Estimating Diphenylamine in Gunshot Residues from a New Tool for Identifying both Inorganic and Organic Residues in the Same Sample
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Chromatographic Conditions
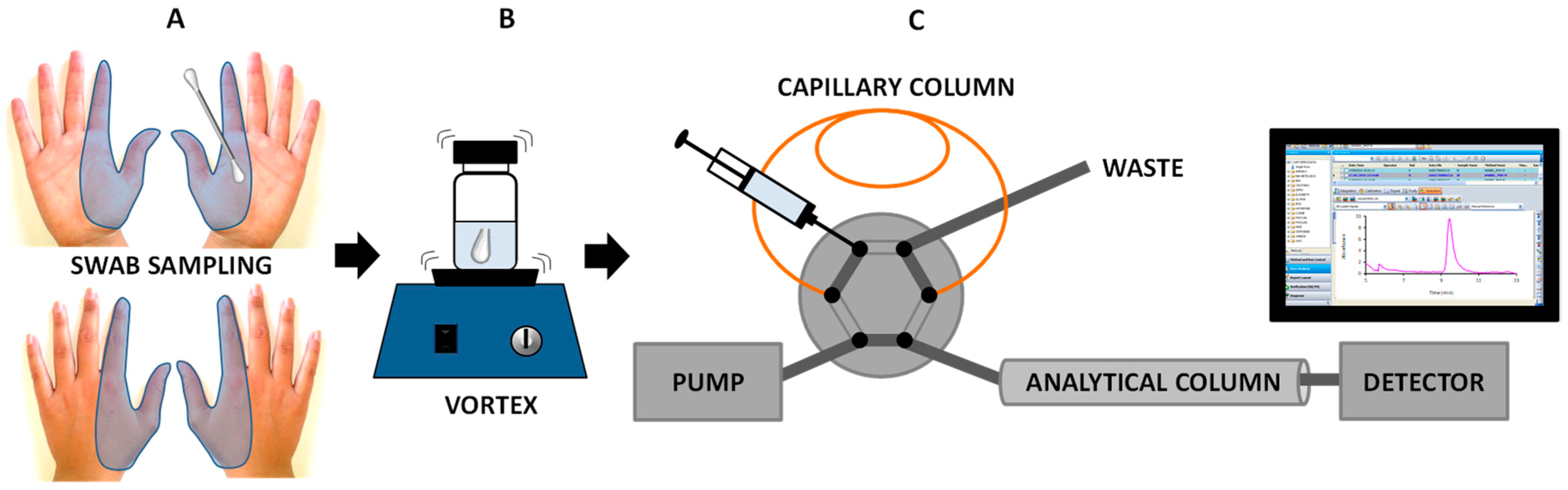
2.3. IT-SPME Procedure
2.4. Shooting and Collection of GSRs from Hands
2.5. Sample Treatment for DPA Analysis
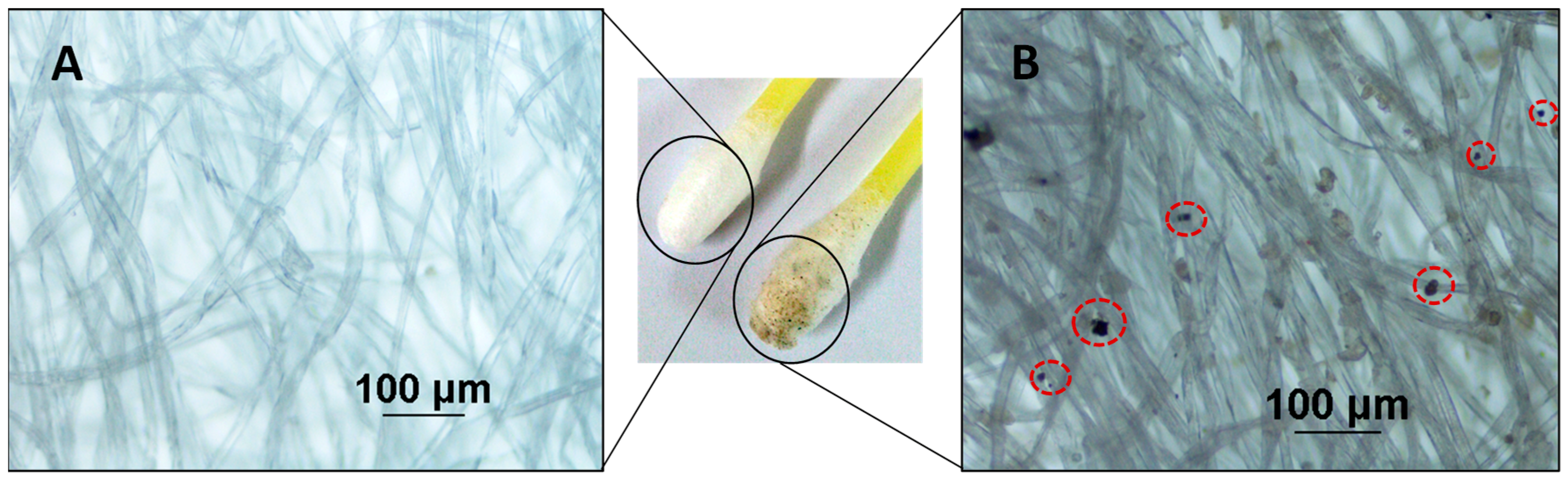
2.6. IGSRs Analysis by SEM/EDX and Optical Microscopy
3. Results and Discussion
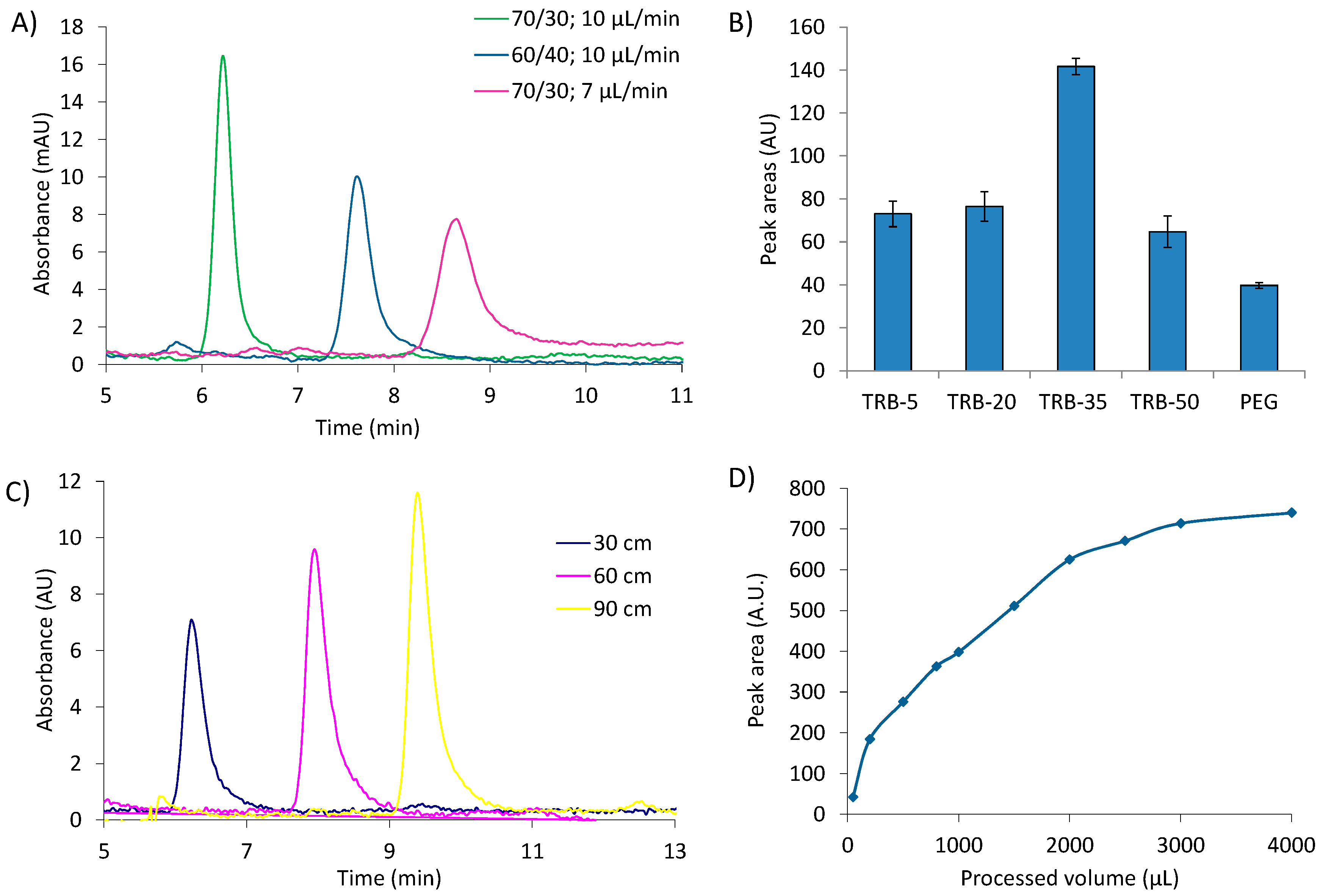
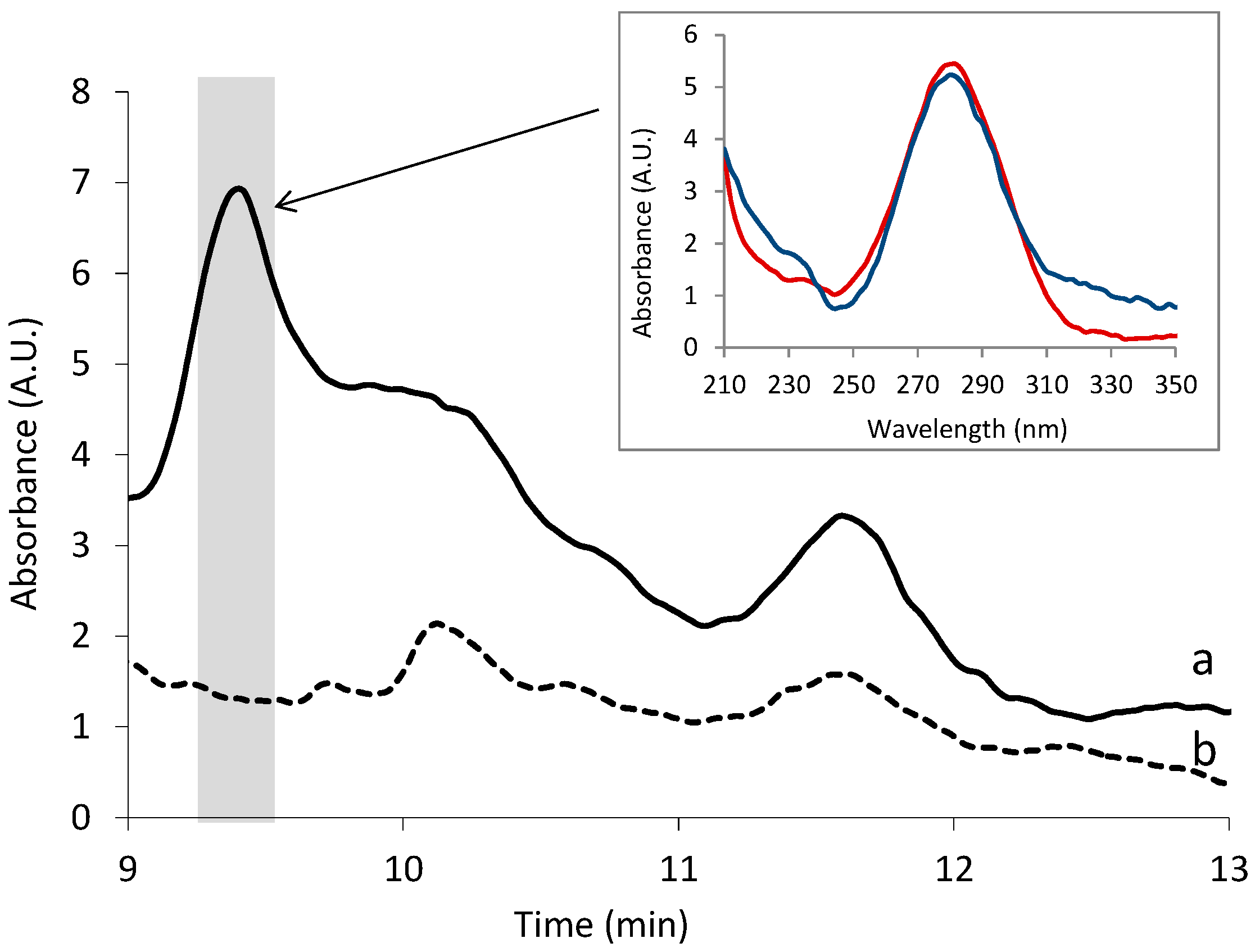
3.1. Optimization of the IT-SPME and Chromatographic Conditions
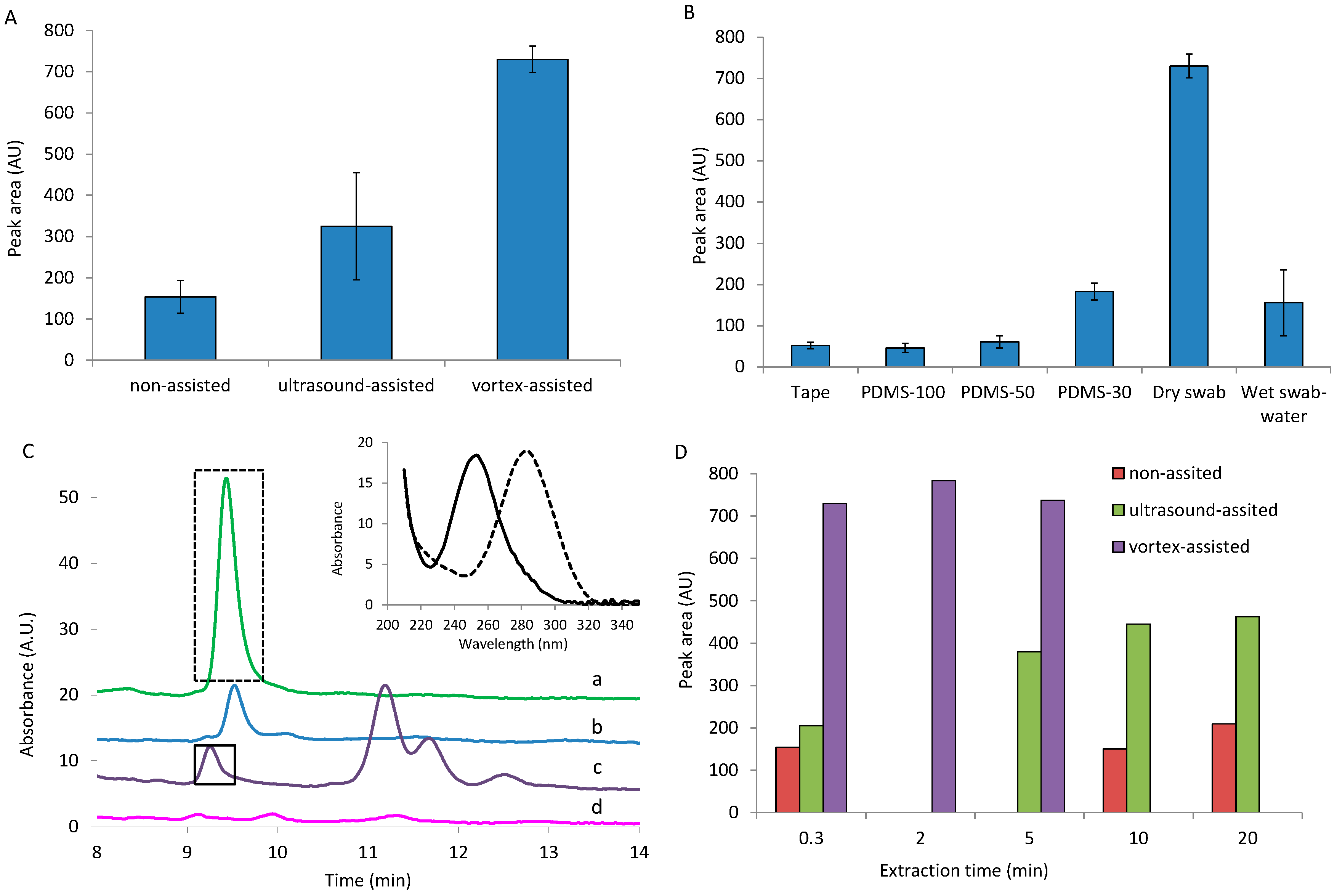
3.2. Optimization of DPA Extraction from Hands
3.2.1. DPA Extraction from Collector
3.2.2. DPA Collecting
3.2.3. Effect of Extraction Solvent on the DPA Extraction from the Sampler
3.3. Analytical Performance of DPA Determination
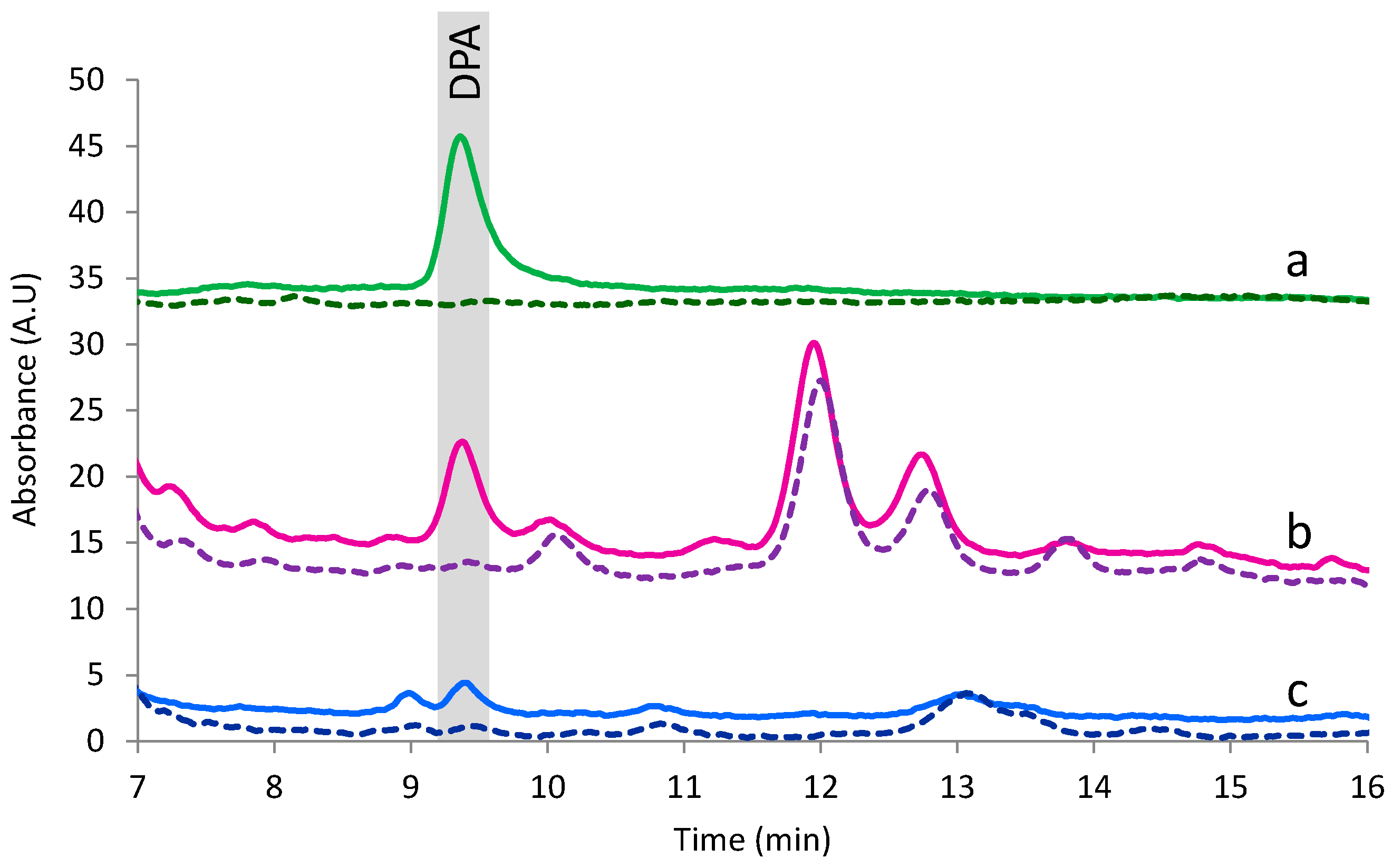
3.4. Analysis of Samples
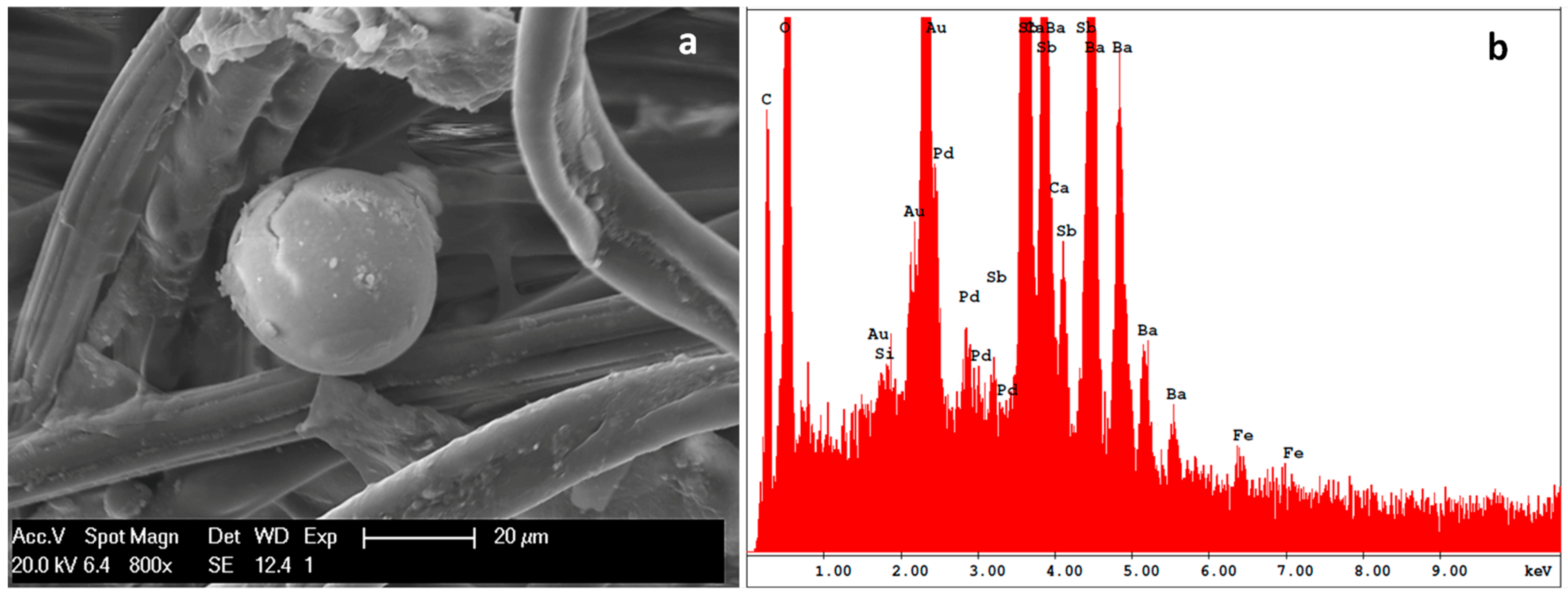
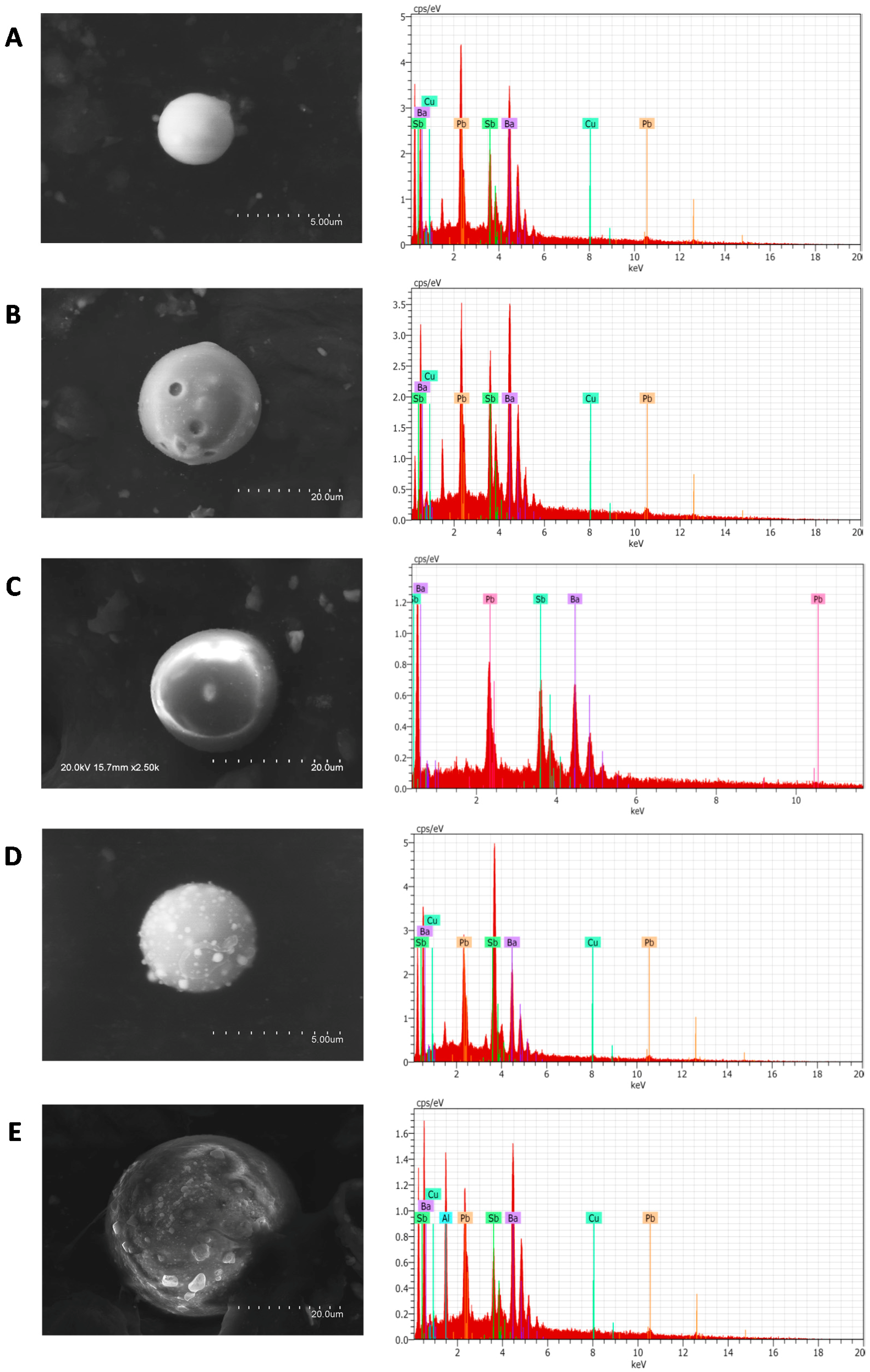
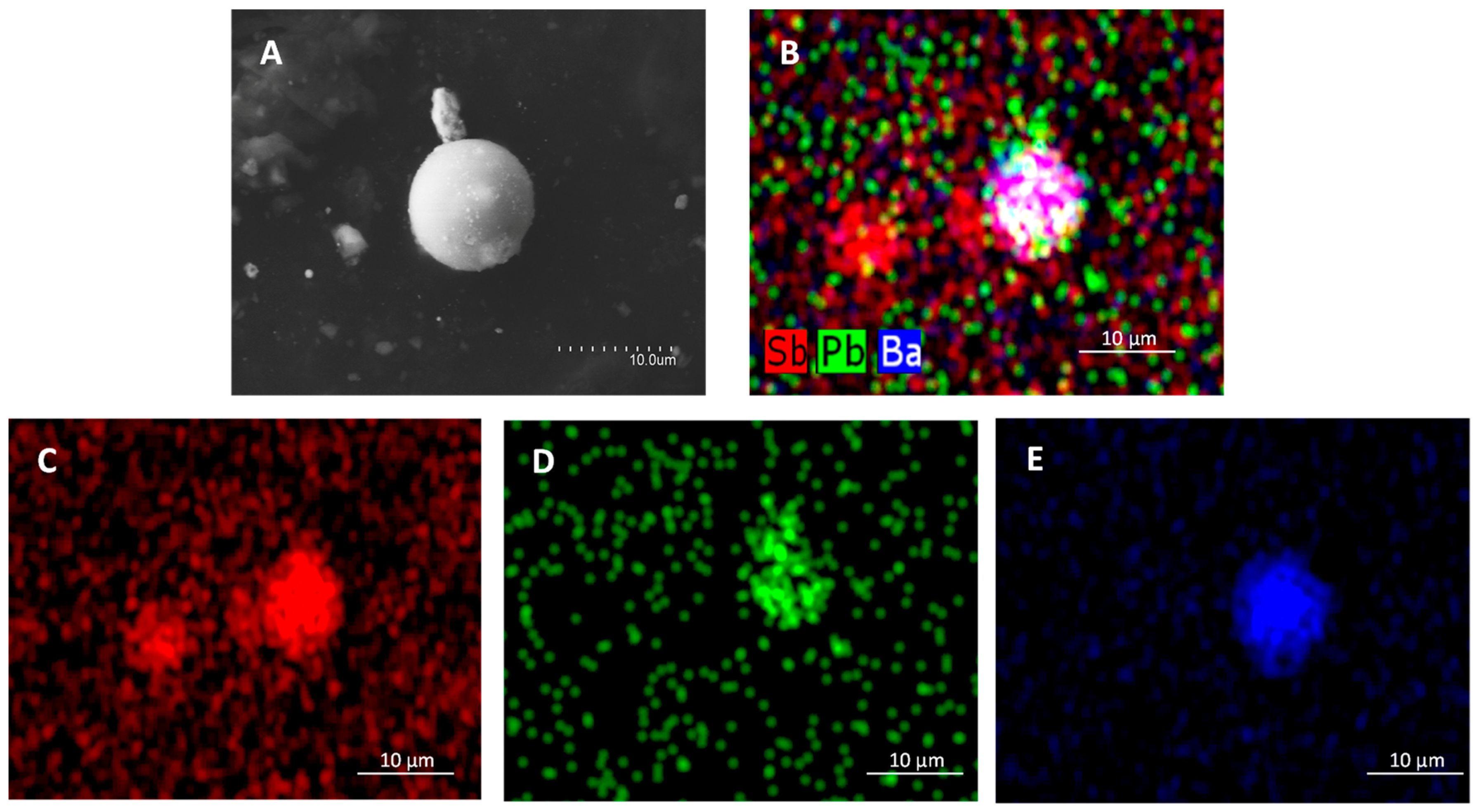
3.5. IGSR Particles’ Identification
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Firearms and the Internal Security of the EU: Protecting Citizens and Disrupting Illegal Trafficking; The European Data Protection Supervisor (EDPS): Brussels, Belgium, 2013.
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Bastian, B.A.; Rep, N.V.S. Deaths: Final Data for 2009. Natl. Vital Stat. Rep. 2016, 64, 1–117. [Google Scholar]
- Salles, M.O.; Naozuka, J.; Bertotti, M. A forensic study: Lead determination in gunshot residues. Microchem. J. 2012, 101, 49–53. [Google Scholar] [CrossRef]
- Tarifa, A.; Almirall, J.R. Fast detection and characterization of organic and inorganic gunshot residues on the hands of suspects by CMV-GC-MS and LIBS. Sci. Justice 2015, 55, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bernal Morales, E.; Revilla Vázquez, A.L. Simultaneous determination of inorganic and organic gunshot residues by capillary electrophoresis. J. Chromatogr. A 2004, 1061, 225–233. [Google Scholar] [CrossRef]
- Dalby, O.; Butler, D.; Birkett, J.W. Analysis of gunshot residue and associated materials—A review. J. Forensic Sci. 2010, 55, 924–943. [Google Scholar] [CrossRef] [PubMed]
- Schwoeble, A.J.; Exline, D.L. Gunshot Methods in Current Methods in Gunshot Residue Analysis; CRC Press: London, UK, 2000. [Google Scholar]
- Bailey, M.J.; Kirkby, K.J.; Jeynes, C. Trace element profiling of gunshot residues by PIXE and SEM-EDS: A feasibility study. X-Ray Spectrom. 2009, 38, 190–194. [Google Scholar] [CrossRef]
- Brożek-Mucha, Z. Trends in analysis of gunshot residue for forensic purposes. Anal. Bioanal. Chem. 2017, 409, 5803–5811. [Google Scholar] [CrossRef] [PubMed]
- Farokhcheh, A.; Alizadeh, N. Determination of diphenylamine residue in fruit samples using spectrofluorimetry and multivariate analysis. LWT—Food Sci. Technol. 2013, 54, 6–12. [Google Scholar] [CrossRef]
- Grima, M.; Butler, M.; Hanson, R.; Mohameden, A. Firework displays as sources of particles similar to gunshot residue. Sci. Justice 2012, 52, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Oommen, Z.; Pierce, S.M. Lead-free primer residues: A qualitative characterization of Winchester WinCleanTM, Remington/UMC LeadLessTM, Federal BallistiCleanTM, and Speer Lawman CleanFireTM handgun ammunition. J. Forensic Sci. 2006, 51, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Blakey, L.S.; Sharples, G.P.; Chana, K.; Birkett, J.W. Fate and Behavior of Gunshot Residue—A Review. J. Forensic Sci. 2017, 63, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Burleson, G.L.; Gonzalez, B.; Simons, K.; Yu, J.C.C. Forensic analysis of a single particle of partially burnt gunpowder by solid phase micro-extraction-gas chromatography-nitrogen phosphorus detector. J. Chromatogr. A 2009, 1216, 4679–4683. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Quan, Y.; Wang, W.; Zhou, H.; Liu, Z.; Shi, H.; Wang, P. Determination of Diphenylamine in Gunshot Residue by HPLC-MS/MS. J. Forensic Sci. Med. 2016, 2, 18. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Z.; Yang, C.; Yu, J.; Zhang, X.; Yang, S.; Wen, Y. Determination of diphenylamine stabilizer and its nitrated derivatives in smokeless gunpowder using a tandem MS method. Analyst 2001, 126, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Morelato, M.; Beavis, A.; Ogle, A.; Doble, P.; Kirkbride, P.; Roux, C. Screening of gunshot residues using desorption electrospray ionisation-mass spectrometry (DESI-MS). Forensic Sci. Int. 2012, 217, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Laza, D.; Nys, B.; De Kinder, J.; Kirsch-De Mesmaeker, A.; Moucheron, C. Development of a Quantitative LCMS⁄MS Method for the Analysis of Common Propellant Powder Stabilizers in Gunshot Residue. J. Forensic Sci. 2007, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent advances of in-tube solid-phase microextraction. TrAC–Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Serra-Mora, P.; Moliner-Martínez, Y.; Molins-Legua, C.; Herráez-Hernández, R.; Verdú-Andrés, J.; Campíns-Falcó, P. Trends in online in-tube solid phase microextraction. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 14; Volume 76, pp. 427–461. [Google Scholar]
- Scherperel, G.; Reid, G.E. Waddell Smith, R. Characterization of smokeless powders using nanoelectrospray ionization mass spectrometry (nESI-MS). Anal. Bioanal. Chem. 2009, 394, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.; Bell, S.; Crookshanks, L.; Lovejoy, M.; Oleska, C.; Tulley, T.; Wolfe, D. Preliminary evaluation of the persistence of organic gunshot residue. Forensic Sci. Int. 2012, 222, 137–145. [Google Scholar] [CrossRef]
- Gandy, L.; Najjar, K.; Terry, M.; Bridge, C. A novel protocol for the combined detection of organic, inorganic gunshot residue. Forensic Chem. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Available online: https://echa.europa.eu/es/information-on-chemicals/registered-substances (accessed on 31 January 2019).
- Galuszka, A.; Konieczka, P.; Migaszewski, Z.M.; Namiesnik, J. Analytical eco-scale for assessing the greenness of analytical procedures. TrAC-Trend. Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J. A new tool for the evaluation of the analytical procedures: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Pla-Tolós, J.; Serra-Mora, P.; Hakobyan, L.; Molins-Legua, C.; Moliner-Martinez, Y.; Campins-Falcó, P. A sustainable on-line CapLC method for quantifying antifouling agents like irgarol-1051 and diuron in water samples: Estimation of the carbon footprint. Sci. Total Environ. 2016, 560–570, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Jornet-Martínez, N.; Bocanegra-Rodríguez, S.; González-Fuenzalida, R.A.; Molins-Legua, C.; Campíns-Falcó, P. In Situ Analysis Devices for Estimating the Environmental Footprint in Beverages Industry. In Processing and Sustainability of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, Chapter 9; pp. 275–317. [Google Scholar]
| Technique/Limit of Detection (LOD) | DPA Extraction | DPA Amount on Hands | Mobile Phase; Flow; Injection Volume | Organic Solvents | Ref. |
|---|---|---|---|---|---|
| High-performance liquid chromatography -tandem mass spectrometry/0.3 ng/mL (solution) | DPA was extracted with cotton swab soaked with acetone, which was evaporated and DPA was dissolved in 0.1 mL methanol. | ˂Limit of quantitation (LOQ) | Methanol-water (90:10); 800 µL/min; 10 µL | Methanol and acetone as extractive solvents and mobile phase | [15] |
| Gas chromatography- mass spectrometry/3 ng | DPA was extracted with cotton swab moistened in water, the swab was heated and capillary microextraction made | ≈1 ng ˂ LOQ | - | Water as extractive solvent | [4] |
| Tandem Mass Spectrometry/1 ng/mL (solution) | Cotton swab soaked with methanol to extract DPA from the hand and dilution to 1 mL of methanol | Not studied | 0.1 mL/min; 20 µL | Methanol as extractive solvent | [16] |
| Mass spectrometry/- | Dabbing an adhesive coated aluminum stub over the hands | Not detected | 4 µL/min | Water:methanol 0.1% formic acid as solvent spray | [17] |
| Liquid chromatography- tandem mass spectrometry/34,000 ng | Cotton swab moistened with isopropyl alcohol:water, 75:25, which was introduced in a tube with 3.2 mL of the mixture and centrifuged. The aliquot was diluted five times with deionized water. SPEC C18 cartridges were conditioned with 250 µL of isopropyl alcohol and deionized water. 5000 µL of aqueous samples were loaded. The sorbent was rinsed with 250 µL of deionized water and dried. The analytes were eluted in acetonitrile:water:methyl alcohol, 80:10:10; 200 µL | 0.29–83 nmol/L | Acetonitrile: methanol: water, acidified by 0.1% of formic acid; 200 µL/min; 20 µL | Isopropylalcohol as extraction solvent, methanol and acetonitrile for mobile phase | [18] |
| Capillary electrophoresis/2387 ng/mL (solution) | Hands were swabbed by a cotton swab embedded in a solvent. The analyte was recuperated by sonication into 2 mL of solvent. Liquid extraction was carried out with 2 mL of ethyl acetate and 50 µL of ethylene glycol; the solvent was evaporated under dry nitrogen. The residues were reconstituted with diaminocyclohexane tetraacetic acid, and borate | Not detected | - | Diaminocyclohexane tetraacetic acid and sodium dodecyl sulfate as sampling solvents | [5] |
| IT-SPMS-CapLC-DAD/0.15 ng/mL (solution) 0.3 ng by cotton swab | DPA was extracted from hands by cotton swab and then DPA was extracted to 2 mL of water under vortex conditions (20 s) | ˂LOD-16.5 ng | Acetonitrile: water gradient; 10 µL/ min; 72 µL | Water as extractive solvent. Acetonitrile as mobile phase | This work |
| Extractive Capillary | Coating | Coating Thickness (µm) |
|---|---|---|
| TRB-5 | 5% diphenyl-95% polydimethylsiloxane | 3 |
| TRB-20 | 20% diphenyl-80% polydimethylsiloxane | 3 |
| TRB-35 | 35% diphenyl-65% polydimethylsiloxane | 3 |
| TRB-50 | 50% diphenyl-50% polydimethylsiloxane | 3 |
| Zebron ZB-WAXplus | polyethylene glycol | 1 |
| Linear Range (ng mL−1) | y = a + bx (ng mL−1) | Precision as % RSD (n = 4, 15 ng mL−1) | LOD (ng mL−1) | LOQ (ng mL−1) | ||||
|---|---|---|---|---|---|---|---|---|
| a ± sa | b ± sb | R2 | Intra-Day | Inter-Day | ||||
| Solution | 0.15–50 | −7 ± 57 | 144 ± 2 | 0.999 | 5 | 10 | 0.05 | 0.15 |
| Swab-vortex | 0.5–25 | −13 ± 24 | 49.2 ± 1.7 | 0.994 | 9 | 14 | 0.15 | 0.5 |
| Police Officer | Sample | Hand | Number of Shots | DPA Concentration (ng **) |
|---|---|---|---|---|
| A | 1 A | left | 25 | 4.4 |
| 2 A | right | 3.8 | ||
| B | 3 B | left | 12 | 2.7 |
| 4 B | right | 1.9 | ||
| C | 5 C | left | 25 | 3.0 |
| 6 C | right | 3.8 | ||
| D | 7 D | left | 25 | 2.8 |
| 8 D | right | ˂LOD | ||
| E | 9 E | left | 25 | 2.5 |
| 10 E | right | 3.2 | ||
| F | 11 F | left | 25 | 16.5 |
| 12 F | right | 13.4 | ||
| G | 13 G | left | 25 | ˂LOD |
| 14 G | right | ˂LOD | ||
| H | 15 H | left | 25 | 4.9 |
| 16 H | right | ˂LOD | ||
| I | 17 I | left | 25 | 8.4 |
| 18 I | right | 9.5 | ||
| J | 19 J | left | 25 | 8.0 |
| 20 J | right | 4.3 | ||
| K * | 21 K | left | 25 | ˂LOD |
| 22 K | right | ˂LOD | ||
| L | 23 L | left and right | 25 | 1.4 |
| M * | 24 M | left and right | 25 | 3.6 |
| N * | 25 N | left and right | 25 | 6.6 |
| O * | 26 O | left and right | 25 | 6.9 |
| P * | 27 P | left and right | 25 | ˂LOD |
| Sample | GSR Particle | Shape | Surface | Elemental Composition (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Major | Minor/Trace | ||||||||
| Ba | Sb | Pb | Cu | Al | Fe | ||||
| 21K | 21K.1 | Irregular | Nonmetallic bumpy | 62.5 | 33.2 | 4.3 | X | ||
| 21K.2 | Spherical | Nonmetallic smooth | 62.4 | 25.7 | 11.9 | X | |||
| 21K.3 | Spherical | Nonmetallic bumpy | 65.9 | 18.8 | 15.3 | X | |||
| 21K.4 | Spherical | Nonmetallic bumpy | 46.8 | 40.2 | 13.0 | X | |||
| 21K.5 | Spheroidal | Nonmetallic smooth | 65.7 | 14.8 | 19.5 | X | |||
| 21K.6 | Spherical | Nonmetallic smooth | 87.5 | 11.1 | 1.4 | X | |||
| 21K.7 | Spherical | Nonmetallic bumpy | 58.4 | 28.8 | 12.8 | X | |||
| 22K | 22K.1 | Spherical | Metallic smooth | 61.5 | 33.5 | 5.0 | X | ||
| 22K.2 | Irregular | Metallic bumpy | 98.8 | 0.7 | 0.5 | ||||
| 22K.3 | Spherical | Nonmetallic smooth | 48.0 | 37.3 | 14.7 | ||||
| 22K.4 | Spherical | Metallic smooth | 57.0 | 37.1 | 5.9 | X | |||
| 22K.5 | Spherical | Metallic smooth | 61.8 | 32.7 | 5.5 | X | |||
| 22K.6 | Spherical | Metallic smooth | 79.0 | 16.8 | 4.2 | X | |||
| 22K.7 | Spherical | Nonmetallic with hollows | 50.6 | 33.4 | 16.0 | X | |||
| 24M | 24M.1 | Spherical | Metallic smooth | 63.0 | 34.8 | 2.1 | |||
| 24M.2 | Spherical | Metallic smooth | 63.2 | 30.6 | 6.3 | ||||
| 24M.3 | Spherical | Nonmetallic bumpy | 0.0 | 96.7 | 3.3 | X | |||
| 24M.4 | Spherical | Metallic smooth | 60.4 | 37.2 | 2.3 | X | |||
| 24M.5 | Spherical | Nonmetallic smooth | 51.1 | 34.5 | 14.3 | X | X | ||
| 24M.6 | Spherical | Metallic smooth | 69.2 | 20.3 | 10.5 | X | X | ||
| 24M.7 | Spherical | Metallic smooth | 54.8 | 37.3 | 7.9 | X | X | ||
| 25N | 25N.1 | Spherical | Metallic smooth | 68.8 | 24.7 | 6.4 | X | ||
| 25N.2 | Irregular | Nonmetallic bumpy | 72.0 | 17.8 | 10.2 | X | |||
| 25N.3 | Spheroidal | Metallic bumpy | 63.3 | 30.0 | 6.7 | ||||
| 25N.4 | Spherical | Metallic smooth | 53.0 | 39.7 | 7.4 | X | |||
| 26O | 26O.1 | Spherical | Metallic smooth | 63.6 | 34.2 | 2.2 | |||
| 26O.2 | Spherical | Metallic smooth | 61.7 | 35.2 | 3.0 | X | |||
| 26O.3 | Irregular | Nonmetallic bumpy | 40.0 | 33.8 | 26.2 | ||||
| 26O.4 | Spherical | Metallic smooth | 0.3 | 4.9 | 94.9 | X | |||
| 26O.5 | Spherical | Metallic smooth | 62.7 | 31.2 | 6.3 | ||||
| 26O.6 | Spherical | Metallic smooth | 50.6 | 35.8 | 13.6 | ||||
| 27P | 27P.1 | Spherical | Nonmetallic bumpy | 53.5 | 38.3 | 8.2 | X | X | |
| 27P.2 | Spherical | Nonmetallic bumpy | 57.4 | 30.9 | 11.7 | X | |||
| 27P.3 | Spherical | Nonmetallic smooth | 60.2 | 36.9 | 2.9 | X | |||
| 27P.4 | Spherical | Nonmetallic smooth | 57.6 | 30.9 | 11.5 | X | |||
| 27P.5 | Spheroidal | Nonmetallic bumpy | 48.9 | 30.7 | 20.5 | X | |||
| 27P.6 | Spherical | Nonmetallic bumpy | 61.3 | 31.7 | 7.0 | X | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argente-García, A.I.; Hakobyan, L.; Guillem, C.; Campíns-Falcó, P. Estimating Diphenylamine in Gunshot Residues from a New Tool for Identifying both Inorganic and Organic Residues in the Same Sample. Separations 2019, 6, 16. https://doi.org/10.3390/separations6010016
Argente-García AI, Hakobyan L, Guillem C, Campíns-Falcó P. Estimating Diphenylamine in Gunshot Residues from a New Tool for Identifying both Inorganic and Organic Residues in the Same Sample. Separations. 2019; 6(1):16. https://doi.org/10.3390/separations6010016
Chicago/Turabian StyleArgente-García, Ana Isabel, Lusine Hakobyan, Carmen Guillem, and Pilar Campíns-Falcó. 2019. "Estimating Diphenylamine in Gunshot Residues from a New Tool for Identifying both Inorganic and Organic Residues in the Same Sample" Separations 6, no. 1: 16. https://doi.org/10.3390/separations6010016
APA StyleArgente-García, A. I., Hakobyan, L., Guillem, C., & Campíns-Falcó, P. (2019). Estimating Diphenylamine in Gunshot Residues from a New Tool for Identifying both Inorganic and Organic Residues in the Same Sample. Separations, 6(1), 16. https://doi.org/10.3390/separations6010016






