Abstract
A method for the analysis of thyroid hormones by liquid chromatography-mass spectrometry was used for the dissolution testing of single- and dual-component thyroid hormone supplements via a two-stage biorelevant dissolution procedure. The biorelevant media consisted of fasted-state simulated gastric fluid and fasted state simulated intestinal fluid at 37 °C, and was investigated using an internationally recognized protocol. The dissolution profiles showed consistent solubilization for both single- and dual-component batches at pH 6.5 in the fasted-state simulated intestinal fluid.
1. Introduction
Thyroid hormones are responsible for the regulation of a variety of metabolic functions, including basal metabolic rate and lipid, glucose and carbohydrate metabolism [1]. This group of compounds contains tyrosine-based compounds including the physiologically active form triiodothyronine (T3) and the prehormone thyroxine (T4). The majority of triiodothyronine is formed enzymatically by the deiodination of thyroxine [2,3] (Figure 1). Hyperthyroidism and hypothyroidism are the two main medical conditions associated with thyroid hormone levels. Hypothyroidism is caused by a depleted level of triiodothyronine, the treatment for which is lifelong thyroid supplement therapy [1,4,5,6,7]. Currently, the favoured treatment for hypothyroidism is the administration of levothyroxine sodium salt, with its cost being a considerable factor [8]. However, a number of studies have found that in order to maintain euthyroid levels of both T4 and T3, an excess of levothyroxine sodium salt must be administered [9,10,11]. This has resulted in products becoming available that contain either T3 alone or a combination of T4 and T3 in a single dose.
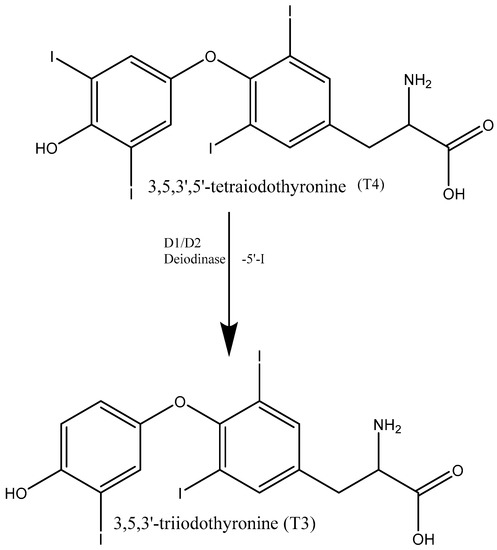
Figure 1.
Conversion of T4 to T3 by deiodination.
Dissolution testing is widely accepted within the pharmaceutical industry as the measure of drug release rate to aid in quality control, formulation and process development [12,13]. Noyes and Whitney, investigating the dissolution of benzoic acid and lead chloride, performed the first reported study into dissolution in 1897 [14]. However, the importance of dissolution testing for pharmaceutical quality control and drug formulation was not established until 70 years later [12,15]. Within the pharmaceutical industry, there are two main categories of dissolution testing performed, biorelevant and quality control [13]. Biorelevant is an abbreviated term for “biologically relevant”, and the selected media mimic the fluids found within the stomach (gastric) and intestinal tract [16].
Biorelevant dissolution is a multiple-stage dissolution test designed to model the different in vivo environments as the dosage passes through the gastrointestinal (GI) tract. A biorelevant dissolution method is utilized during early formulation selection and optimization but due to the cost and complexity of the media plus variability of physiological parameters it is replaced by a quality control dissolution method once formulation has been developed [13,15]. A quality control dissolution method consists of one medium that is designed for detecting variations in routine manufacturing or changes during stability testing (e.g., to detect incorrect granulation or compression [13]).
This paper investigates the bioavailability profile, using simulated in vitro gastrointestinal extraction media, of thyroid hormone supplements that contain either a single or a dual combination of the two hormones using biorelevant dissolution media. In addition, the bioavailability profile is compared against the standard United States Pharmacopoeia (USP) quality control test specification of 70% release within 45 min for individual dosage forms of thyroid hormone supplements [17,18].
2. Materials and Methods
2.1. Chemicals and Reagents
Triiodothyronine and thyroxine/triiodothyronine tablets were purchased from RX Cart (Sagunto, Valencia, Spain), specifically Cytomel containing 25 µg of T3. Tiromel containing 25 µg of T3 and Dithyron containing 12.5 µg of T3 and 50 µg of T4 were purchased from http://www.buyt3.co.uk/. Organic LC–MS grade solvents (methanol, acetonitrile, acetic acid and formic acid) were purchased from Fisher Scientific (Loughborough, Leicestershire, UK). Standards of T3 and T4 were purchased from Sigma Aldrich (Poole, Dorset, UK) with a purity of ≥98%. For dissolution media, FaSSIF/FeSSIF/FaSSGF powder was purchased from Biorelevant (London, UK) and additives (sodium hydroxide pellet and monobasic sodium phosphate monohydrate) were purchased from Sigma Aldrich (Poole, Dorset) while sodium chloride, hydrochloric acid and sodium hydroxide were purchased from Fisher Scientific (Loughborough, Leicestershire, UK).
2.2. Instrumentation
LC–MS analysis chromatographic separation was achieved on a reversed phase pentafluorophenyl column (Supelco 2.1 µm F5, 100 × 2.1 mm) from Sigma Aldrich (Poole, Dorset, UK). Thermo Surveyor LC (Thermo Scientific, Hemel Hempsted, UK) consisted of a quaternary MS pump, vacuum degasser, thermostated autosampler (set to 5 °C) and a thermostated column oven (set to 25 °C). Mass spectrometry was performed using an LTQ XL ion trap mass spectrometer (Thermo Scientific, Hemel Hempsted, UK) equipped with a heated electrospray ionization (HESI) source maintained at 200 °C. The solvent evaporation was aided with auxiliary gas, sheath gas and sweep gas set to an arbitrary flow rate of 15, 60 and 1, respectively. The mass spectrometer was operated in selected reaction monitoring (SRM) MS/MS in negative mode, with collision energies of 28 eV for T4 and 27 eV for T3. The monitored transitions were 776 → 604 and 650 → 633 for T4 and T3, respectively. In SRM MS/MS mode the precursor ion is isolated and subjected to a specified amount of collision energy to induce fragmentation. The MS method is then set to monitor the precursor and a minimum of two stable product ions. Sample aliquots of 10 µL were introduced onto the column at a flow rate of 200 µL/min. The analytes were separated using an isocratic method using water +0.2% formic acid (A) and methanol +0.2% formic acid (B) as the mobile phase.
2.3. Dissolution
Dissolution was carried out using a SOTAX Smart AT7™ dissolution bath from Sotax (Finchley, London). The dissolution bath was set to 37 °C and 250 mL of fasted-state simulated gastric fluid (FaSSGF) was added to each vessel; the rotor speed set to 75 RPM and allowed to equilibrate for at least 1 h. The initial and final temperatures and pH were recorded to ensure consistent temperature and buffer control. Tablet weights were recorded prior to being released into the relevant vessels simultaneously. Samples were taken at the following time points through probe filters and syringe filters: 5, 10, 20 and 30 min. The paddles were stopped and 250 mL of fasted-state simulated intestinal fluid (FaSSIF), added to each vessel and the paddle was resumed. Samples were taken at the following time points through probe filters and syringe filters: 35, 40, 50, 60, 90, 120, 150, 180, 210 and 240 min.
2.4. Preparation of Stock Solutions and Dissolution Media
Stock solutions of each hormone were prepared at a concentration approximately 0.5 mg/mL in methanol, aliquoted into 100 µL aliquots and stored at −20 °C, as recommended by the manufacturer to increase the working life of the standard solutions. A fresh working standard solution of both standards was prepared each week by dilution of stock solutions in mobile phase (70% methanol: 30% water, v/v). Calibration standards were prepared daily for each analysis from the working stock solution ranging from 1 to 200 ng/mL for LC–MS/MS. A quality control standard containing both thyroid hormones was also prepared at a concentration of 50 ng/mL.
To prepare the fasted-state small intestinal fluid (FaSSIF) buffer, 0.84 g of sodium hydroxide pellet, 7.90 g of monobasic sodium phosphate monohydrate and 12.38 g of sodium chloride were added to approximately 1.8 L of Type 1 water. The solution was pH adjusted to 6.5 with 1 N sodium hydroxide and made up to 2 L with Type 1 water. To make the final FaSSIF medium, 4.48 g of FaSSIF/FeSSIF/FaSSGF powder was added to approximately 1 L of FaSSIF buffer. The solution was stirred until completely dissolved and made up to 2 L with FaSSIF buffer. The solution was allowed to stand for 2 h prior to use [19]. To prepare the fasted-state gastric fluid (FaSSGF) buffer, 3.2 g of sodium chloride was added to approximately 1.8 L of Type 1 water. The solution was pH adjusted to 1.6 with 1 N hydrochloric acid and made up to 2 L with Type 1 water. To make the final FaSSGF medium, 0.12 g of FaSSIF/FeSSIF/FaSSGF powder was added to approximately 1 L of FaSSGF buffer. The solution was stirred until completely dissolved and made up to 2 L with FaSSGF buffer [19].
2.5. Tablet Analysis
For each batch of tablets, three tablets were weighed and crushed using a pestle and mortar. In triplicate for each tablet, a fifth of the weight was transferred to a 10 mL volumetric flask, made up to volume with water, and sonicated for 20 min. The solutions were allowed to cool, then 10 µL was transferred to a separate 10 mL volumetric flask and made up to volume with water. The solution was then filtered through a 0.22 µm nylon filter and placed in autosampler vials for analysis by LC–MS/MS.
3. Results & Discussion
3.1. Tablet Analysis
Calibration data was generated for both T3 and T4 over the concentration range 0–250 ng/mL, based on 11 data points. Subsequently, the calibration curve obtained gave an r2 value of 0.9962 and 0.9973 for T3 and T4, respectively (Table 1). The developed LC–MS/MS method was both sensitive and selective for T3 and T4 analysis, with limit of quantitation (LOQ) values of 1.6 and 1.3 ng/mL and limit of detection (LOD) values of 0.2 and 0.8 ng/mL, respectively. Calculations were based on the standard curve method: LOD = (3.3σ)/S and LOQ = (10σ)/S, where σ is the standard deviation and S is the slope of the curve [20]. In addition, a quality control standard (50 ng/mL) was analyzed throughout the experimental duration and given average recoveries of 99.6% and 102.6% for T3 and T4, respectively. Tablet analysis was performed prior to dissolution testing to ensure that the stated dosage was present, as this would be used to indicate 100% of release under dissolution testing. The tablet analysis was consistent with the dosages stated on the packaging for all four batches of thyroid supplement with a % content of >93.3% for all batches and replicate preparations (Table 2). Good inter-batch and inter-tablet precision were also observed with % (relative standard deviation) RSD of <1.8% and <3.4%, respectively.

Table 1.
Analytical data for T3 and T4 by LC–MS/MS.

Table 2.
Tablet analysis: single- and dual-component thyroid hormone supplements.
3.2. Dissolution
In accordance with the United States Pharmacopeia (USP), the tablets are considered to be dissolved when 75% of the stated dosage has been released [17,18]. The USP method is used as a quality control method, which is normally deployed to identify variations during manufacturing or storage stability, and does not mimic the different physiological conditions of the gastrointestinal tract. The USP-stated method uses alkaline borate buffer (pH 10) and 0.01 N hydrochloric acid containing 0.2% sodium lauryl sulfate for T3 and T4, respectively, with no proposed method for dual-component tablets [20]. Therefore, to ensure consistency and allow a direct comparison between single- and dual-component tablets, biorelevant dissolutions were performed using simulated gastric and intestinal fluids. The results from the dissolution testing using the simulated gastric and intestinal fluids of the tablets for the single- and dual-component thyroid hormones are shown in Figure 2. It is noted that the dissolution for T3 and T4 occurs within approximately 45 min (i.e., 75% dissolution), which is in agreement with the USP method [17,18]. From Figure 2 it can also be seen that total release of T3 and T4 from the tablet formulations was obtained within 120 min.
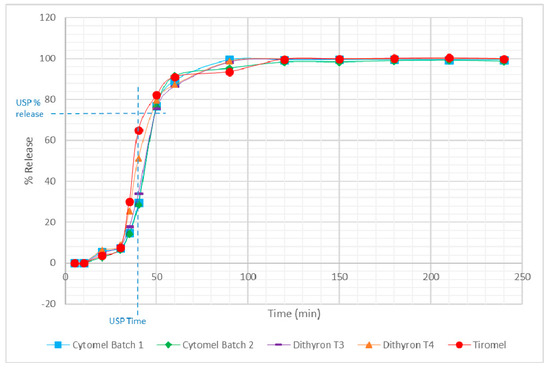
Figure 2.
Dissolution testing profile for T3 and T4 from single- and dual-supplement thyroid hormone supplements.
The kinetics of dissolution have been investigated using a first-order rate constant (Figure 3). It was observed that a two-stage dissolution process occurs with crossing points determined by extrapolation of the line of best fit. The initial rate constant corresponds to tablet coating dissolution while the second rate constant is indicative of tablet breakdown. The rate constants (k) were calculated to be between 5.3–6.1 h−1 and 0.4–0.8 h−1 for coating removal and tablet solubilization, respectively. The rate constant was calculated based on the change in cumulative % drug remaining over time ((y2 − y1)/(x2 − x1)) with y calculated using the equation of the line.
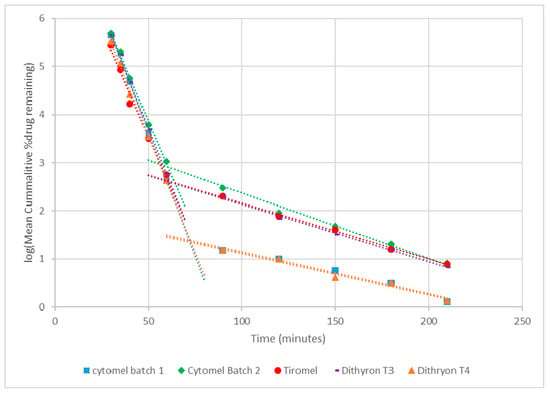
Figure 3.
Kinetics of dissolution for T3 and T4 from single- and dual-supplement thyroid hormone supplements.
4. Conclusions
The tablet analysis shows that the thyroid hormone supplements contain T3 and T4 content corresponding to the stated dosage. The dissolution testing profiles and kinetic of dissolution plots show that there is consistent solubilization of the active pharmaceutical ingredient across both single- and dual-component batches for thyroid hormone supplements. Therefore, it is concluded that the use of simulated in vitro gastric intestinal fluids has no influence on dual-component thyroid hormone supplement extraction and recovery. It was also noted, from the dissolution testing profile, that there was minimal solubilization of T3 and T4 in the gastric fluid. However, rapid release of the active compounds was observed within 15 min of the addition of the intestinal fluid.
Author Contributions
Conceptualization, J.R.D., M.G.C. and S.L.B.; methodology, S.L.B.; validation, S.L.B.; formal analysis, S.L.B.; investigation, S.L.B.; resources, S.L.B., J.R.D. and M.G.C.; data curation, S.L.B.; writing—original draft preparation, S.L.B.; writing—review and editing, J.R.D., S.L.B. and M.G.C.; supervision, J.R.D. and M.G.C.; project administration, J.R.D.
Funding
This research received no external funding.
Acknowledgments
We acknowledge GlaxoSmithKline for the donation of the dissolution bath to Northumbria University. We also gratefully acknowledge financial support from Northumbria University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol. Metab. 2014, 25, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.M. Basic Medical Endocrinology, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2009; p. 17. [Google Scholar]
- Gu, J.; Soldin, O.P.; Soldin, S.J. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin. Biochem. 2007, 40, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017. [Google Scholar] [CrossRef]
- Dunn, D.; Turner, C. Hypothyroidism in women. Nurs. Women’s Health 2016, 20, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J. Hypothyroidism. Medicine 2017, 45, 506–509. [Google Scholar] [CrossRef]
- National Health Service. Hypothyroidism. Available online: https://www.nhs.uk/conditions/underactive-thyroid-hypothyroidism/ (accessed on 24 October 2018).
- Escobar-Morreale, H.F.; Botella-Carretero, J.I.; Morreale de Escobar, G. Treatment of hypothyroidism with levothyroxine or a combination of levothyroxine plus L-triiodothyronine. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregon, M.J.; Escobar del Rey, F.; Morreale de Escobar, G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroididm in all tissues, as studied in thyroidectomized rats. J. Clin. Investig. 1995, 96, 2828–2838. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregon, M.J.; Hernandez, A.; Escobar del Rey, F.; Morreale de Escobar, G. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology 1997, 138, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Obregon, M.J.; del Escobar Rey, F.; Morreale de Escobar, G. Tissue-specific patterns of changes in 3,5,3-triiodo-L-thyronine concentrations. Biochimie 1999, 81, 453–462. [Google Scholar] [CrossRef]
- Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the biopharmaceutics classification system. Int. J. Pharm. 2006, 321, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Grady, H.; Elder, D.; Webster, G.K.; Mao, Y.; Lin, Y.; Flanagan, T.; Mann, J.; Blanchard, A.; Cohen, M.J.; Lin, J.; et al. Industry’s view on using quality control, biorelevant, and clinically relevant dissolution tests for pharmaceutical development, registration, and commercialization. J. Pharm. Sci. 2018, 107, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Dressman, J.B.; Kramer, J. Pharmaceutical Dissolution Testing, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 4–15. [Google Scholar]
- Leigh, M.; Kloefer, B.; Schaich, M. Comparison of the solubility and dissolution of drugs in fasted-state biorelevant media (FaSSIF and FaSSIF-V2). Diss. Technol. 2013, 20, 44–50. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP36 NF31. In Official Monographs/Levothyroxine; United States Pharmacopeia: Rockville, MD, USA, 2013; pp. 4109–4110. [Google Scholar]
- United States Pharmacopeia. USP36 NF31. In Official Monographs/Liothyronine; United States Pharmacopeia: Rockville, MD, USA, 2013; pp. 4121–4123. [Google Scholar]
- Biorelevant. Biorelevant Instructions V1.1. Available online: https://biorelevant.com/ (accessed on 30 October 2018).
- ICH Guideline Validation of Analytical Procedures—Test and Methodology. Available online: http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html (accessed on 11 February 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
