Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Materials and Chemicals
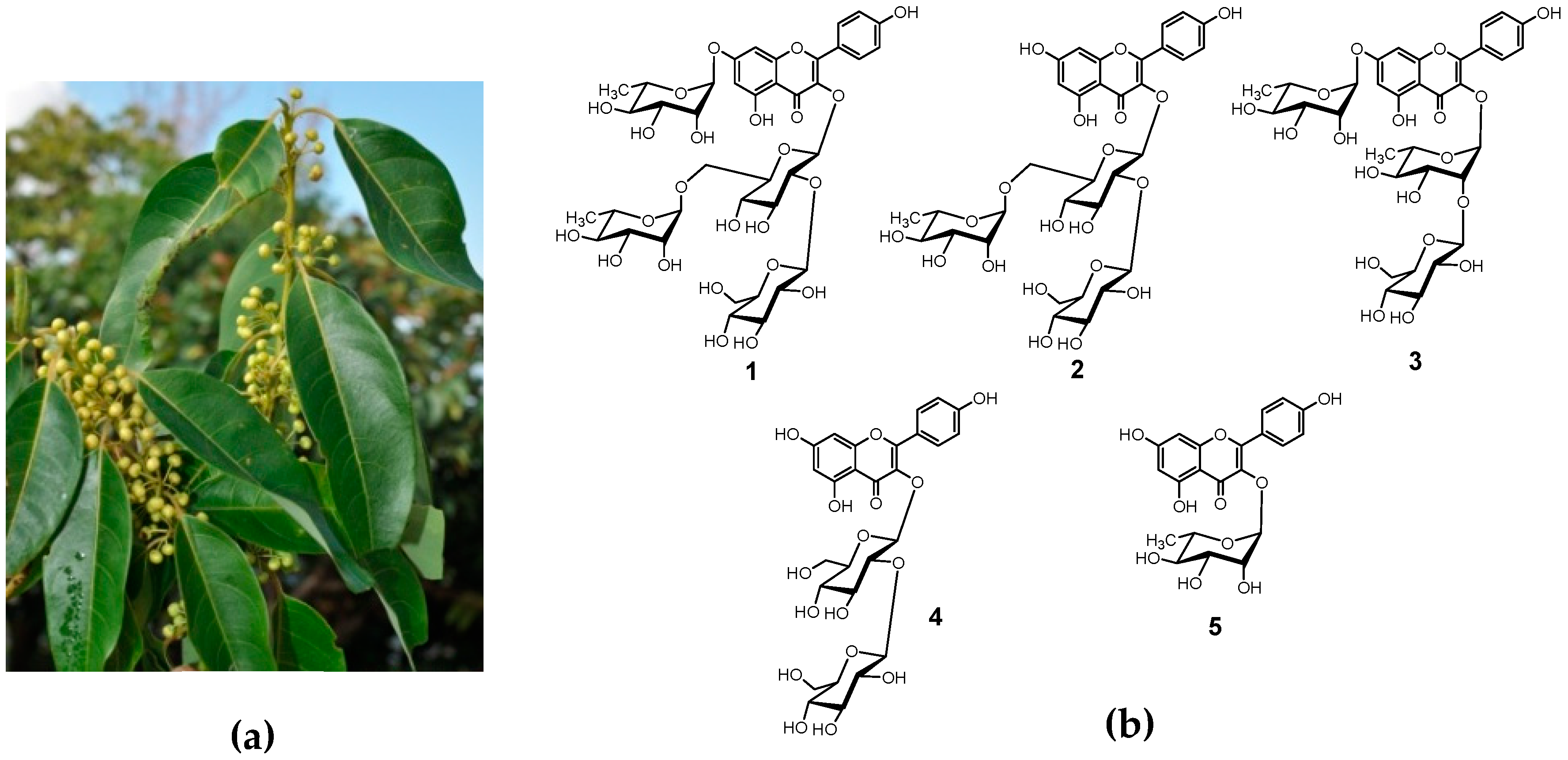
2.3. Extraction and Isolation
2.4. Measurement of DPPH Free Radical Scavenging and Pancreartic Lipase Inhibitory Activities
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: a source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Manandhar, N.P. Plants and People of Nepal; Timber Press, Inc.: Portland, OR, USA, 2002. [Google Scholar]
- Wang, J.W.; Chen, X.Y.; Hu, P.Y.; Tan, M.M.; Tang, X.G.; Huang, M.C.; Lou, Z.H. Effects of linderae radix extracts on a rat model of alcoholic liver injury. Exp. Ther. Med. 2016, 11, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chang, F.R.; Wu, Y.C. The constituents of Lindera glauca. J. Chin. Chem. Soc. 2000, 47, 373–380. [Google Scholar] [CrossRef]
- Gan, L.S.; Zheng, Y.L.; Mo, J.X.; Liu, X.; Li, X.H.; Zhou, C.X. Sesquiterpene lactones from the root tubers of Lindera aggregata. J. Nat. Prod. 2009, 72, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.H.; Seguin, E.; Tillequin, F.; Koch, M. Aporphine alkaloids from Lindera myrrha. Phytochemistry 1994, 35, 1363–1365. [Google Scholar] [CrossRef]
- Huh, G.W.; Park, J.H.; Kang, J.H.; Jeong, T.S.; Kang, H.C.; Baek, N.I. Flavonoids from Lindera glauca Blume as low-density lipoprotein oxidation inhibitors. Nat. Prod. Res. 2014, 28, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Ichino, K. Two flavonoids from two Lindera umbellata varieties. Phytochemistry 1989, 28, 955–956. [Google Scholar] [CrossRef]
- Li, Y.M.; Ohno, Y.; Minatoguchi, S.; Fukuda, K.; Ikoma, T.; Ohno, T.; Akao, S.; Takemura, G.; Gotou, K.; Fujiwara, H. Extracts from the roots of Lindera strychifolia induces apoptosis in lung cancer cells and prolongs survival of tumor-bearing mice. Am. J. Chin. Med. 2003, 31, 857–869. [Google Scholar] [CrossRef]
- Yan, R.; Yang, Y.; Zeng, Y.; Zou, G. Cytotoxicity and antibacterial activity of Lindera strychnifolia essential oils and extracts. J. Ethnopharmacol. 2009, 121, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yamazaki, M.; Katagata, Y. Kuromoji (Lindera umbellata) essential oil inhibits LPS-induced inflammation in RAW 264.7 cells. Biosci. Biotechnol. Biochem. 2013, 77, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Huang, G.J.; Huang, H.C.; Chen, Y.C.; Chang, C.I.; Wang, S.Y.; Chen, I.S.; Tseng, Y.H.; Chien, S.C.; Kuo, Y.H. A new butanolide compound from the aerial part of Lindera akoensis with anti-inflammatory activity. Molecules 2012, 17, 6585–6592. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Ushikoshi, H.; Hattori, A.; Murata, I.; Ohno, Y.; Aoyama, T.; Kawasaki, M.; Nishigaki, K.; Takemura, G.; Fujiwara, T.; et al. Treatment with Lindera strychnifolia Reduces Blood Pressure by Decreasing Sympathetic Nerve Activity in Spontaneously Hypertensive Rats. Am. J. Chin. Med. 2010, 38, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ruehl, M.; Erben, U.; Kim, K.; Freise, C.; Dagdelen, T.; Eisele, S.; Trowitzsch-Kienast, W.; Zeitz, M.; Jia, J.; Stickel, F.; et al. Extracts of Lindera obtusiloba induce antifibrotic effects in hepatic stellate cells via suppression of a TGF-β-mediated profibrotic gene expression pattern. J. Nutr. Biochem. 2009, 20, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, B.; Zhu, W.; Li, X.; Tian, J.; Zhang, L. Characterisation of polyphenol constituents of Linderae aggregate leaves using HPLC fingerprint analysis and their antioxidant activities. Food Chem. 2015, 186, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Hadi, A.H.; Mohamad, J.; Khalilzadeh, M.A.; Cheahd, S.C.; Fadaeinasab, M. Flavonoids and linderone from Lindera oxyphylla and their bioactivities. Comb. Chem. High Throughput Screen 2013, 16, 160–166. [Google Scholar] [PubMed]
- Watanabe, T.; Rajbhandari, K.R.; Malla, K.J.; Devkota, H.P.; Yahara, S. A Handbook of Medicinal Plants of Nepal Supplement I; Ayurseed L.E.I.: Kanagawa, Japan, 2013. [Google Scholar]
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Dall’Acqua, S.; Grillo, A.; Castagliuolo, I.; Gurung, K.; Innocenti, G. Essential oil of Lindera neesiana fruit: Chemical analysis and its potential use in topical applications. Fitoterapia 2010, 81, 11–16. [Google Scholar] [CrossRef]
- Devkota, H.P.; Kurizaki, A.; Tsuhiro, K.; Hori, K.; Wada, M.; Watanabe, T. Medicinal Plants as Source of Potent Bioactive Natural Products and Functional Foods. In Proceedings of the Proceeding of Japan-Turkey International Symposium on Pharmaceutical and Biomedical Sciences, Kumamoto, Japan, 2–3 October 2016; p. 16. [Google Scholar]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Kite, G.C.; Veitch, N.C.; Boalch, M.E.; Lewis, G.P.; Leon, C.J.; Simmonds, M.S.J. Flavonol tetraglycosides from fruits of Styphnolobium japonicum (Leguminosae) and the authentication of Fructus Sophorae and Flos Sophorae. Phytochemistry 2009, 70, 785–794. [Google Scholar] [CrossRef]
- Taylor, W.G.; Fields, P.G.; Sutherland, D.H. Fractionation of lentil seeds (Lens culinaris Medik.) for insecticidal and flavonol tetraglycoside components. J. Agric. Food Chem. 2007, 55, 5491–5498. [Google Scholar] [CrossRef]
- Kucukislamoglu, M.; Yayli, N.; Şentürk, H.B.; Genç, H.; Özden, S. Flavonol glycosides from Consolida armeniaca. Turk. J. Chem. 2000, 24, 191–197. [Google Scholar]
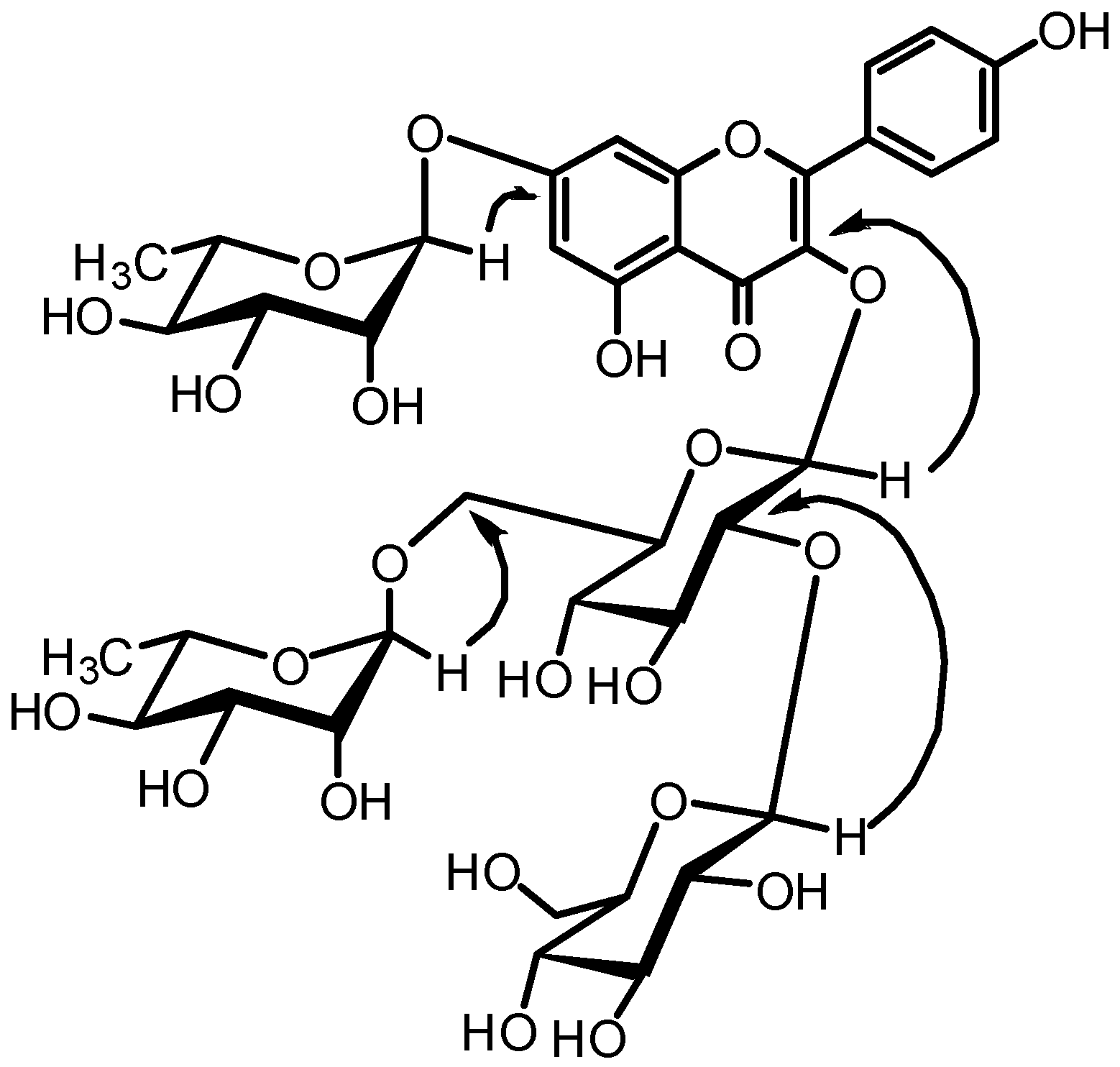
| Position | δC | δH, mult. (J in Hz) | Position | δC | δH, mult. (J in Hz) |
|---|---|---|---|---|---|
| 2 | 159.6 | (2→1) Rha | |||
| 3 | 135.0 | 1 | 104.6 | 4.75, d (7.5) | |
| 4 | 179.7 | 2 | 75.5 | 3.38, m | |
| 5 | 162.8 | 3 | 77.9 | 3.38, m | |
| 6 | 100.6 | 6.46, d (2.1) | 4 | 71.2 | 3.36, m |
| 7 | 163.5 | 5 | 78.4 | 3.30, m | |
| 8 | 95.8 | 6.74, d (2.1) | 6 | 62.6 | 3.80, m; 3.70, m |
| 9 | 158.0 | (6→1) Rha | |||
| 10 | 107.4 | 1 | 102.1 | 4.47, d (1.3) | |
| 1’ | 122.6 | 2 | 72.0 | 3.54, m | |
| 2’ | 132.4 | 8.05, d (8.8) | 3 | 72.2 | 3.54, m |
| 3’ | 116.3 | 6.91, d (8.8) | 4 | 73.6 | 3.22, m |
| 4’ | 161.6 | 5 | 69.6 | 3.40, m | |
| 5’ | 116.3 | 6.91, d (8.8) | 6 | 17.8 | 1.07, d (6.1) |
| 6’ | 132.4 | 8.05, d (8.8) | 7-O-Rha | ||
| 3-O-Glc | 1 | 99.9 | 5.56, d (1.5) | ||
| 1 | 100.9 | 5.40, d (7.6) | 2 | 71.6 | 4.02, dd (1.5, 3.3) m |
| 2 | 82.4 | 3.74, m | 3 | 72.0 | 3.82, m |
| 3 | 77.7 | 3.60, m | 4 | 73.8 | 3.47, m |
| 4 | 71.3 | 3.30, m | 5 | 71.3 | 3.62, m |
| 5 | 77.0 | 3.30, m | 6 | 18.1 | 1.26, d (6.1) |
| 6 | 68.0 | 3.80, m; 3.36, m |
| Samples | DPPH Free Radical Scavenging Activity a | Lipase Inhibitory Activity a |
|---|---|---|
| 60% EtOH extract | 29.5 ± 1.04 | 3.14 ± 0.76 |
| 1 | 130.22 ± 7.078 | NA b |
| 2 | NA b | NA b |
| 3 | 354.26 ± 38.55 | NA b |
| 4 | 131.65 ± 7.67 | 19.56 ± 0.40 |
| 5 | 200.96 ± 17.52 | 22.35 ± 0.85 |
| Trolox c | 12.03 ± 0.25 | - |
| Cetilistat c | - | 3.85 ± 0.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari-Devkota, A.; Dirar, A.I.; Kurizaki, A.; Tsushiro, K.; Devkota, H.P. Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations 2019, 6, 10. https://doi.org/10.3390/separations6010010
Adhikari-Devkota A, Dirar AI, Kurizaki A, Tsushiro K, Devkota HP. Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations. 2019; 6(1):10. https://doi.org/10.3390/separations6010010
Chicago/Turabian StyleAdhikari-Devkota, Anjana, Amina Ibrahim Dirar, Ayumi Kurizaki, Kazuki Tsushiro, and Hari Prasad Devkota. 2019. "Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana" Separations 6, no. 1: 10. https://doi.org/10.3390/separations6010010
APA StyleAdhikari-Devkota, A., Dirar, A. I., Kurizaki, A., Tsushiro, K., & Devkota, H. P. (2019). Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations, 6(1), 10. https://doi.org/10.3390/separations6010010






