [Google Scholar] [CrossRef]
- García-Alvarez-Coque, M.C.; Ruiz-Angel, M.J.; Berthod, A.; Carda-Broch, S. On the use of ionic liquids as mobile phase additives in high-performance liquid chromatography. Anal. Chim. Acta 2015, 883, 1–21. [Google Scholar] [CrossRef]
- Poole, C.F. Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids. J. Chromatogr. A 2004, 1037, 49–82. [Google Scholar] [CrossRef]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic liquids in analytical chemistry: Fundamentals, advances, and perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr. A 2018, 1559, 2–16. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of ionic liquids. Clean 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Fernández-Navarro, J.J.; García-Álvarez-Coque, M.C.; Ruiz-Ángel, M.J. The role of the dual nature of ionic liquids in the reversed-phase liquid chromatographic separation of basic drugs. J. Chromatogr. A 2011, 1218, 398–407. [Google Scholar] [CrossRef]
- Ubeda-Torres, M.T.; Ortiz-Bolsico, C.; García-Alvarez-Coque, M.C.; Ruiz-Angel, M.J. Gaining insight in the behaviour of imidazolium-based ionic liquids asadditives in reversed-phase liquid chromatography for the analysis of basic compounds. J. Chromatogr. A 2015, 1380, 96–103. [Google Scholar] [CrossRef]
- Mehvar, R.; Brocks, D.R. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Sci. 2001, 4, 185–200. [Google Scholar]
- Berthod, A.; García-Álvarez-Coque, M.C. Micellar Liquid Chromatography; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Armstrong, D.W.; Nome, F. Partitioning behavior of solutes eluted with micellar mobile phases in liquid chromatography. Anal. Chem. 1981, 53, 1662–1666. [Google Scholar] [CrossRef]
- Arunyanart, M.; Cline-Love, L.J. Model for micellar effects on liquid chromatography capacity factors and for determination of micelle-solute equilibrium constants. Anal. Chem. 1984, 56, 1557–1561. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Huguet, S. Nonmolecular solvents in separation methods: Dual nature of room temperature ionic liquids. Anal. Chem. 2005, 77, 4071–4080. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Stine, G.Y. Selectivity in pseudophase liquid chromatography. Anal. Chem. 1983, 55, 2317–2320. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).