Chemometric Characterization of Synthetic Dye Sorption onto Slovakian River Sediments: A Laboratory Batch Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. River Sediments and Waters
2.2. Chemicals
2.3. Characterization of River Sediments
2.4. Characterization of River and Model Waters
2.5. Sorption and Desorption of Dyes
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of River Sediments
3.2. Characterization of River and Model Waters
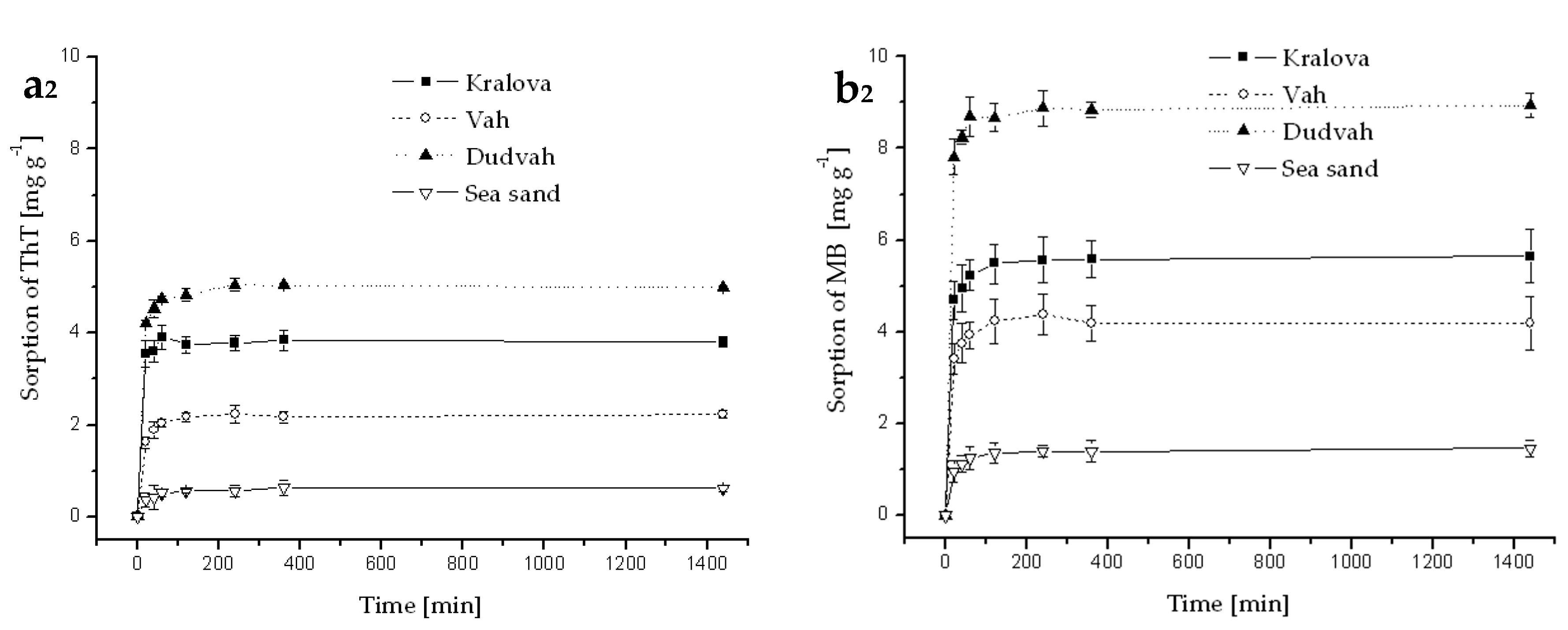
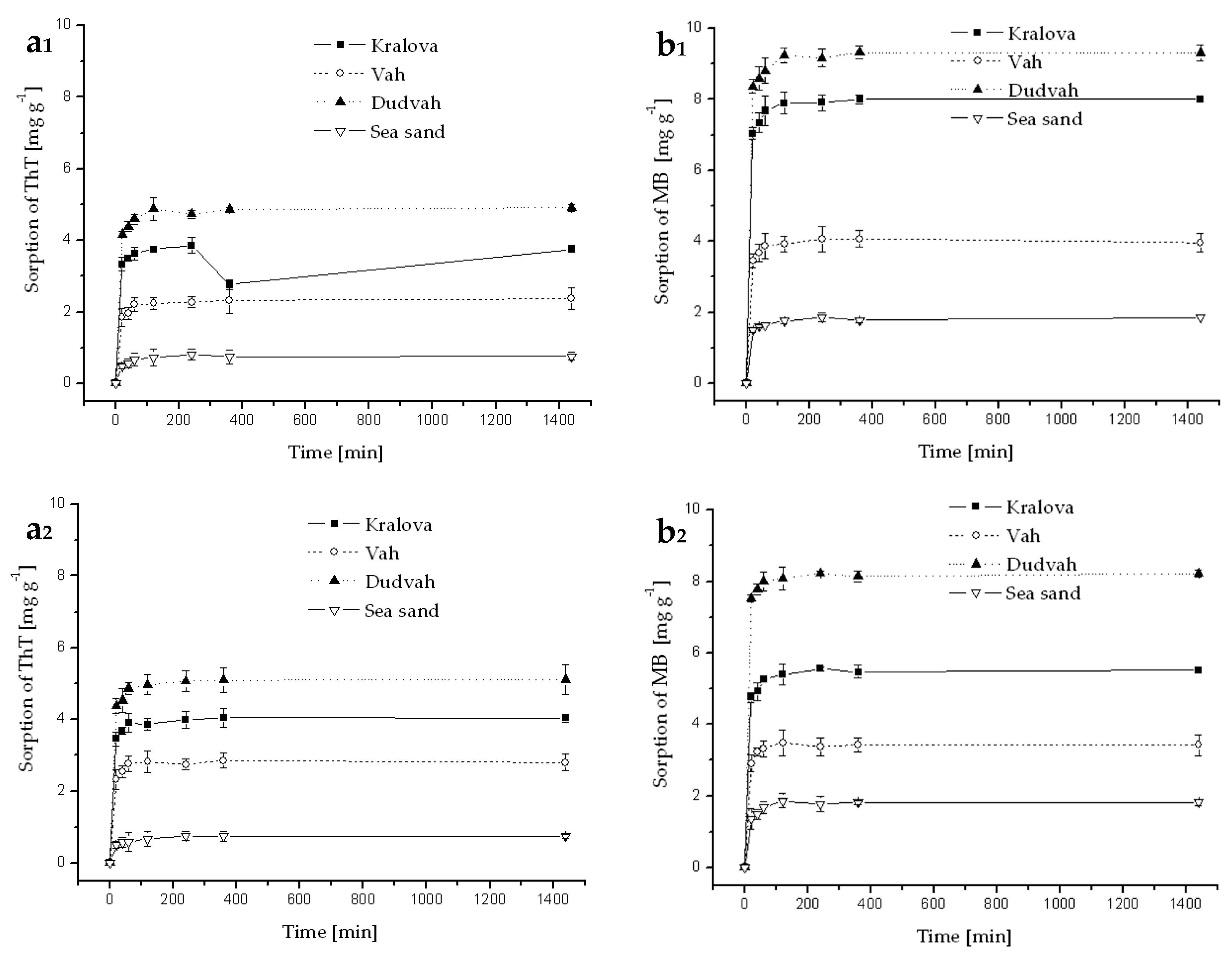
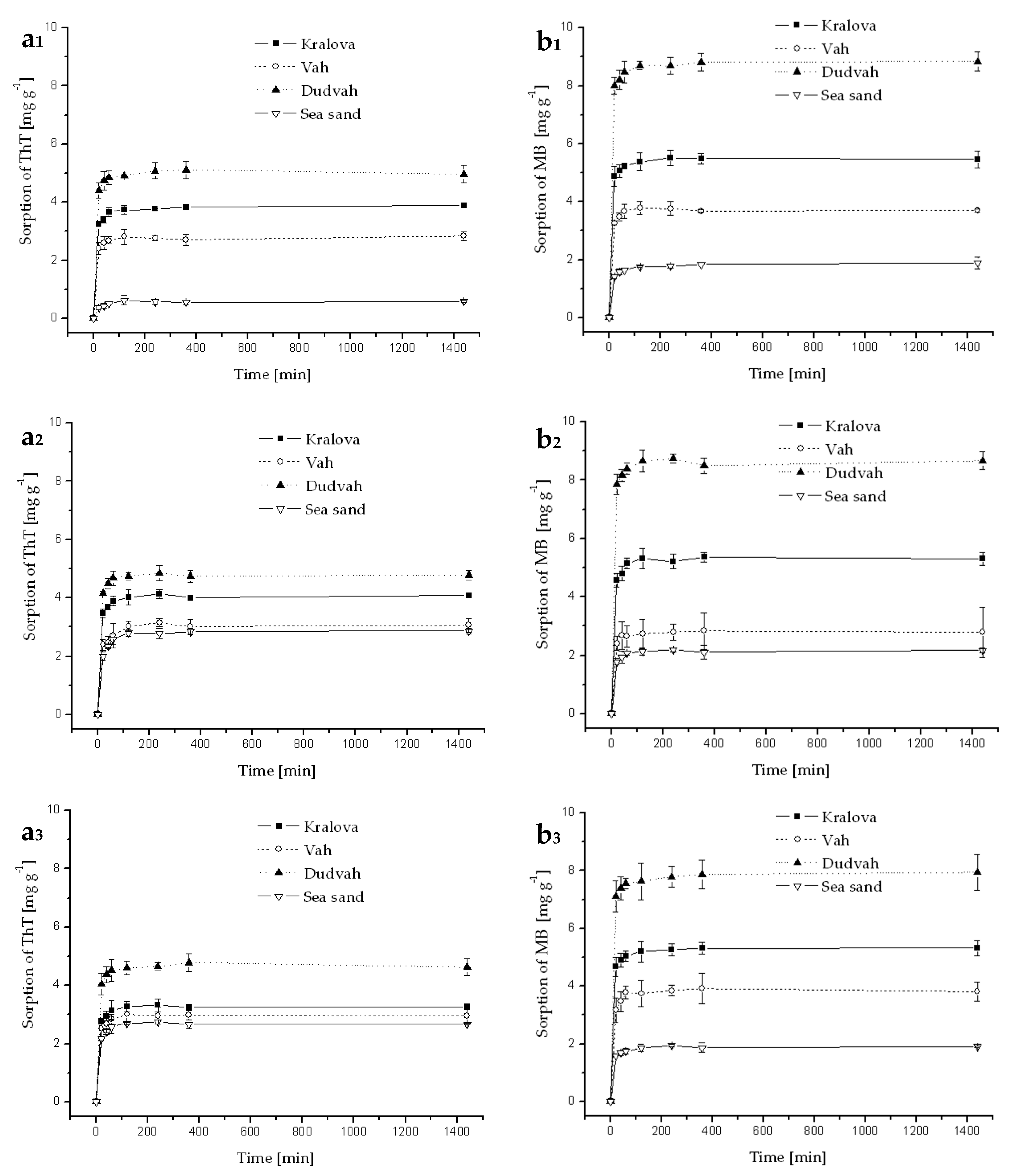
3.3. Sorption and Desorption of Dyes
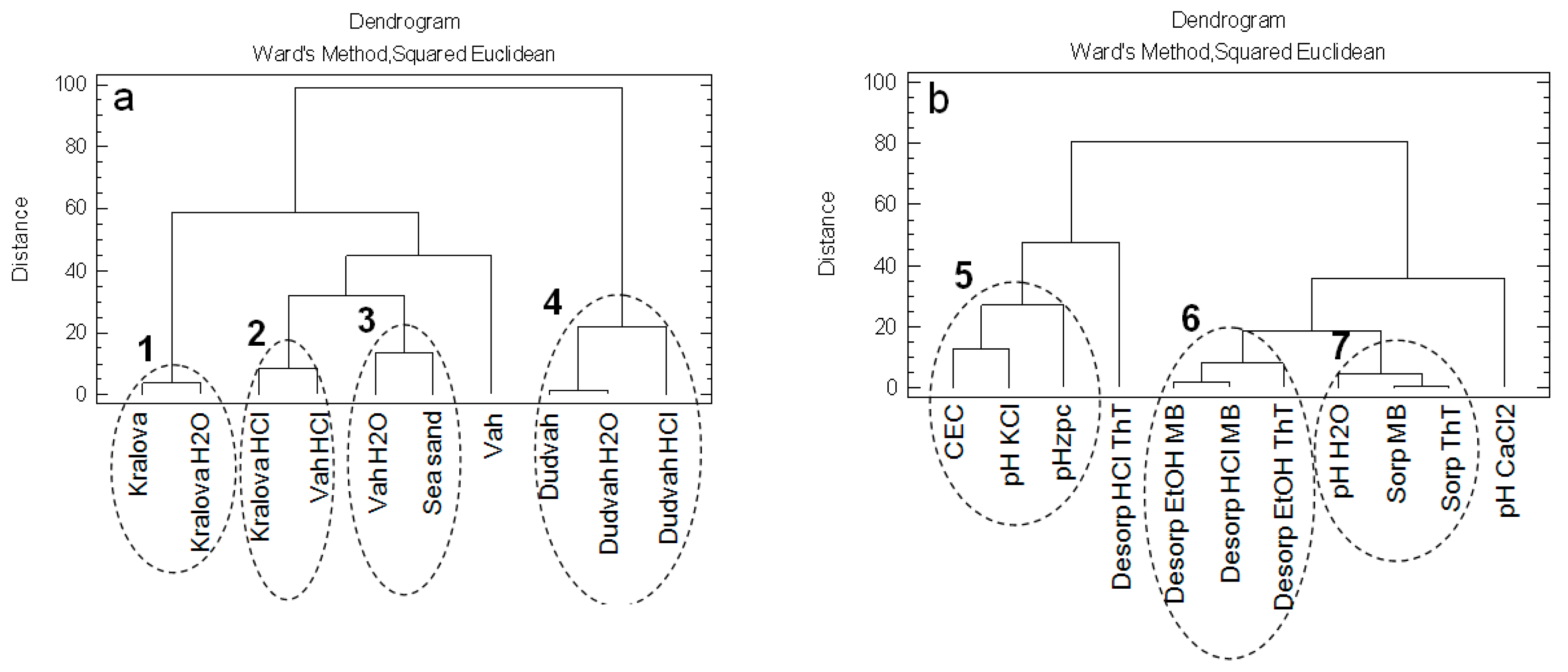
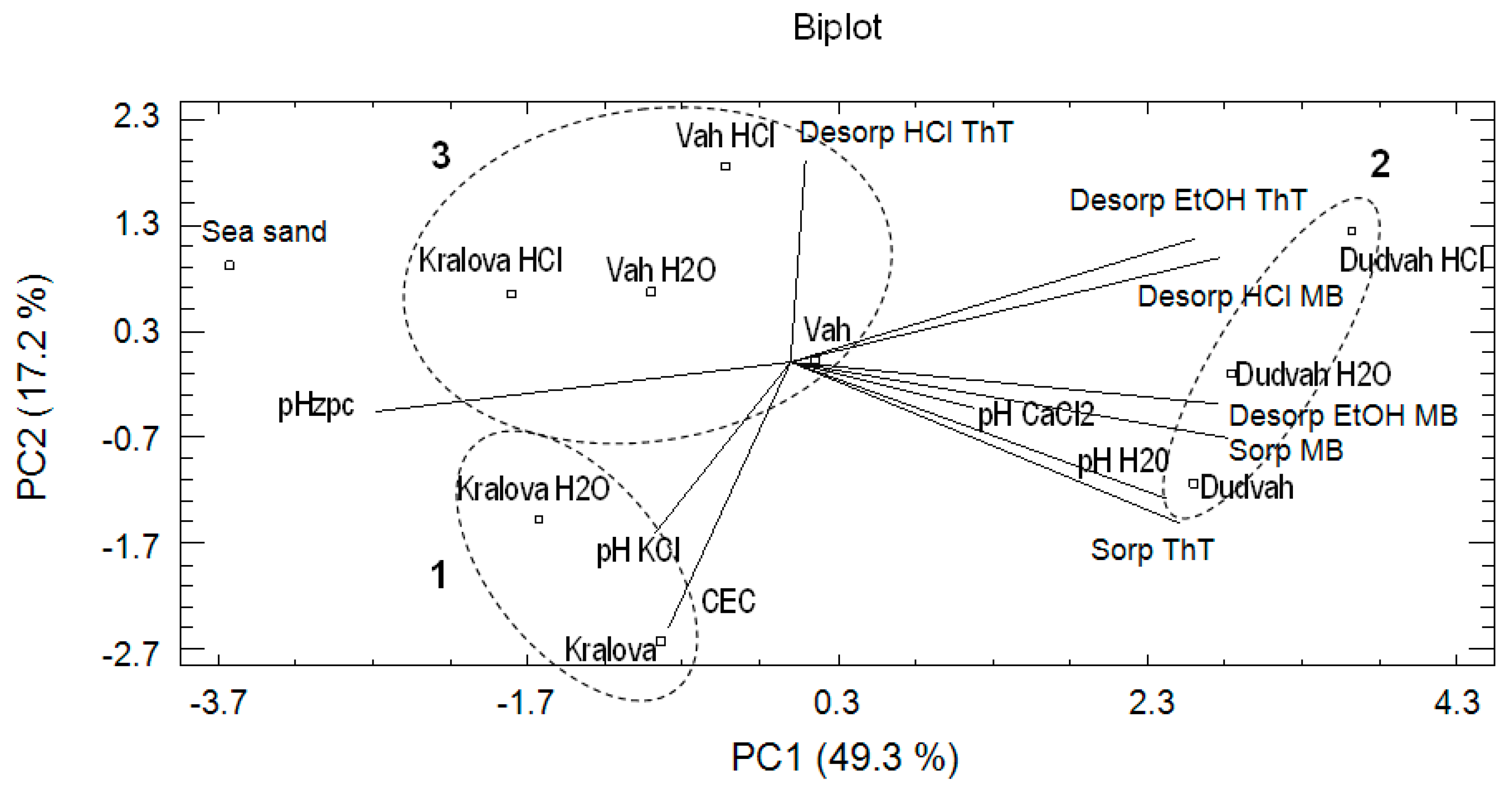
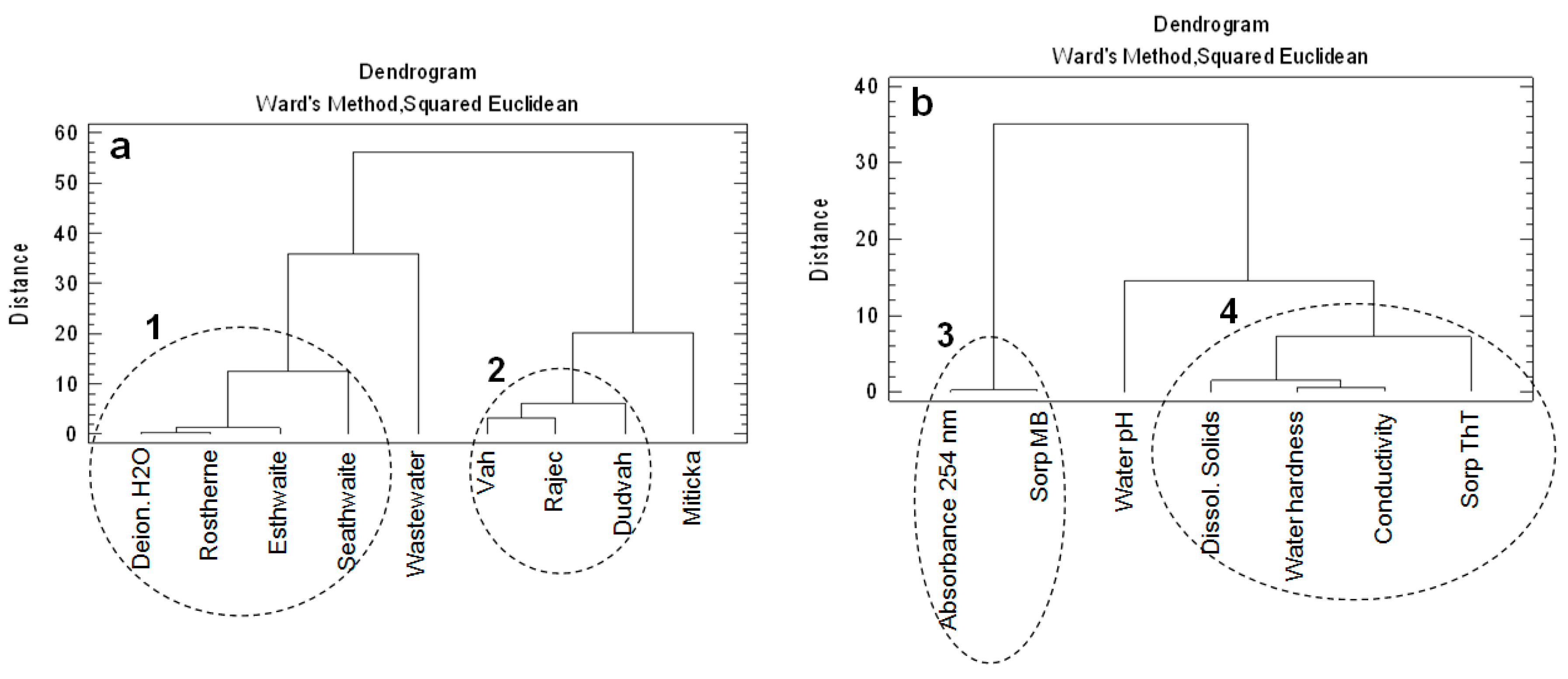
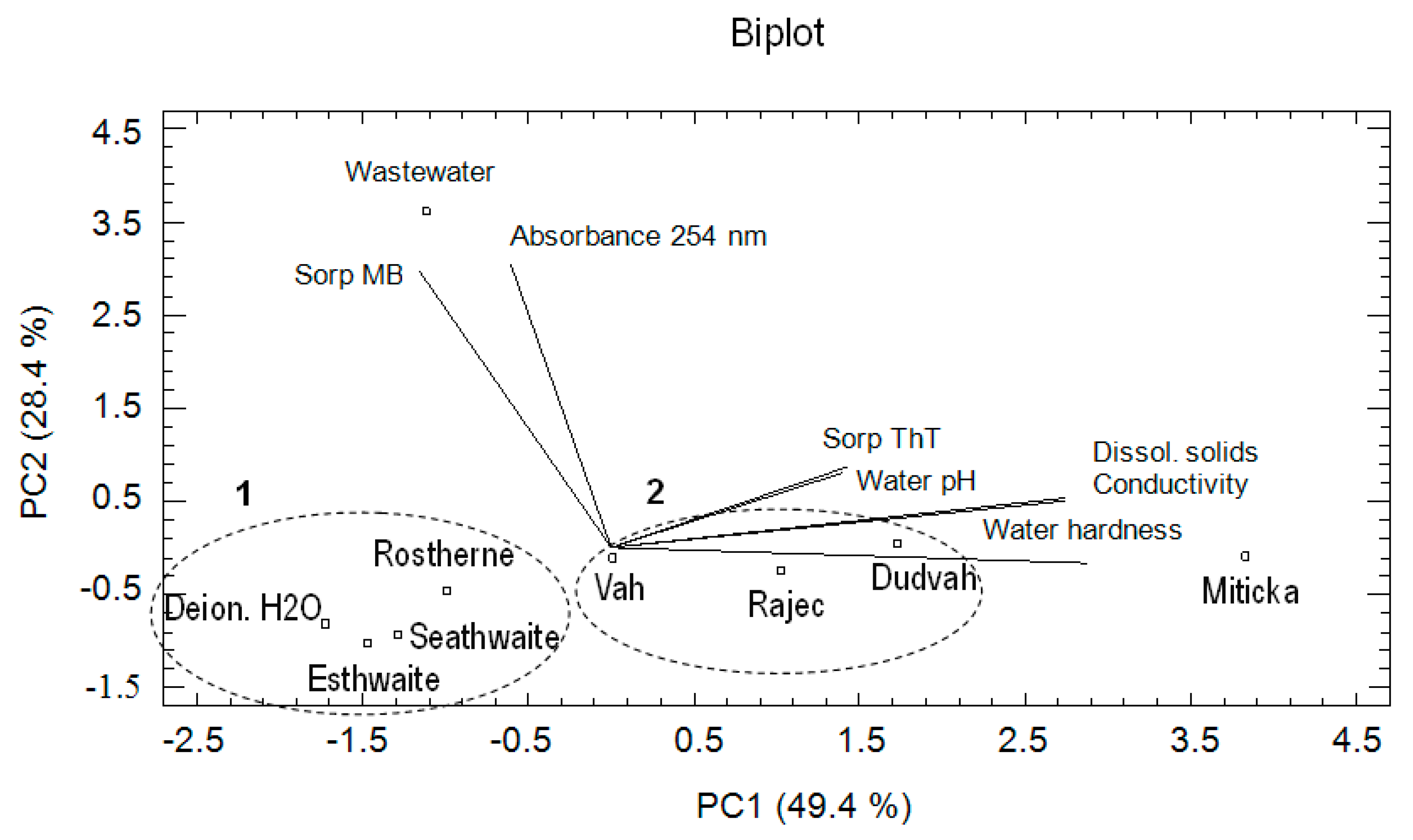
3.4. Chemometric Analysis of the Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
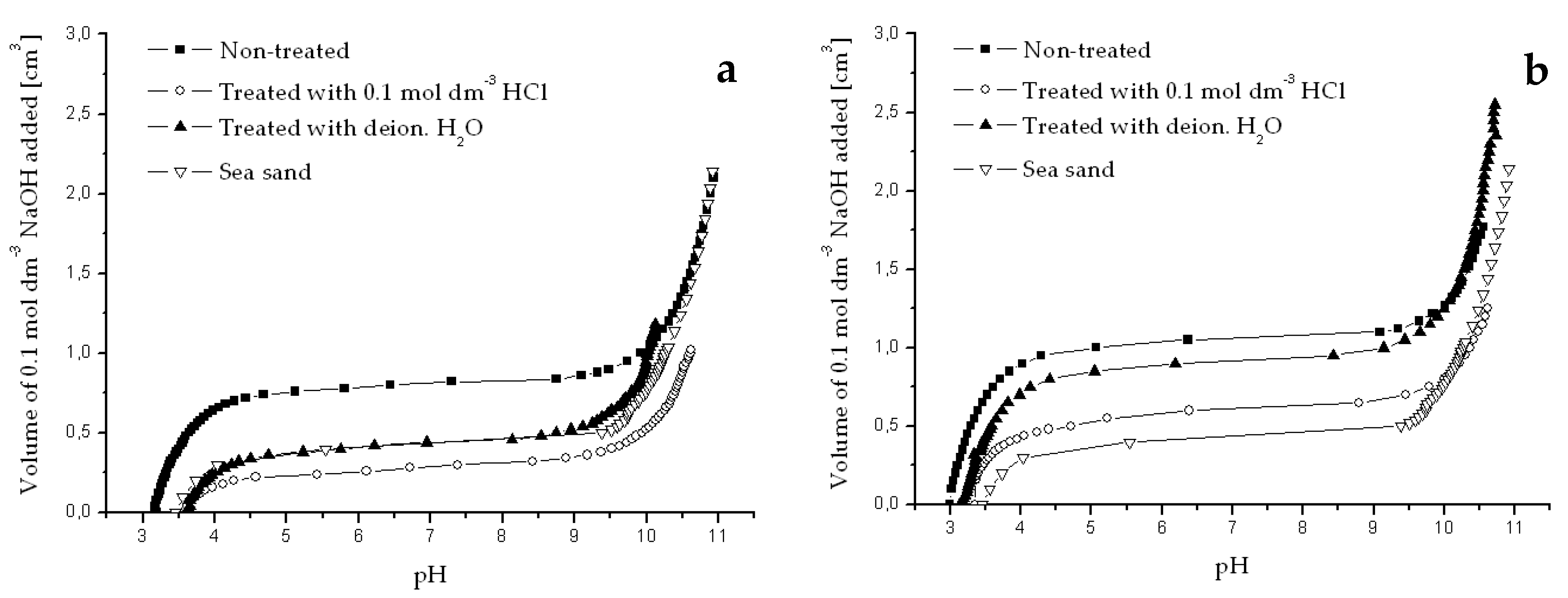
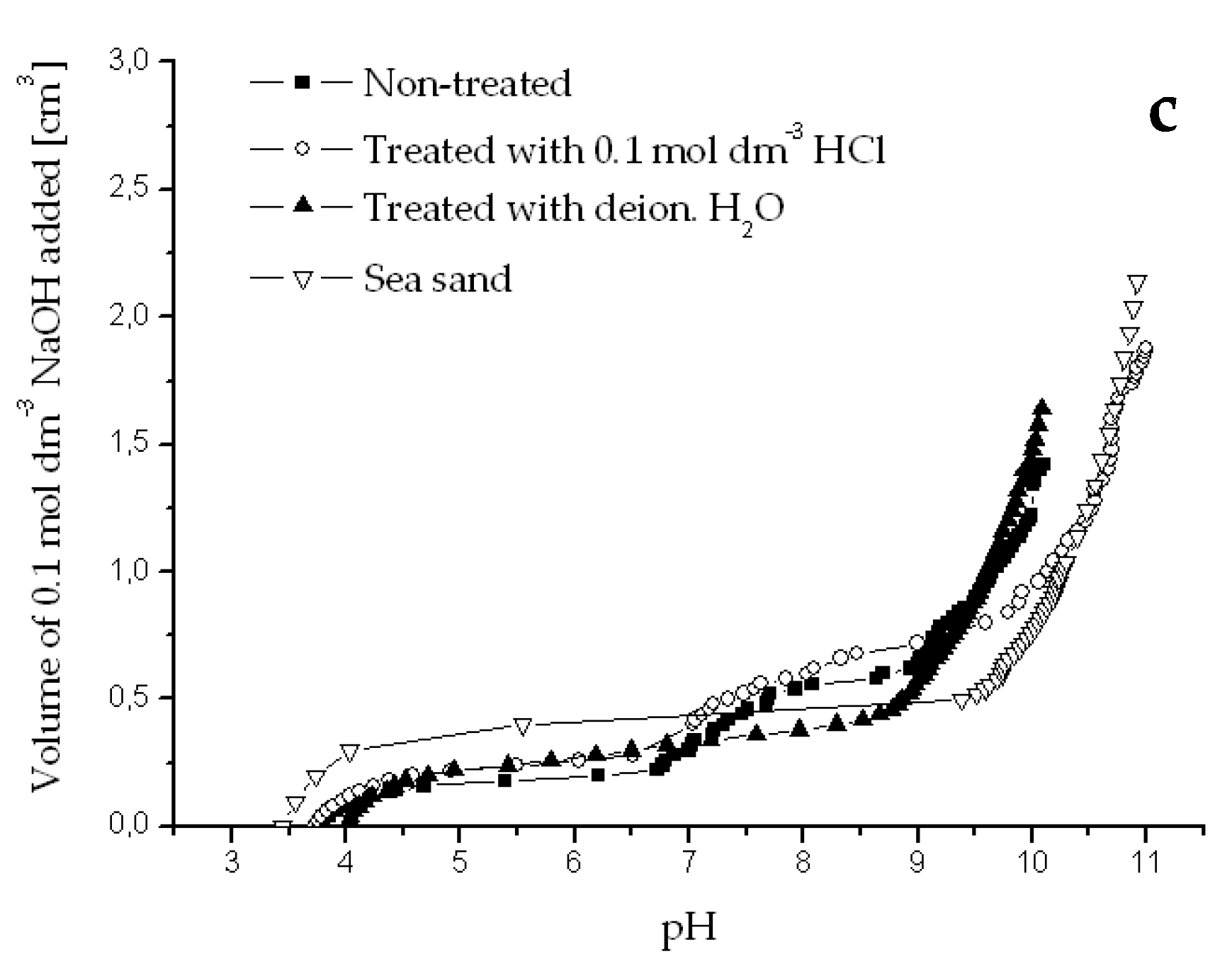
Appendix B
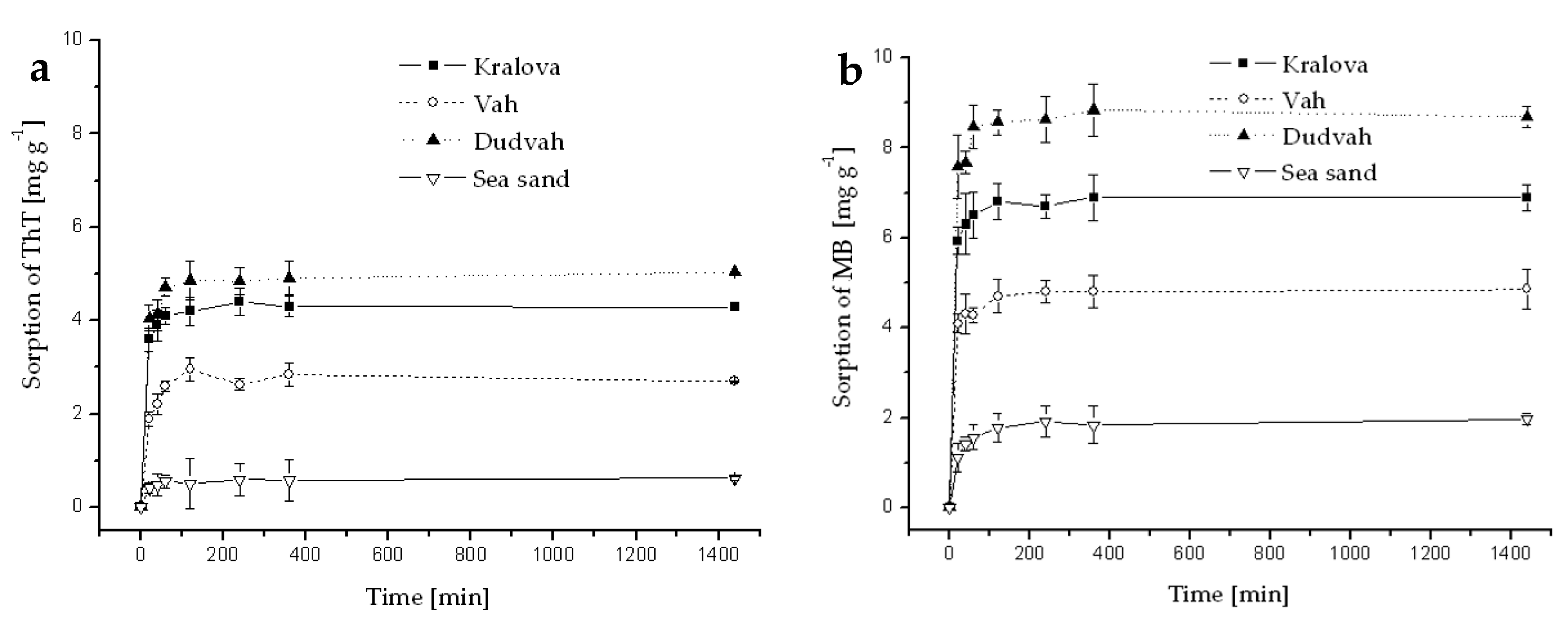
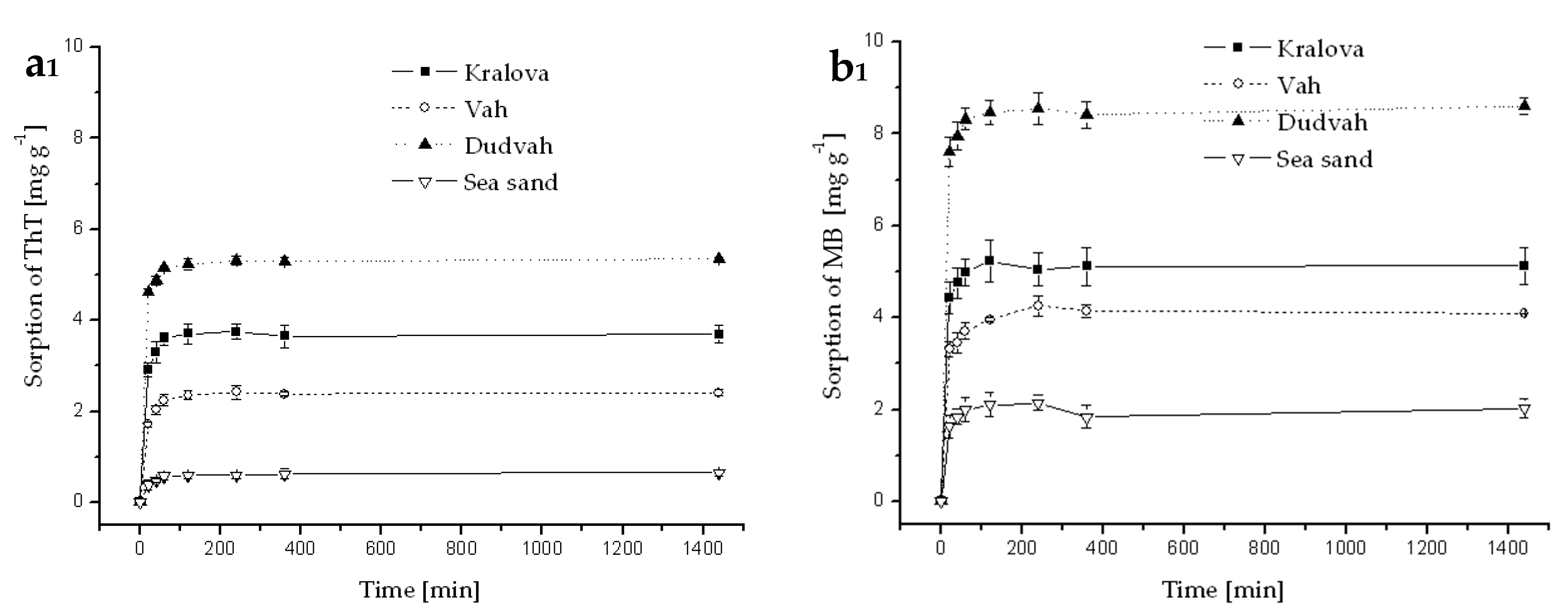

References
- Pointing, S.B. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, M.; Liang, S.C.; Zhao, L.Y.B.; Zhang, B.B. Production and synthetic dyes decolourization capacity of a recombinant laccase from Pichia pastoris. J. Appl. Microbiol. 2009, 107, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Jain, A.K. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Colloid Interface Sci. 2005, 281, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Marungrueng, K.; Pavasant, P. Removal of basic dye (Astrazon Blue FGRL) using macroalga Caulerpa lentillifera. J. Environ. Manag. 2006, 78, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.H.; Tang, H.X. Effect of dye compounds on the adsorption of atrazine by natural sediment. Chemosphere 2004, 56, 31–38. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L. Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour. Technol. 2008, 99, 2357–2363. [Google Scholar] [CrossRef]
- Scheytt, T.; Mersmann, P.; Lindstädt, R.; Heberer, T. Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere 2005, 60, 245–253. [Google Scholar] [CrossRef]
- Mutavdžić Pavlović, D.; Glavač, A.; Gluhak, M.; Runje, M. Sorption of albendazole in sediments and soils: Isotherms and kinetics. Chemosphere 2018, 193, 635–644. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, L.; Liu, L.; Shi, L.; Guang, A.; Mu, Z. Bisphenol A in the Yellow River: Sorption characteristics and influential factors. J. Hydrol. 2018, 564, 307–313. [Google Scholar] [CrossRef]
- Lewandowski, K.K.; Cieślikiewicz, W.; Kobusińska, M.E.; Niemirycz, E. Sorption of pentachlorophenol (PCP) in the marine bottom sediments—Batch sorption experiment at varying pressure. Environ. Sci. Pollut. Res. 2018, 25, 10799–10807. [Google Scholar] [CrossRef] [PubMed]
- Mutavdžić Pavlović, D.; Ćurković, L.; Grčić, I.; Šimić, I.; Župan, J. Isotherm, kinetic, and thermodynamic study of ciprofloxacin sorption on sediments. Environ. Sci. Pollut. Res. 2017, 24, 10091–10106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H. Factors influencing adsorption and desorption of trimethoprim on marine sediments: Mechanisms and kinetics. Environ. Sci. Pollut. Res. 2017, 24, 21929–21937. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Endo, S.; Gocht, T.; Barth, J.A.; Lacorte, S.; Barcelo, D.; Grathwohl, P. Sorption of alkylphenols on Ebro River sediments: Comparing isotherms with field observations in river water and sediments. Environ. Pollut. 2009, 157, 698–703. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Nema, A.K. Assessment of heavy metal contamination in Hindon River sediments: A chemometric and geochemical approach. Chemosphere 2012, 87, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G. Chemometrics: Applications of Mathematics and Statistics to Laboratory Systems; Ellis Horwood: Chichester, UK, 1990; 307p, ISBN 0-13-131350-9. [Google Scholar]
- Mrozik, W.; Kotłowska, A.; Kamysz, W.; Stepnowski, P. Sorption of ionic liquids onto soils: Experimental and chemometric studies. Chemosphere 2012, 88, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Wajda, L.; Duda-Chodak, A.; Tarko, T.; Kamiński, P. Application of principal component analysis for the optimisation of lead(II) biosorption. World J. Microbiol. Biotechnol. 2017, 33, 193. [Google Scholar] [CrossRef] [PubMed]
- Horník, M.; Šuňovská, A.; Partelová, D.; Pipíška, M.; Augustín, J. Continuous sorption of synthetic dyes on dried biomass of microalga Chlorella pyrenoidosa. Chem. Pap. 2013, 67, 254–264. [Google Scholar] [CrossRef]
- Bachratá, M.; Šuňovská, A.; Horník, M.; Pipíška, M.; Augustín, J. Sorption of synthetic dyes onto river sediments: A laboratory study. Nova Biotechnol. Chim. 2013, 12, 12–29. [Google Scholar] [CrossRef]
- Smith, E.J.; Davison, W.; Hamilton-Taylor, J. Methods for preparing synthetic freshwaters. Water Res. 2002, 36, 1286–1296. [Google Scholar] [CrossRef]
- Bracklow, U.; Drews, A.; Vocks, M.; Kraume, M. Comparison of nutrients degradation in small scale membrane bioreactors fed with synthetic/domestic wastewater. J. Hazard. Mater. 2007, 144, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R. Optimum nutrient solutions for plants. Science 1920, 52, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Xu, M.; Zheng, F.; Zhao, M. Study of the mechanisms of Cu2+ biosorption by ethanol/caustic-pretreated baker’s yeast biomass. J. Hazard. Mater. 2010, 178, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.F.; Fein, J.B. Protofit: A program for determining surface protonation constants from titration data. Comput. Geosci. 2006, 32, 1344–1356. [Google Scholar] [CrossRef]
- ISO. ISO Standard Method STN No. 11260: Kvalita pôdy. Stanovenie výmennej kapacity katiónov a hodnoty nasýtenia zásadami pomocou roztoku chloride barnatého; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- ISO. ISO Standard Method STN No. 10390: Kvalita pôdy. Stanovenie pH; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- ISO. ISO Standard Method STN No. 6059: Kvalita vody. Stanovenie sumy vápnika a horčíka. Titračná metóda s EDTA; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- Slovak Office of Standards, Metrology and Testing. Standard Method STN No. 75 7360: Kvalita vody. Stanovenie absorbancie; Slovak Office of standards, Metrology and Testing: Bratislava, Slovak Republic, 1991. [Google Scholar]
- Slovak Office of Standards, Metrology and Testing. Standard Method STN No. 75 7373: Kvalita vody. Stanovenie rozpustených látok; Slovak Office of standards, Metrology and Testing: Bratislava, Slovak Republic, 2007. [Google Scholar]
- Raux, J.; Copard, Y.; Laignel, B.; Fournier, M.; Masseï, N. Classification of worldwide drainage basins through the multivariate analysis of variables controlling their hydrosedimentary response. Glob. Planet. Change 2011, 76, 117–127. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis—Mineralogical, Organic and Inorganic Methods; Springer: Heidelberg, Germany, 2006; 993p, ISBN 978-3-540-31211-6. [Google Scholar]
- Cornelissen, G.; Gustafsson, O.; Bucheli, T.D.; Jonker, M.T.O.; Koelmans, A.A.; Van Noort, P.C.M. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005, 39, 6881–6895. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; 548p, ISBN 978-1-420-09370-4. [Google Scholar]
- US EPA. Mid-Atlantic Integrated Assessment (MAIA) Estuaries, 1997–98: Summary Report; U.S. EPA: Research Triangle Park, NC, USA, 2002.
- Zaharescu, D.G.; Hooda, P.S.; Soler, A.P.; Fernandez, J.; Burghelea, C.I. Trace metals and their source in the catchment of the high altitude Lake Respomuso, Central Pyrenees. Sci. Total Environ. 2009, 407, 3546–3553. [Google Scholar] [CrossRef]
- Yin, H.; Gao, Y.; Fan, C. Distribution, sources and ecological risk assessment of heavy metals in surface sediments from Lake Taihu, China. Environ. Res. Lett. 2011, 6, 044012. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Kavitha, D.; Swaminathan, K.; Yun, S.E. Aerated and rotated mode decolorization of a textile dye solution by native and modified mycelial biomass of Trametes versicolor. J. Chem. Technol. Biot. 2007, 82, 350–359. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Mao, J.; Yun, Y.S. Biosorption of methylene blue from aqueous solution using free and polysulfone-immobilized Corynebacterium glutamicum: Batch and column studies. Bioresour. Technol. 2008, 99, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Malekbala, M.R.; Hosseini, S.; Yazdi, S.K.; Soltani, S.M.; Malekbala, M.R. The study of the potential capability of sugar beet pulp on the removal efficiency of two cationic dyes. Chem. Eng. Res. Des. 2012, 90, 704–712. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Huang, Q.H.; Li, W.Y.; Tang, Y.J.; Zhao, J.F. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai, China. Sci. Total Environ. 2009, 407, 2931–2938. [Google Scholar] [CrossRef] [PubMed]

| Synthetic Freshwater | Stock Solution | Solution Composition | Concentration g dm−3 | Solution Preparation * |
|---|---|---|---|---|
| Seathwaite Tarn water (pH = 4.3 χ = 276 µS cm−1) | A1 | NaCl MgCl2·6H2O CaCl2 KCl NaNO3 | 4.27 6.67 6.12 0.746 3.06 | 1 cm3 |
| A2 | Na2SO4 | 5.97 | 1 cm3 | |
| A3 | AlCl3·6H2O HCl | 1.45 7 cm3 1 mol dm−3 HCl | 1 cm3 | |
| Esthwaite water (pH = 6.6 χ = 137 µS cm−1) | S1 | MgCl2·6H2O CaCl2 Ca(NO3)2·4H2O | 12.2 17.5 3.54 | 1 cm3 |
| S2 | CaCO3 | 0.0187 | 910 cm3 | |
| S3 | Na2SO4 KHCO3 NaHCO3 | 16.3 2.50 1.68 | 1 cm3 | |
| Rostherne Mere water (pH = 7.0 χ = 111 µS cm−1) | H1 | CaCl2 Ca(NO3)2·4H2O | 7.49 1.18 | 10 cm3 |
| H2 | CaCO3 | 0.0916 | 910 cm3 | |
| H3 | KHCO3 KH2PO4 | 0.751 0.408 | 10 cm3 | |
| H4 | NaHCO3 Na2SO4 | 2.27 2.81 | 10 cm3 | |
| H5 | MgSO4·7H2O | 10.0 | 10 cm3 |
| Matrix | Treated with | pHH2O | pHCaCl2 | pHKCl | CEC meq/100 g | pHzpc |
|---|---|---|---|---|---|---|
| Dudvah | Non-treated | 8.40 | 7.63 | 7.95 | 3.20 | 8.58 |
| Deion. H2O | 8.44 | 7.60 | 7.97 | 2.88 | 8.61 | |
| 0.1 mol dm−3 HCl | 8.44 | 7.63 | 7.99 | 2.88 | 7.48 | |
| Vah | Non-treated | 7.98 | 8.35 | 7.63 | 2.93 | 10.5 |
| Deion. H2O | 8.04 | 7.47 | 8.30 | 3.20 | 10.6 | |
| 0.1 mol dm−3 HCl | 7.19 | 7.38 | 7.49 | 4.00 | 8.63 | |
| Kralova | Non-treated | 7.91 | 7.55 | 8.56 | 12.6 | 9.78 |
| Deion. H2O | 8.06 | 7.38 | 8.44 | 7.60 | 10.2 | |
| 0.1 mol dm−3 HCl | 7.24 | 7.10 | 7.37 | 4.00 | 10.8 | |
| Sea sand | Non-treated | 7.33 | 7.39 | 8.60 | 2.60 | 10.8 |
| Environment | pH | Conductivity μS cm−1 | CCa+Mg mmol dm−3 | Dissolved Solids g dm−3 | Absorbance at 254 nm |
|---|---|---|---|---|---|
| Dudvah River water | 7.56 | 718 | 4.44 | 0.686 | 0.053 |
| Vah River water | 7.18 | 401 | 2.07 | 0.286 | 0.076 |
| Deion. H2O | 7.14 | 0.054 | 0.19 | 0.032 | 0.000 |
| Esthwaite 1 | 6.60 | 137 | 0.71 | 0.054 | 0.007 |
| Rostherne Mere 1 | 7.00 | 111 | 1.29 | 0.234 | 0.004 |
| Seathwaite Tarn 1 | 4.30 | 276 | 0.49 | 0.038 | 0.001 |
| Mineral water A 2 | 7.79 | 434 | 2.80 | 0.292 | 0.012 |
| Mineral water B 3 | 7.20 | 1138 | 10.1 | 0.750 | 0.013 |
| Wastewater 4 | 6.92 | 356 | 0.51 | 0.238 | 0.865 |
| Water | Anions mg dm−3; ±SD | |||||||||||||
| Cl− | NO3− | SO42− | CO32− | HCO3− | H2PO4− | NO2− | F− | |||||||
| Dudvah River water | 35.7 ± 1.0 | 20.7 ± 0.8 | 76.3 ± 2.2 | n.a. | n.a. | n.a. | n.a. | n.a. | ||||||
| Vah River water | 3.18 ± 0.10 | 2.32 ± 0.07 | 8.28 ± 0.29 | n.a. | n.a. | n.a. | n.a. | n.a. | ||||||
| Deion. H2O | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ||||||
| Esthwaite 1 | 15.5 | 1.86 | 11.0 | 10.2 | 2.75 | n.a. | n.a. | n.a. | ||||||
| Rostherne Mere 1 | 47.9 | 23.6 | 58.0 | 50.0 | 20.7 | 2.90 | n.a. | n.a. | ||||||
| Seathwaite Tarn 1 | 9.82 | 2.23 | 4.04 | n.a. | n.a. | n.a. | n.a. | n.a. | ||||||
| Mineral water A 2 | 4.30 ± 0.13 | 8.90 ± 0.26 | 20.3 ± 0.7 | n.a. | 321 | n.a. | < 0.01 | 0.10 ± 0.01 | ||||||
| Mineral water B 3 | 17.7 ± 0.7 | 4.11 ± 0.12 | 18.6 ± 0.5 | n.a. | 1370 | n.a. | < 0.01 | n.a. | ||||||
| Wastewater 4 | 8.58 | n.a. | 18.2 | n.a. | n.a. | 16.5 | n.a. | n.a. | ||||||
| Water | Cations/metals mg dm−3; * µg dm−3; ±SD | |||||||||||||
| Cd2+ | Pb2+ | Cu2+ | Zn2+ | Ni2+ | Mg2+ | Ca2+ | Na+ | K+ | Al3+ | NH4+ | Fe2+ | Co2+ | Mn2+ | |
| Dudvah River water | n.d. | 2.19 ± 0.06 * | 2.43 ± 0.08 * | 15.0 ± 0.4 * | 2.58 ± 0.06 * | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Vah River water | n.d. | 1.65 ± 0.05 * | 1.94 ± 0.05 * | 6.96 ± 0.19 * | 2.31 ± 0.07 * | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Deion. H2O | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Esthwaite 1 | n.a. | n.a. | n.a. | n.a. | n.a. | 1.46 | 13.7 | 5.75 | 0.98 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Rostherne Mere 1 | n.a. | n.a. | n.a. | n.a. | n.a. | 9.87 | 62.5 | 15.3 | 4.10 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Seathwaite Tarn 1 | n.a. | n.a. | n.a. | n.a. | n.a. | 0.80 | 2.21 | 4.44 | 0.39 | 0.16 | n.a. | n.a. | n.a. | n.a. |
| Mineral water A 2 | n.a. | n.a. | n.a. | n.a. | n.a. | 19.0 | 87.4 | 2.20 | 0.70 | n.a. | < 0.02 | n.a. | n.a. | n.a. |
| Mineral water B 3 | n.a. | n.a. | n.a. | n.a. | n.a. | 105 | 271 | 27.6 | 2.30 | n.a. | < 0.05 | n.a. | n.a. | n.a. |
| Wastewater 4 | n.a. | n.a. | 20.4 * | 15.0 * | n.a. | 4.05 | n.a. | 1.14 * | 6.91 | n.a. | 16.5 | 1.17 | 74.3 * | 0.11 |
| Object/Process | Maximum Qs Values | Minimum Qs Values |
| Dye | MB | ThT |
| Sediment | Sediment from Dudvah River | Sea sand |
| Environment | Deion. H2O | Mineral water B 1 |
| Sediment treatment | Non-treatment | Treatment with 0.1 mol dm−3 HCl |
| Object/process | Maximum D% values | Minimum D% values |
| Desorption agent | 96% vol. Ethanol | 0.1 mol dm−3 HCl |
| Sediment | Dudvah River | Sea sand |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koprdová, A.; Bachratá, M.; Adamcová, V.; Valica, M.; Pipíška, M.; Horník, M. Chemometric Characterization of Synthetic Dye Sorption onto Slovakian River Sediments: A Laboratory Batch Experiment. Separations 2018, 5, 57. https://doi.org/10.3390/separations5040057
Koprdová A, Bachratá M, Adamcová V, Valica M, Pipíška M, Horník M. Chemometric Characterization of Synthetic Dye Sorption onto Slovakian River Sediments: A Laboratory Batch Experiment. Separations. 2018; 5(4):57. https://doi.org/10.3390/separations5040057
Chicago/Turabian StyleKoprdová, Anna, Miroslava Bachratá, Vanda Adamcová, Martin Valica, Martin Pipíška, and Miroslav Horník. 2018. "Chemometric Characterization of Synthetic Dye Sorption onto Slovakian River Sediments: A Laboratory Batch Experiment" Separations 5, no. 4: 57. https://doi.org/10.3390/separations5040057
APA StyleKoprdová, A., Bachratá, M., Adamcová, V., Valica, M., Pipíška, M., & Horník, M. (2018). Chemometric Characterization of Synthetic Dye Sorption onto Slovakian River Sediments: A Laboratory Batch Experiment. Separations, 5(4), 57. https://doi.org/10.3390/separations5040057




