Dissolution Testing of Single- and Dual-Component Thyroid Hormone Supplements
Abstract
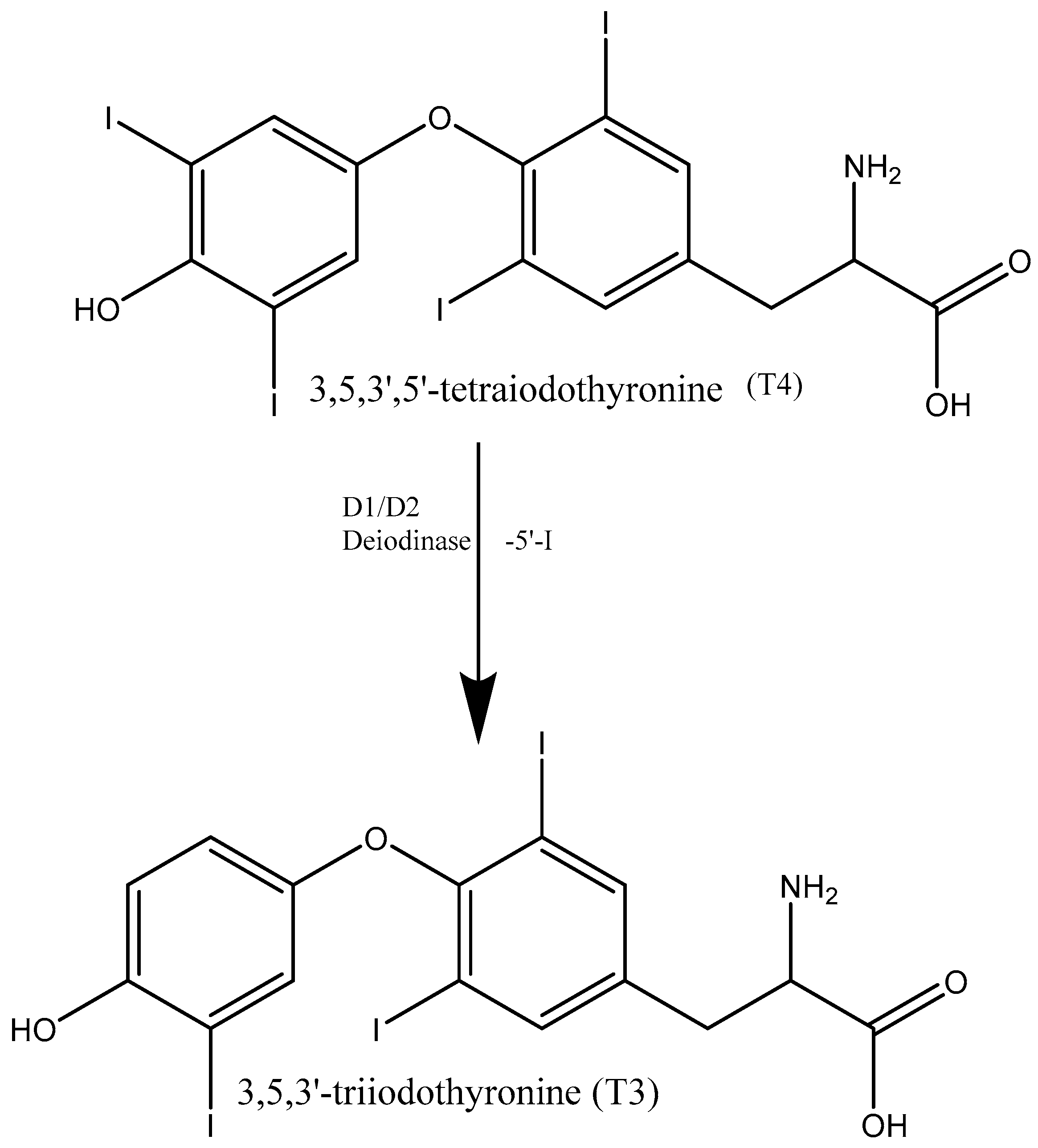
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
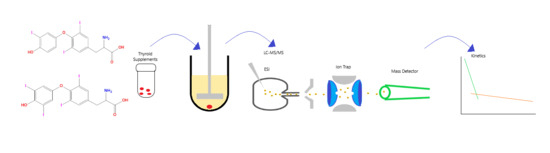
2.3. Dissolution
2.4. Preparation of Stock Solutions and Dissolution Media
2.5. Tablet Analysis
3. Results & Discussion
3.1. Tablet Analysis
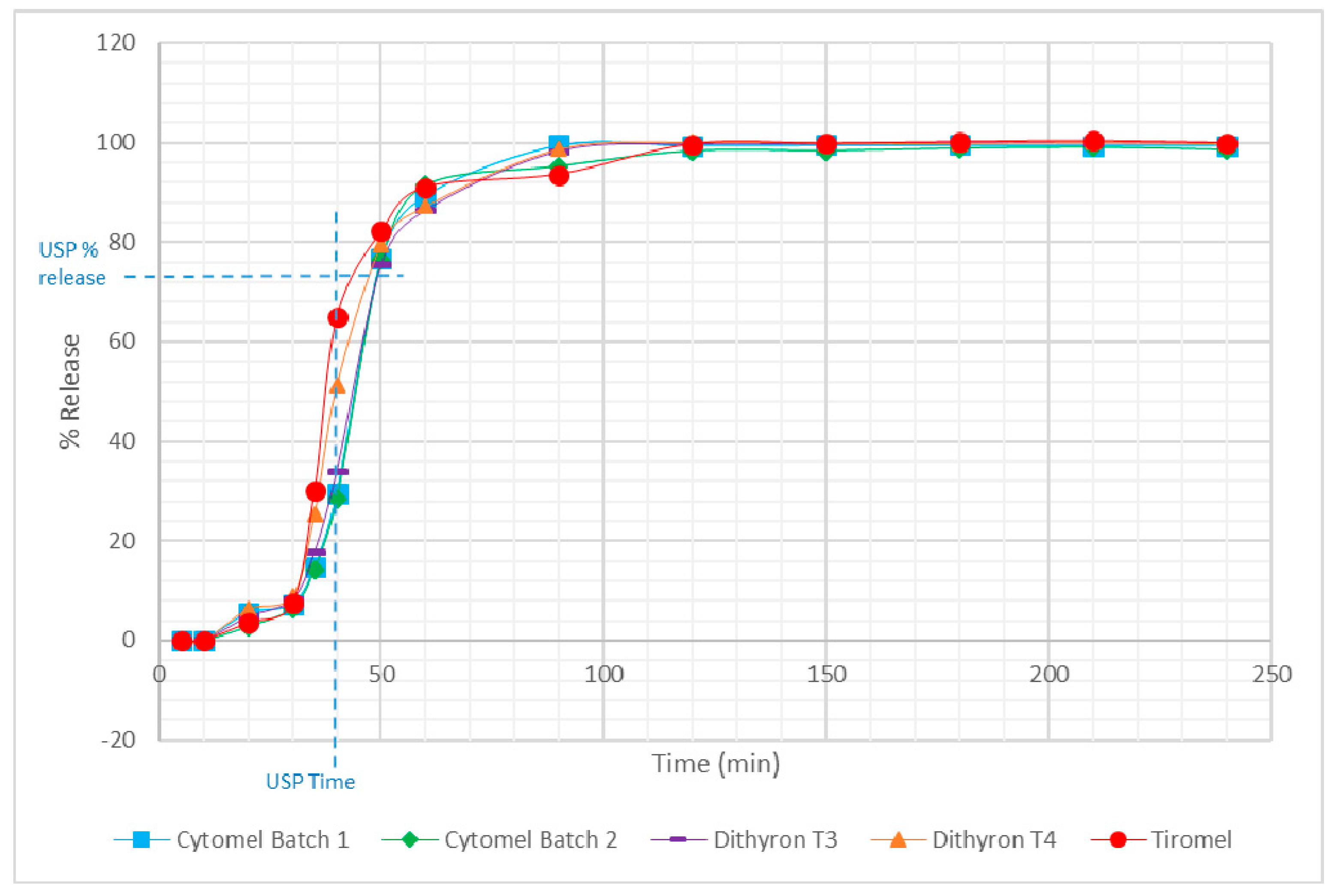
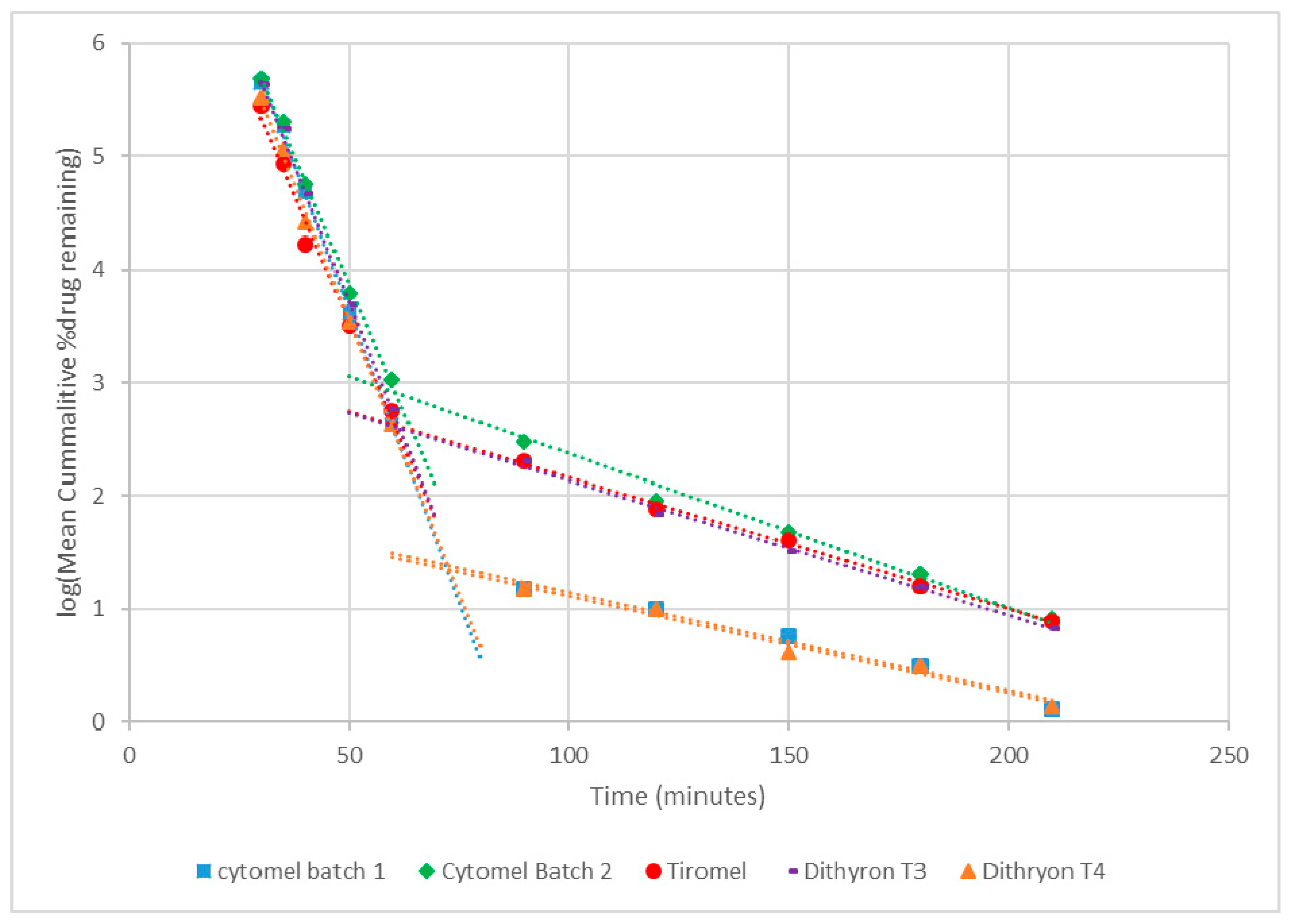
3.2. Dissolution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol. Metab. 2014, 25, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.M. Basic Medical Endocrinology, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2009; p. 17. [Google Scholar]
- Gu, J.; Soldin, O.P.; Soldin, S.J. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin. Biochem. 2007, 40, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017. [Google Scholar] [CrossRef]
- Dunn, D.; Turner, C. Hypothyroidism in women. Nurs. Women’s Health 2016, 20, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J. Hypothyroidism. Medicine 2017, 45, 506–509. [Google Scholar] [CrossRef]
- National Health Service. Hypothyroidism. Available online: https://www.nhs.uk/conditions/underactive-thyroid-hypothyroidism/ (accessed on 24 October 2018).
- Escobar-Morreale, H.F.; Botella-Carretero, J.I.; Morreale de Escobar, G. Treatment of hypothyroidism with levothyroxine or a combination of levothyroxine plus L-triiodothyronine. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregon, M.J.; Escobar del Rey, F.; Morreale de Escobar, G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroididm in all tissues, as studied in thyroidectomized rats. J. Clin. Investig. 1995, 96, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregon, M.J.; Hernandez, A.; Escobar del Rey, F.; Morreale de Escobar, G. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology 1997, 138, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregon, M.J.; del Escobar Rey, F.; Morreale de Escobar, G. Tissue-specific patterns of changes in 3,5,3-triiodo-L-thyronine concentrations. Biochimie 1999, 81, 453–462. [Google Scholar] [CrossRef]
- Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the biopharmaceutics classification system. Int. J. Pharm. 2006, 321, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grady, H.; Elder, D.; Webster, G.K.; Mao, Y.; Lin, Y.; Flanagan, T.; Mann, J.; Blanchard, A.; Cohen, M.J.; Lin, J.; et al. Industry’s view on using quality control, biorelevant, and clinically relevant dissolution tests for pharmaceutical development, registration, and commercialization. J. Pharm. Sci. 2018, 107, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Dressman, J.B.; Kramer, J. Pharmaceutical Dissolution Testing, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 4–15. [Google Scholar]
- Leigh, M.; Kloefer, B.; Schaich, M. Comparison of the solubility and dissolution of drugs in fasted-state biorelevant media (FaSSIF and FaSSIF-V2). Diss. Technol. 2013, 20, 44–50. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP36 NF31. In Official Monographs/Levothyroxine; United States Pharmacopeia: Rockville, MD, USA, 2013; pp. 4109–4110. [Google Scholar]
- United States Pharmacopeia. USP36 NF31. In Official Monographs/Liothyronine; United States Pharmacopeia: Rockville, MD, USA, 2013; pp. 4121–4123. [Google Scholar]
- Biorelevant. Biorelevant Instructions V1.1. Available online: https://biorelevant.com/ (accessed on 30 October 2018).
- ICH Guideline Validation of Analytical Procedures—Test and Methodology. Available online: http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html (accessed on 11 February 2019).
| Compound | Calibration Range (ng/mL) | No of Data Points | Linearity | R2 Value | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| T3 | 0–250 | 11 | Y = 83.622x − 196.97 | 0.9962 | 0.2 | 1.6 |
| T4 | 0–250 | 11 | Y = 27.338x + 10.442 | 0.9973 | 0.8 | 1.3 |
| Single or Dual Thyroid Hormone Component | Sample | Thyroid Hormone | Replicate | µg per Tablet (n = 3) ± SD | % Content (n = 3) (%RSD) | % Content per Batch (n = 9) (%RSD) |
|---|---|---|---|---|---|---|
| single | Cytomel (batch 1) | T3 | 1 | 24.4 ± 0.83 | 97.6 (3.4) | 95.6 (1.8) |
| 2 | 23.7 ± 0.27 | 95.0 (1.2) | ||||
| 3 | 23.6 ± 0.58 | 94.3 (2.4) | ||||
| single | Cytomel (batch 2) | T3 | 1 | 23.4 ± 0.46 | 93.6 (2.0) | 94.0 (0.8) |
| 2 | 23.7 ± 0.38 | 94.8 (1.6) | ||||
| 3 | 23.4 ± 0.51 | 93.5 (2.2) | ||||
| single | Tiromel | T3 | 1 | 23.3 ± 0.12 | 93.3 (0.5) | 94.4 (1.3) |
| 2 | 23.9 ± 0.06 | 95.7 (0.3) | ||||
| 3 | 23.5 ± 0.19 | 94.1 (0.8) | ||||
| dual | Dithyron | T3 | 1 | 12.4 ± 0.08 | 99.2 (0.6) | 96.3 (2.7) |
| 2 | 11.8 ± 0.13 | 94.4 (1.1) | ||||
| 3 | 11.9 ± 0.07 | 95.2 (0.6) | ||||
| Dithyron | T4 | 1 | 49.3 ± 0.21 | 98.6 (0.4) | 98.4 (0.9) | |
| 2 | 48.7 ± 0.09 | 97.4 (0.2) | ||||
| 3 | 49.6 ± 0.16 | 99.2 (0.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowerbank, S.L.; Carlin, M.G.; Dean, J.R. Dissolution Testing of Single- and Dual-Component Thyroid Hormone Supplements. Separations 2019, 6, 18. https://doi.org/10.3390/separations6010018
Bowerbank SL, Carlin MG, Dean JR. Dissolution Testing of Single- and Dual-Component Thyroid Hormone Supplements. Separations. 2019; 6(1):18. https://doi.org/10.3390/separations6010018
Chicago/Turabian StyleBowerbank, Samantha L., Michelle G. Carlin, and John R. Dean. 2019. "Dissolution Testing of Single- and Dual-Component Thyroid Hormone Supplements" Separations 6, no. 1: 18. https://doi.org/10.3390/separations6010018
APA StyleBowerbank, S. L., Carlin, M. G., & Dean, J. R. (2019). Dissolution Testing of Single- and Dual-Component Thyroid Hormone Supplements. Separations, 6(1), 18. https://doi.org/10.3390/separations6010018