Alternative Green Extraction Phases Applied to Microextraction Techniques for Organic Compound Determination
Abstract
1. Introduction
2. Biosorbent-Based Extraction Phases
2.1. Cork as a Biosorbent
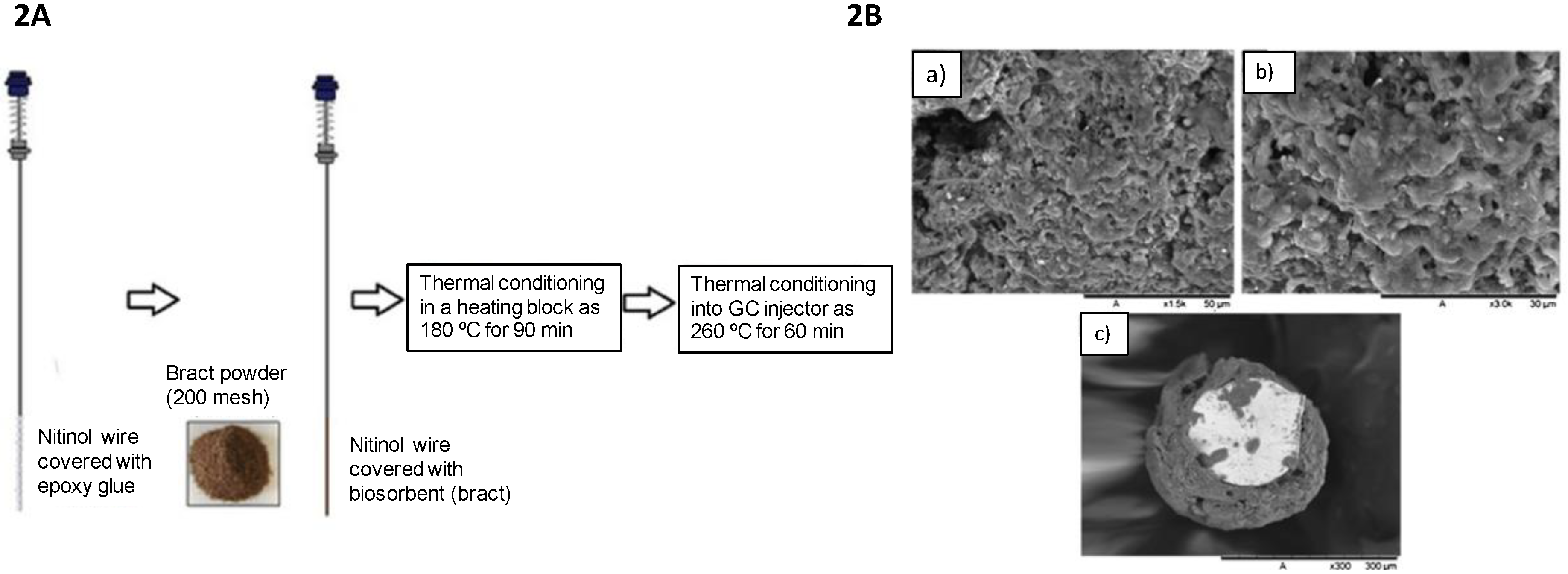
2.2. Bract as a Biosorbent
2.3. Recycled Diatomaceous Earth as a Biosorbent Material
2.4. Other Materials Used as Biosorbents
2.5. Concluding Remarks about Biosorbents
3. Ionic Liquids (ILs) as Green Extraction Phase
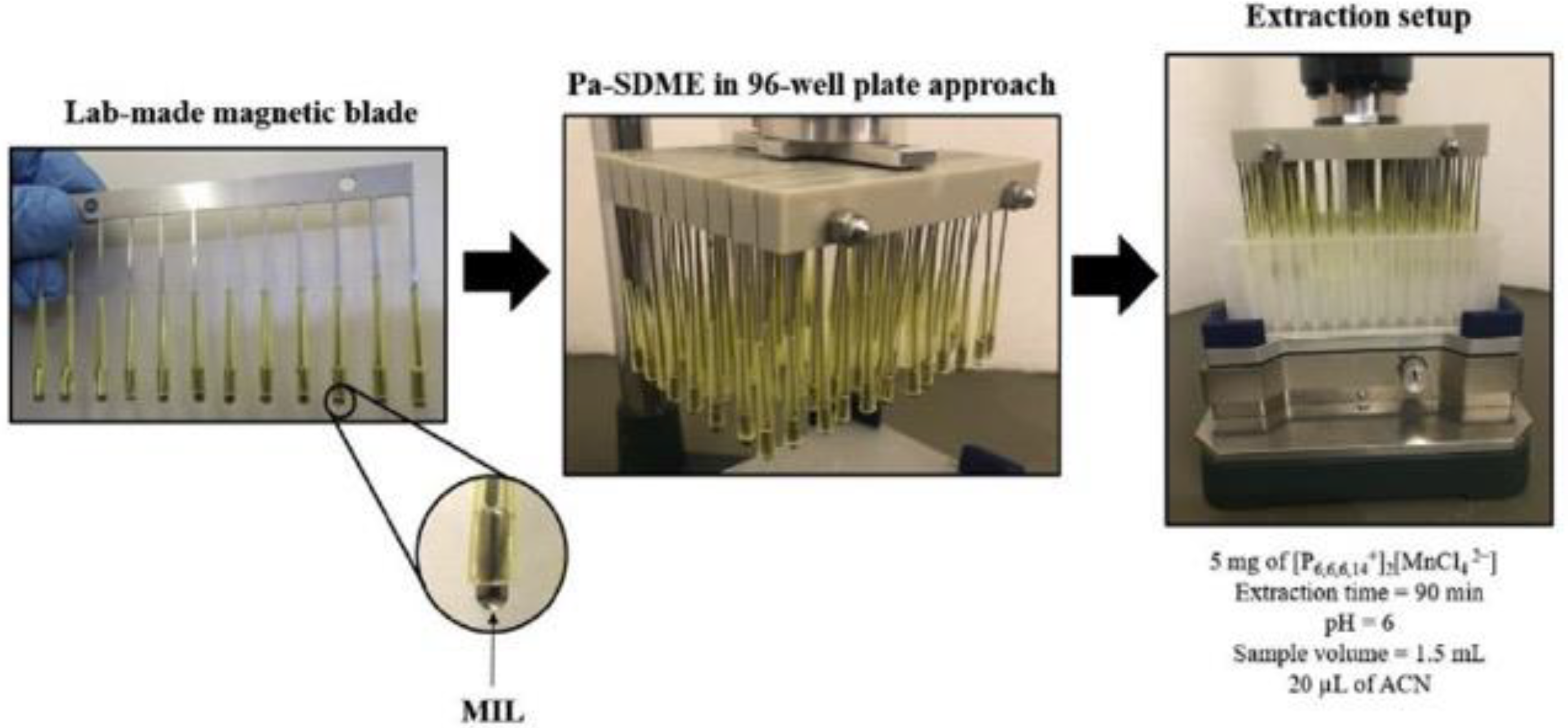
3.1. Magnetic Ionic Liquids (MILs) as Green Extraction Phase
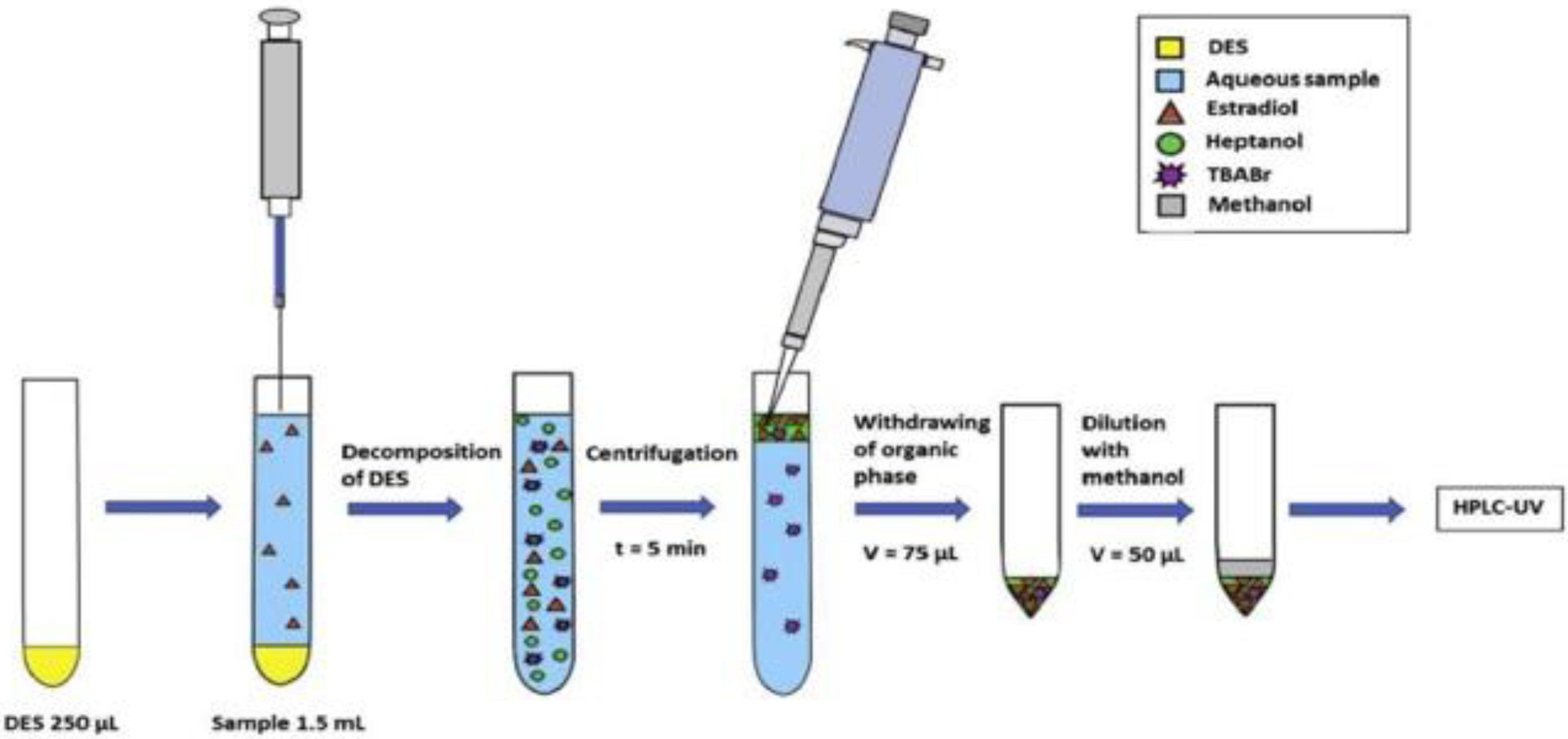
3.2. Deep Eutectic Solvent (DES) and Natural Deep Eutectic Solvents (NADES) as a Green Extraction Solvent
4. Supramolecular Solvent (SUPRAS) as Extraction Phase
5. Bio-Based Solvents
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Filippou, O.; Dimitrios, B.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 4–62. [Google Scholar] [CrossRef] [PubMed]
- Stocka, J.; Tankiewicz, M.; Biziuk, M.; Namieśnik, J. Green Aspects of Techniques for the Determination of Currently Used Pesticides in Environmental Samples. Int. J. Mol. Sci. 2011, 12, 7785–7805. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green extraction techniques in green analytical chemistry. Trac-Trend Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyaci, E. Thin film microextraction: Towards faster and more sensitive microextraction. Trac-Trend Anal. Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- Carasek, E.; Merib, J.; Mafra, G.; Spudeit, D. A recent overview of the application of liquid-phase microextraction to the determination of organic micro-pollutants. Trac-Trend Anal. Chem. 2018, 108, 203–209. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Hosseinia, M.R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A. 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, M.A.; Cantwell, F.F. Solvent microextraction into a single drop. Anal. Chem. 1996, 68, 2236–2240. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A. Green Extraction Techniques Principles, Advances and Applications. In Comprehensive Analytical Chemistry, 1st ed.; Ibanez, E., Cifuentes, A., Eds.; Elsevier: New York, NY, USA, 2017; Volume 76, pp. 519–573. [Google Scholar]
- Armenta, S.; de la Guardia, M.; Mamiesnik, J. Green Microextractions. In Analytical Microextraction Techniques; Valcárcel, M., Cárdenas, S., Lucena, R., Eds.; Bentham Science Publishers: Sharjah, UAE, 2017; pp. 4–22. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Araujo, L.A.; Bezerra, C.O.; Cusioli, L.F.; Silva, M.F.; Nishi, L.; Gomes, R.G.; Bergamasco, R. Moringa oleifera biomass residue for the removal of pharmaceuticals from water. J. Environ. Chem. Eng. 2018, 6, 7192–7199. [Google Scholar] [CrossRef]
- Neto, C.P.; Rocha, J.; Gil, A.; Cordeiro, N.; Esculcas, A.P.; Rocha, S.; Delgadillo, I.; De Jesus, J.D.P.; Correia, A.J.F. 13C solid-state nuclear magnetic resonance and Fourier transform infrared studies of the thermal decomposition of cork. Solid State Nucl. Mag. Reson. 1995, 4, 143–151. [Google Scholar] [CrossRef]
- Dias, A.N.; Simão, V.; Merib, J.; Carasek, E. Cork as a new (green) coating for solid-phase microextraction: Determination of polycyclic aromatic hydrocarbons in water samples by gas chromatography–mass spectrometry. Anal. Chim. Acta 2013, 772, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.N.; Simão, V.; Merib, J.; Carasek, E. Use of green coating (cork) in solid-phase microextraction for the determination of organochlorine pesticides in water by gas chromatography-electron capture detection. Talanta 2015, 134, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Dias, A.N.; Carasek, E. Exploiting Cork as Biosorbent Extraction Phase for Solid-Phase Microextraction to Determine 3-(4-Methylbenzylidene)camphor and 2-Ethylhexyl 4-(Dimethylamino)benzoate in River Water by Gas Chromatography-Mass Spectrometry. J. Braz. Chem. Soc. 2017, 28, 2341–2347. [Google Scholar] [CrossRef]
- Morés, L.; Dias, A.N.; Carasek, E. Development of a high-throughput method based on thin-film microextraction using a 96-well plate system with a cork coating for the extraction of emerging contaminants in river water samples. J. Sep. Sci. 2018, 41, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.N.; da Silva, A.C.; Simão, V.; Merib, J.; Carasek, E. A novel approach to bar adsorptive microextraction: Cork as extractor phase for determination of benzophenone, triclocarban and parabens in aqueous samples. Anal. Chim. Acta 2015, 888, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Oenning, A.L.; Morés, L.; Dias, A.N.; Carasek, E. A new configuration for bar adsorptive microextraction (BAμE) for the quantification of biomarkers (hexanal and heptanal) in human urine by HPLC providing an alternative for early lung cancer diagnosis. Anal. Chim. Acta 2017, 965, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.N.; Merib, J.; Dias, A.N.; Stolberg, J.; Budziak, D.; Carasek, E. A low-cost biosorbent-based coating for the highly sensitive determination of organochlorine pesticides by solid-phase microextraction and gas chromatography-electron capture detection. J. Chromatogr. A 2017, 1525, 23–31. [Google Scholar] [CrossRef]
- Suterio, N.; do Carmo, S.; Budziak, D.; Merib, J.; Carasek, E. Use of a Natural Sorbent as Alternative Solid-Phase Microextraction Coating for the Determination of Polycyclic Aromatic Hydrocarbons in Water Samples by Gas Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2000, 38, 55–60. [Google Scholar] [CrossRef]
- Do Carmo, S.N.; Merib, J.; Carasek, E. Bract as a novel extraction phase in thin-film SPME combined with 96-well plate system for the high-throughput determination of estrogens in human urine by liquid chromatography coupled to fluorescence detection. J. Chromatogr. B 2019, 1118–1119, 17–24. [Google Scholar] [CrossRef]
- Reinert, N.P.; Vieira, C.M.S.; Da Silveira, C.B.; Budziak, D.; Carasek, E. A Low-Cost Approach Using Diatomaceous Earth Biosorbent as Alternative SPME Coating for the Determination of PAHs in Water Samples by GC-MS. Separations 2018, 5, 55. [Google Scholar] [CrossRef]
- Kirschner, N.; Dias, A.N.; Budziak, D.; da Silveira, C.B..; Merib, J.; Carasek, E. Novel approach to high-throughput determination of endocrine disruptors using recycled diatomaceous earth as a green sorbent phase for thin-film solid-phase microextraction combined with 96-well plate system. Anal. Chim. Acta 2017, 996, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mafra, G.; Oenning, A.L.; Dias, A.N.; Merib, J.; Budziak, D.; da Silveira, C.B.; Carasek, E. Low-cost approach to increase the analysis throughput of bar adsorptive microextraction (BAµE) combined with environmentally-friendly renewable sorbent phase of recycled diatomaceous earth. Talanta 2018, 178, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.S.; Liu, Y.L.; Zhou, J.B.; Chen, X.F.; Wang, X. Bamboo charcoal as a novel solid-phase microextraction coating material for enrichment and determination of eleven phthalate esters in environmental water samples. Anal. Bioanal. Chem. 2013, 405, 4993–4996. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Basheer, C.; Alshara, A.; Narasimhan, K.; Buhmeida, A.; Al Qahtani, M.; Al-Ahwal, M.S. Development of natural sorbent based micro-solid-phase extraction for determination of phthalate esters in milk samples. Anal. Chim. Acta 2016, 924, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fiscal-Ladino, J.A.; Obando-Ceballos, M.; Rosero-Moreano, M.; Montaño, D.F.; Cardona, W.; Giraldo, L.F.; Richter, P. Ionic liquids intercalated in montmorillonite as the sorptive phase for the extraction of low-polarity organic compounds from water by rotating-disk sorptive extraction. Anal. Chim. Acta 2017, 953, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.M.S.; Mazurkievicz, M.; Lopez, A.; Debatin, V.; Micke, G.; Richter, P.; Rosero Moreano, M.; Carasek, E. Exploiting green sorbents in rotating-disk sorptive extraction for the determination of parabens by high performance liquid chromatography tandem electrospray ionization triple quadrupole mass spectrometry. J. Sep. Sci. 2018, 41, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic Liquids in Analytical Chemistry: Fundamentals, Advances, and Perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Han, D.; Tang, B.; Lee, Y.R.; Row, K.H. Application of ionic liquid in liquid phase microextraction technology. J. Sep. Sci. 2012, 35, 2949–2961. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, L.; Lu, R.; Zhou, W.; Gao, H. Application of ionic liquids for liquid–liquid microextraction. Anal. Methods 2013, 5, 5376–5385. [Google Scholar] [CrossRef]
- Escudero, L.B.; Grijalba, A.C.; Martinis, E.M.; Wuilloud, R.G. Bioanalytical separation and preconcentration using ionic liquids. Anal. Bioanal. Chem. 2013, 405, 7597–7613. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Emaus, M.N.; Varona, M.; Bowers, A.N.; Anderson, J.L. Ionic Liquids: Solvents and Sorbents in Sample Preparation. J. Sep. Sci. 2018, 41, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, G.; Chi, Y.; Cai, Y.; Zhou, Q.; Hu, J.-T. Use of Ionic Liquids for Liquid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Anal. Chem. 2003, 75, 5870–5876. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, L.; Pena-Pereira, F.; Kloskowski, A.; Namiesnik, J. Opportunities and shortcomings of ionic liquids in single-drop microextraction. Trac-Trend Anal. Chem. 2015, 72, 153–168. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. Trac-Trend Anal. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Abujaber, F.; Ricardo, A.I.C.; Ríos, A.; Bernardo, F.J.G.; Martín-Doimeadios, R.C.R. Ionic liquid dispersive liquid-liquid microextraction combined with LC-UV-Vis for the fast and simultaneous determination of cortisone and cortisol in human saliva samples. J. Pharm. Biomed. Anal. 2019, 165, 141–146. [Google Scholar] [CrossRef]
- Clark, K.D.; Nacham, O.; Purslow, J.A.; Pierson, S.A.; Anderson, J.L. Magnetic ionic liquids in analytical chemistry: A review. Anal. Chim. Acta 2016, 934, 9–21. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Xu, B.; Li, X.; Jin, R.; Zhang, H.; Song, D. Magnetic ionic liquid-based dispersive liquid-liquid microextraction for the determination of triazine herbicides in vegetable oils by liquid chromatography. J. Chromatogr. A 2014, 1373, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M. Magnetic ionic liquids in analytical sample preparation: A literature review. Trends Anal. Chem. 2019, 113, 210–223. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Anderson, J.L. In situ formation of hydrophobic magnetic ionic liquids for dispersive liquid-liquid microextraction. J. Chromatogr. A 2019, 1588, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mafra, G.; Vieira, A.A.; Merib, J.; Anderson, J.L.; Carasek, E. Single drop microextraction in a 96-well plate format: A step toward automated and high-throughput analysis. Anal. Chim. Acta 2019, 1063, 159–166. [Google Scholar] [CrossRef]
- Cao, D.; Xua, X.; Xuea, S.; Fenga, X.; Zhanga, L. An in situ derivatization combined with magnetic ionic liquid-based fast dispersive liquid-liquid microextraction for determination of biogenic amines in food samples. Talanta 2019, 199, 212–219. [Google Scholar] [CrossRef]
- Silva, A.C.; Mafra, G.; Spudeit, D.; Merib, J.; Carasek, E. Magnetic ionic liquids as an efficient tool for the multiresidue screening of organic contaminants in river water samples. Sep. Sci. Plus 2019, 2, 51–58. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Anderson, J.L. In situ generation of hydrophobic magnetic ionic liquids in stir bar dispersive liquid-liquid microextraction coupled with headspace gas chromatography. Talanta 2019, 196, 420–428. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Pierson, S.A.; Anderson, J.L.; Stalikas, C.D. Enhanced magnetic ionic liquid-based dispersive liquid-liquid microextraction of triazines and sulfonamides through a one-pot, pH-modulated approach. J. Chromatogr. A 2018, 1571, 47–54. [Google Scholar] [CrossRef]
- Merib, J.; Spudeit, D.A.; Corazza, G.; Carasek, E.; Anderson, J.L. Magnetic ionic liquids as versatile extraction phases for the rapid determination of estrogens in human urine by dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography-diode array detection. Anal. Bioanal. Chem. 2018, 410, 4689–4699. [Google Scholar] [CrossRef]
- Benedé, J.L.; Anderson, J.L.; Chisvert, A. Trace determination of volatile polycyclic aromatic hydrocarbons in natural waters by magnetic ionic liquid-based stir bar dispersive liquid microextraction. Talanta 2018, 176, 253–261. [Google Scholar] [CrossRef]
- An, J.; Rahn, K.L.; Anderson, J.L. Headspace single drop microextraction versus dispersive liquid-liquid microextraction using magnetic ionic liquid extraction solvents. Talanta 2017, 167, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Chisvert, A.; Benedé, J.L.; Anderson, J.L.; Pierson, S.A.; Salvador, A. Introducing a new and rapid microextraction approach based on magnetic ionic liquids: Stir bar dispersive liquid microextraction. Anal. Chim. Acta 2017, 983, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Pino, V.; Anderson, J.L. Magnetic ionic liquids as extraction solvents in vacuum headspace single drop microextraction. Talanta 2017, 172, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, A.P.G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; McIntosh, A.J.S.; Welton, T.; Branco, L.C.; Marrucho, I.M. A closer look into deep eutectic solvents: Exploring intermolecular interactions using solvatochromic probes. Phys. Chem. Chem. Phys. 2018, 20, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Sattari, D.M.; Yadeghari, A. Deep eutectic solvent based gas-assisted dispersive liquid-phase microextraction combined with gas chromatography and flame ionization detection for the determination of some pesticide residues in fruit and vegetable samples. J. Sep. Sci. 2017, 40, 2253–2260. [Google Scholar] [CrossRef]
- Ge, D.; Zhang, Y.; Dai, Y.; Yang, S. Air-assisted dispersive liquid-liquid microextraction based on a new hydrophobic deep eutectic solvent for the preconcentration of benzophenone-type UV filters from aqueous samples. J. Sep. Sci. 2018, 41, 1635–1643. [Google Scholar] [CrossRef]
- Shishov, A.; Chromá, R.; Vakha, C.; Kuchár, J.; Simon, A.; Andruch, A.; Bulatov, A. In situ decomposition of deep eutectic solvent as a novel approach in liquid-liquid microextraction. Anal. Chim. Acta 2019, 1065, 49–55. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Dehdashtian, S. Application of green solvents as sorbent modifiers in sorptive-based extraction techniques for extraction of environmental pollutants. Trac-Trend Anal. Chem. 2018, 109, 50–61. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trac-Trend Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Lamei, N.; Ezoddin, M.; Abdi, K. Air assisted emulsification liquid-liquid microextraction based on deep eutectic solvent for preconcentration of methadone in water and biological samples. Talanta 2017, 165, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, L.; Liu, X.; Yin, S.; Lu, R.; Zhang, S.; Zhou, W.; Gao, H. Deep eutectic solvent-based ultrasound-assisted dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for the determination of ultraviolet filters in water samples. J. Chromatogr. A 2017, 1516, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Ahmadi, R.; Ramezani, A.M. Vortex-assisted liquid-liquid microextraction based on hydrophobic deep eutectic solvent for determination of malondialdehyde and formaldehyde by HPLC-UV approach. Microchem. J. 2018, 143, 166–174. [Google Scholar] [CrossRef]
- Rajabi, M.; Ghassa, N.; Hemmati, M.; Asghari, A. Emulsification microextraction of amphetamine and methamphetamine in complex matrices using an up-to-date generation of eco-friendly and relatively hydrophobic deep eutectic solvent. J. Chromatogr. A 2018, 1576, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Torbatia, M.; Mohebbi, A.; Farajzadeh, M.A.; Mogaddam, M.R.A. Simultaneous derivatization and air–assisted liquid–liquid microextraction based on solidification of lighter than water deep eutectic solvent followed by gas chromatography–mass spectrometry: An efficient and rapid method for trace analysis of aromatic amines in aqueous samples. Anal. Chim. Acta 2018, 1032, 48–55. [Google Scholar] [CrossRef]
- Makoś, P.; Przyjazny, A.; Boczkj, G. Hydrophobic deep eutectic solvents as “green” extraction media for polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 2018, 1570, 28–37. [Google Scholar] [CrossRef]
- Yaripour, A.M.S.; Farajzadeh, M.A.; Mogaddam, M.R.A. Combination of dispersive solid phase extraction and deep eutectic solvent–based air–assisted liquid–liquid microextraction followed by gas chromatography–mass spectrometry as an efficient analytical method for the quantification of some tricyclic antidepressant drugs in biological fluids. J. Chromatogr. A 2018, 1571, 84–93. [Google Scholar] [CrossRef]
- Yanga, D.; Wang, Y.; Peng, J.; Xun, C.; Yang, Y. A green deep eutectic solvents microextraction coupled with acid-base induction for extraction of trace phenolic compounds in large volume water samples. Ecotoxicol. Environ. Safe 2019, 178, 130–136. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Qian, H.; Lu, R.; Zhang, S.; Zhou, W.; Gao, H.; Xu, D. Vortex-assisted deep eutectic solvent reversed-phase liquid–liquid microextraction of triazine herbicides in edible vegetable oils. J. Chromatogr. A 2019, 1589, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Torbati, M.; Farajzadeh, M.A.; Mogaddam, M.R.A.; Torbati, M. Development of microwave-assisted liquid-liquid extraction combined with lighter than water in syringe dispersive liquid-liquid microextraction using deep eutectic solvents: Application in extraction of some herbicides from wheat. Microchem. J. 2019, 147, 1103–1108. [Google Scholar] [CrossRef]
- Deng, W.; Yu, L.; Li Ji, X.; Xuanxuan, C.; Zixin, W.; Xiao, D.Y. Hexafluoroisopropanol-based hydrophobic deep eutectic solvents for dispersive liquid-liquid microextraction of pyrethroids in tea beverages and fruit juices. Food Chem. 2019, 274, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Qian, H.; Qu, Y.; Zhang, S.; Lu, R.; Gao, H.; Zhou, W. Ultrasound-assisted dispersive liquid-liquid microextraction based on a hydrophobic deep eutectic solvent for the preconcentration of pyrethroid insecticides prior to determination by high-performance liquid chromatography. Microchem. J. 2019, 146, 614–621. [Google Scholar] [CrossRef]
- Soylak, M.; Khan, M.; Yilmaz, E. Switchable solvent based liquid phase micro- extraction of uranium in environmental samples: A green approach. Anal. Methods 2016, 8, 979–986. [Google Scholar] [CrossRef]
- Costi, E.M.; Sicilia, M.D.; Rubio, S. Supramolecular solvents in solid sample microextractions: Application to the determination of residues of oxolinic acid and flumequine in fish and shellfish. J. Chromatogr. A 2010, 1217, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Moral, A.; Sicilia, M.D.; Rubio, S. Determination of benzimidazolic fungicides in fruits and vegetables by supramolecular solvent-based microextraction/liquid chromatography/fluorescence detection. Anal. Chim. Acta 2009, 650, 207–213. [Google Scholar] [CrossRef]
- Feizi, N.Y.; Moradi, Y.M.; Karimi, M.; Salamata, Q.; Amanzadeh, H. A new generation of nano–structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal. Chim. Acta 2017, 953, 1–9. [Google Scholar] [CrossRef]
- Caballero-Casero, N.; Çabuk, H.; Martínez-Sagarra, G.; Devesa, J.A.; Rubio, S. Nanostructured alkyl carboxylic acid–based restricted access solvents: Application to the combined microextraction and cleanup of polycyclic aromatic hydrocarbons in mosses. Anal. Chim. Acta 2015, 890, 124–133. [Google Scholar] [CrossRef]
- Zohrabi, P.; Shamsipur, M.; Hashemi, M.; Hashemi, B. Liquid–phase microextraction of organophosphorus pesticides using supramolecular solvent as a carrier for ferrofluid. Talanta 2016, 160, 340–346. [Google Scholar] [CrossRef]
- Salatti-Dorado, J.A.; Caballero-Casero, N.; Sicilia, M.D.; Lunar, M.L.; Rubio, S. The use of a restricted access volatile supramolecular solvent for the LC/MS–MS assay of bisphenol A in urine with a significant reduction of phospholipid–based matrix effects. Anal. Chim. Acta 2017, 950, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gissawong, N.; Boonchiangma, S.; Mukdasai, S.; Srijaranai, S. Vesicular supramolecular solvent-based microextraction followed by high performance liquid chromatographic analysis of tetracyclines. Talanta 2019, 200, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, W.; Li, X.; Wang, X.; Xiao, Y. Hexafluoroisopropanol/Brij-35 based supramolecular solvent for liquid-phase microextraction of parabens in different matrix samples. J. Chromatogr. A 2019, 1591, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio- derived solvents in solvent extraction applications: A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Chemat, S.; Tomao, V.; Chemat, F. Limonene as Green Solvent for Extraction of Natural Products. In Green Solvents I; Mohammad, A., Inamuddin, Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 175–186. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total lipid extraction of food using d- limonene as an alternative to n-hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Pourreza, N.; Naghdi, T. D-Limonene as a green bio-solvent for dispersive liquid–liquid microextraction of b-cyclodextrin followed by spectrophotometric determination. J. Ind. Eng. Chem. 2017, 51, 71–76. [Google Scholar] [CrossRef]
- Tobiszewski, M. Analytical chemistry with biosolvents. Anal. Bioanal. Chem. 2019, 1–6. [Google Scholar] [CrossRef]

| Biosorbent | Technique | Analyte | Matrix | LOQ | LOD | Linear Range | Recovery (%) | Precision (RSD%) | Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Cork | SPME | polycyclic aromatic hydrocarbons (PAHs) | River water | 0.1 μg L−1 | 0.03 μg L−1 | 0.1–10 μg L−1 | 70–103 | 1.9–15.7 | GC-MS | [14] |
| Organochlorine pesticides | River water | 1–10 ng L−1 | 0.3–3 ng L−1 | 1–50 ng L−1 | 60–112 | 0.5–25.5 | GC-ECD | [15] | ||
| UV filters | River water | 0.01–0.1 μg L−1 | 0.004–0.03 μg L−1 | 0.01–0.5 μg L−1 | 67–107 | 3–18 | GC-MS | [16] | ||
| TFME | Emerging contaminants | River water | 0.8–15 μg L−1 | 0.3–5.5 µg L−1 | 5–400 μg L−1 | 72–125 | 4–18 | HPLC-DAD | [17] | |
| BAμE | Parabens, benzophenone and triclocarban | Lake water, effluent, wastewater | 1.6–20 μg L−1 (15 mm) | 0.5–6.5 µg L−1 (15 mm) | 1.6–500 µg L−1 (15 mm) | 65–123 (7.5 mm) | 3–22 (7.5 mm) | HPLC-DAD | [18] | |
| 0.64–8 μg L−1 (7.5 mm) | 0.2–2.5 µg L−1 (7.5 mm) | 0.64–400 µg L−1 (7.5 mm) | ||||||||
| Hexanal and heptanal | Human urine | 2.19–3 μmol L−1 | 0.73–1 μmol L−1 | 2.19–8 μmol L−1 | 88–111 | 3–7 | HPLC-DAD | [19] | ||
| Bract | SPME | Organochlorine pesticides | River and lake water | 0.65–2.38 ng L−1 | 0.19–0.71 ng L−1 | 5–100 ng L− | 60–110 | 5–19 | GC-ECD | [20] |
| PAHs | Lake water | 0.01–0.1 μg L−1 | 0.003–0.03 μg L−1 | 0.01–4 μg L−1 | 68–117 | 0.6–17 | GC-MS | [21] | ||
| TFME | Steroid estrogens | Human urine | 0.1–10 µg L−1 | 0.3–3 µg L−1 | 0.1–400 μg L−1 | 71–105 | 1–17 | HPLC-FLD | [22] | |
| Diatomaceous earth | SPME | PAHs | River water | 0.1–0.5 µg L−1 | 0.03–0.16 µg L−1 | 0.1–25 μg L−1 | 83–100 | 2–15 | GC-MS | [23] |
| TFME | Endocrine disruptors | River water | 3–23 µg L−1 | 1–8 µg L−1 | 5–285 µg L−1 | 70–117 | 1–21 | HPLC-DAD | [24] | |
| BAμE | Methyl and ethyl paraben, benzophenone, triclocarban | Lake water | 0.63–6.9 µg L−1 | 0.19–2 µg L−1 | 0.63–100 µg L−1 | 63–124 | 1–20 | HPLC-DAD | [25] | |
| Bamboo charcoal | SPME | Phthalate esters | Tap and river water | 0.004–0.023 µg L−1 | 0.1–100 µg L−1 | 61–87 | 1.89–9.85 | GC-MS | [26] | |
| Moringa oleifera seeds | µ-SPE | Phthalate esters | Milk | 0.1–3.7 µg L−1 | 0.01–1.2 µg L−1 | 1–100 µg L−1 | 77–103 | 3.6–9.4 | GC-MS | [27] |
| MMT clay | RDSE | polychlorinated biphenyl (PCB) | Wastewater | 6.5–103.8 ng L−1 | 3 ng L−1 to 43 ng L−1 | 80–86 | 2–24 | GC-ECD | [28] | |
| Cork and MMT clay | RDSE | Parabens | River and tap water | 0.8 µg L−1 (cork) | 0.24 µg L−1 (cork) | 0.8–75 µg L−1 (cork) | 80–118 (cork) | 1.15–14.29 (cork) | LC-MS/MS | [29] |
| 3 µg L−1 (MMT clay) | 0.90 µg L−1 (MMT clay) | 3–100 µg L−1 (MMT clay) | 80–119 (MMT clay) | 3.24–18.14 (MMT clay) |
| Method | MIL | Analyte | Matrix | LOD | Instrumentation | Ref. |
|---|---|---|---|---|---|---|
| In situ DLLME | [P6,6,6,14+]2[CoCl42−] | Biogenic amines | Wine fish | 1.3–3.9 μg L−1 1.2–3.8 μg kg−1 | HPLC-UV | [47] |
| DLLME | [P6,6,6,14+] [Cl−] | Estriol Estrone Parabens Carbamazepine Diazepam Ketoprofen Ibuprofen 17α-Ethynylestradiol Triclocarban Aldicarb Methyl parathion Metolachlor Diuron Bisphenol A | River water | 1.5–15 μg L−1 | HPLC-DAD | [48] |
| In situ SB-DLLME | [Ni(C4IM)42+]2[Cl−] [Ni(C8IM)42+]2[Cl−] [Co(C8IM)42+]2[Cl−] | Naphthalene Acenaphthene Fluorene 1-chloro-4-nitrobenzene Biphenyl 5-Bromoacenaphthene 3-Tert-butylphenol | Tap and mineral water | 4.8–15 μg L−1 1–10 μg L−1 5.9–30 μg L−1 | HS-GC-MS | [49] |
| DLLME | P66614+] [Dy(III)(hfacac)4−] | Triazines and sulfonamides | Lake water, effluent wastewater | 0.011–0.03 μg L−1 | HPLC-DAD | [50] |
| DLLME | [P6,6,6,14+]2[MnCl42−] | Estrogens | Human urine | 2 ng mL−1 | HPLC-DAD | [51] |
| SB-DLME | [P6,6,6,14+] [Ni(II)(hfaca)3−] | PAHs | River water and rain water | 1.7–28.7 ng L−1 | GC-MS | [52] |
| HS-SDME and DLLME | [P6,6,6,14+]2[MnCl42−] | Aromatic compounds | Lake water | 0.005–1 μg L−1 and 0.04–1 μg L−1 | HPLC-DAD | [53] |
| SB-DLME | [P6,6,6,14+] [Ni(hfacac)3−] | UV filters | River and sea water | 9.9–26.7 ng L−1 | GC-MS | [54] |
| Vacum-HS-SDME | [P6,6,6,14+] [Mn(hfacac)3−] | Free fatty acids | Milk | 14.5–216 μg L−1 | GC-MS | [55] |
| Technique | DES/NADES Composition | Analytes | Matrix | LOD | Instrumentation | Ref. |
|---|---|---|---|---|---|---|
| DES-ALLME | Ch-Cl: TNO 1 | Methadone | Water and biologic | 0.7 µg L−1 | GC-FID | [65] |
| UA-DLLME | trioctylmethylammonium chloride: decanoic acid | UV filters | Water | 0.15–0.30 ng mL−1 | HPLC-UV | [66] |
| VA-LLME | Decanoic acid: Methyltrioctylammonium bromide | Malondialdehyde (MDA) and Formaldehyde (FA) | Human urine, apple juice and rain water | 2.0 and 10.0 ng mL−1 | HPLC-UV | [67] |
| AA-EME | ChCl: Ph-EtOH | Amphetamine-type stimulants (Ats) | Human plasma and pharmaceutical wastewater | 2.0–5.0 ng mL−1 | HPLC-UV | [68] |
| SFO–AALLME | Ch-Cl: n-butyric acid | Aromatic amines | Aqueous samples | 1.8–6.0 ng L−1 | GC-MS | [69] |
| UA-DLLME | thymol, ±camphor, decanoic: 10-undecylenic acids | PAHs | Industrial effluents | 0.0039–0.0098 μg L−1 | GC-MS | [70] |
| DSPE-DES-AALLME | ChCl: 4-chlorophenol | Tricyclic antidepressant drugs | Human urine and plasma | 8–15 and 32–60 ng L−1 | GC-MS | [71] |
| DES-GALLME | Mixture of two or three different carboxylic acids (C8, C9, C10, C11 and C12) | Phenolic compounds | Water | 0.22–0.53 μg L−1 | HPLC-UV | [72] |
| VA-RP-LLME | [N4444]Cl, TBA 2: ethylene glycol (EG) | Triazine herbicides | Vegetable oil samples | 0.60–1.50 μg L−1 | HPLC-UV | [73] |
| MA-in syringe DLLME | ChCl: phenol and ChCl: butyric acid | Herbicides | Wheat | 1.6–12 ng kg−1 | GC-MS | [74] |
| DLLME | Hexafluoro isopropanol: l-carnitine/betaine | Pyrethroids | Tea beverages and fruit juices | 0.06–0.17 ng mL−1 | HPLC | [75] |
| UA-DLLME-DES | Quaternary phosphonium salts: straight-chain monobasic acids | Pyrethroids | Water | 0.30–0.60 μg/L | HPLC-UV | [76] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carasek, E.; Bernardi, G.; do Carmo, S.N.; Vieira, C.M.S. Alternative Green Extraction Phases Applied to Microextraction Techniques for Organic Compound Determination. Separations 2019, 6, 35. https://doi.org/10.3390/separations6030035
Carasek E, Bernardi G, do Carmo SN, Vieira CMS. Alternative Green Extraction Phases Applied to Microextraction Techniques for Organic Compound Determination. Separations. 2019; 6(3):35. https://doi.org/10.3390/separations6030035
Chicago/Turabian StyleCarasek, Eduardo, Gabrieli Bernardi, Sângela N. do Carmo, and Camila M.S. Vieira. 2019. "Alternative Green Extraction Phases Applied to Microextraction Techniques for Organic Compound Determination" Separations 6, no. 3: 35. https://doi.org/10.3390/separations6030035
APA StyleCarasek, E., Bernardi, G., do Carmo, S. N., & Vieira, C. M. S. (2019). Alternative Green Extraction Phases Applied to Microextraction Techniques for Organic Compound Determination. Separations, 6(3), 35. https://doi.org/10.3390/separations6030035





